Open Access Article
Open Access ArticleSynthesis and characterization of Mn-doped CsPb(Cl/Br)3 perovskite nanocrystals with controllable dual-color emission
Pengchao Wang ,
Bohua Dong*,
Zhenjie Cui,
Rongjie Gao,
Ge Su,
Wei Wang and
Lixin Cao*
,
Bohua Dong*,
Zhenjie Cui,
Rongjie Gao,
Ge Su,
Wei Wang and
Lixin Cao*
School of Materials Science and Engineering, Ocean University of China, 238 Songling Road, Qingdao, 266100, P. R. China. E-mail: dongbohua@ouc.edu.cn; caolixin@ouc.edu.cn
First published on 9th January 2018
Abstract
Metal-halide perovskite nanocrystals (NCs) are considered to be promising types of optoelectronic and photonic materials. The emission colors of the cesium lead halide perovskite (CsPbX3, X = Cl, Br, I) NCs depend on the joint influence of the emission peaks of the host and its dopant ions. Herein, we report a phosphine-free strategy to synthesize Mn-doped CsPb(Cl/Br)3 NCs to tune their optical properties in a wide color gamut. Colloidal Mn-doped CsPb(Cl/Br)3 NCs were synthesized by injecting Cs-oleate solution into the MnCl2 and PbBr2 precursor solution. The as-prepared Mn-doped CsPb(Cl/Br)3 NCs are highly crystalline and uniform sized nanocubes with two emission peaks, including the host emission around 450 nm and the Mn2+ dopant emission around 600 nm, which are sensitive to the MnCl2-to-PbBr2 molar feed ratio and the reaction temperature. By varying the MnCl2-to-PbBr2 molar feed ratio or the reaction temperature, the relative PL intensities of dual color emission can be manipulated, showing their ability in tunable color output.
1. Introduction
Solution-processed hybrid and inorganic lead halide perovskites have attracted much attention in the past several years due to their excellent emission properties, high photoluminescence quantum yields (PLQY), small exciton binding energy and balanced electron and hole mobility lifetime,1–8 which means that these materials have great potential in photophysical applications, including light emitting diodes, lasers and photodetectors.9–19 However, the hybrid lead halide perovskites are extremely sensitive to oxygen and moisture,20–22 which greatly restricts their applications. Compared with the organic–inorganic perovskite nanocrystals, the all-inorganic cesium lead halide (CsPbX3, X = Cl, Br, I) nanocrystals are much more stable. In addition, due to the high photoluminescence quantum yields without any additional surface passivation and tunable optical properties throughout the entire visible spectrum, the CsPbX3 perovskite nanocrystals have great potential in optoelectronic applications.23,24Up to date, the synthetic protocols for colloidal perovskite NCs are focused on hot-injection,25,26 anion–exchange reactions,27–31 room-temperature synthesis,32 and ultrasonication.33,34 Kovalenko and his coworkers first synthesized the all-inorganic CsPbX3 perovskite NCs by injecting the prepared Cs-oleate into an octadecene solution containing PbX2 (X = Cl, Br, I) and ligands (oleic acid and oleylamine) at a temperature of 140–200 °C.25 Under this method, they prepared monodisperse CsPbX3 nanocrystals with bright photoluminescence (PL) and narrow full width at half maximum (FWHM). Following their pioneering work, great processes have been made and a variety of shapes of CsPbX3 NCs, such as cubes,25,35 nanowires26,36–39 and nanoplatelets, are obtained. Furthermore, the PL spectra of the CsPbX3 NCs can be tuned over nearly the entire visible spectral region by tuning the composition of the perovskite NCs through the anion–exchange reactions.27,29
The introduction of transition metal ions in II–VI and III–V semiconductor NCs provides another method to adjust the optical, electronic and magnetic properties to afford the parent NCs with brand new functions.40–42 The most commonly used transition metal ion is Mn2+. And the Mn-doped II–VI semiconductors (ZnS, ZnSe, CdS, CdSe) could generate intense doped luminescence of Mn2+ ions, which is attribute to energy transfer from excited host semiconductors to Mn2+ ions.43 In consideration of the similar ionic radius and same valence state of Mn2+ and Pb2+ ions, it is possible to synthesis the Mn-doped CsPbX3 NCs. The recent progress in synthesizing Mn-doped CsPbCl3 NCs also confirms its feasibility.44,45 Despite the successes in the preparation of Mn-doped CsPbCl3 NCs, detailed research into the synthesis of Mn-doped CsPb(Cl/Br)3 NCs and tailoring of its optical properties is still rare.
In this paper, we report the phosphine-free colloidal synthesis of Mn-doped CsPb(Cl/Br)3 NCs to tune their optical properties in a wide color gamut. The synthesis was performed by injecting Cs-oleate solution into the PbBr2 and MnCl2 precursor solution. The as-prepared Mn-doped CsPb(Cl/Br)3 NCs are highly crystalline and uniform sized nanocubes with two emission peaks, including the host emission around 450 nm and the Mn2+ dopant emission around 600 nm, which are both sensitive to the MnCl2-to-PbBr2 molar feed ration or the reaction temperature. By changing the MnCl2-to-PbBr2 molar feed ration or the reaction temperature, the relative PL intensities of dual color emission can be well manipulated and shows a wide color gamut, indicating their potential in light emitting devices.
2. Experimental
2.1 Chemicals
Cesium carbonate (Cs2CO3, 99%), lead bromide (PbBr2, 99%), manganese chloride (MnCl2, ≧99%), 1-octadecene (ODE, ≧90%), oleic acid (OA, 90%), oleylamine (OAm, 80–90%) and cyclohexane (CYH, ≧99%) were purchased from Shanghai Aladdin Industrial Corporation Co., Ltd. Acetone was purchased from Yantai Far Eastern Fine Chemical Co., Ltd. All chemicals were used without any further purification.2.2 Preparation method
2.3 Characterization
X-ray diffraction (XRD) patterns were measured using a BRUKER D8 ADVANCE X-ray diffractometer with a CuKα source.Transmission electron microscope (TEM) images were obtained on a JEOL-JEM 1200 TEM at an accelerating voltage of 100 kV.
Photoluminescence (PL) spectra were collected using an Edinburgh FLS 980 fluorescence spectrophotometer.
High-resolution TEM (HRTEM) images were taken with a FEI Tecnai TEM at an accelerating voltage of 200 kV.
Ultraviolet and visible absorption (UV-vis) spectra were performed by a Shimadzu UV-2550 ultraviolet-visible spectrometer with an integrating sphere.
Inductive coupled plasma (ICP) were taken with a Thermal ICAP 6300 ICP spectrometer to determine the Mn/Pb elemental ratio.
The photoluminescence quantum yields (PLQY) of the nanocrystal samples were obtained by comparing the integrated PL intensities of the nanocrystals and the standard dye (quinine sulfate), with 55.7% PLQY in 0.1 mol L−1 H2SO4 under 320 nm exciton source. Refractive index of 0.1 mol L−1 H2SO4 and cyclohexane were 1.344 and 1.427, respectively. According to the following formula, PLQY could be obtained:
| Φf1/Φf2 = F1/F2 × A2/A1(η1/η2)2 |
3. Results and discussion
The Mn-doped CsPb(Cl/Br)3 NCs are prepared in colloidal solution by introducing MnCl2 into PbBr2 precursors. In a typical synthesis, MnCl2 and PbBr2 with the molar feed ratio of 1.35 are mixed in a mixture of OA, OAm and ODE under vacuum at 120 °C to remove the water. Then it is vital important to raise the temperature over 200 °C for the complete solubilization of the metal salts. Afterwards, the temperature is lowed to 180 °C and Cs-oleate solution is rapidly injected into the precursor solution to get the Mn-doped CsPb(Cl/Br)3 NCs. The nanostructures of the as-prepared Mn-doped CsPb(Cl/Br)3 NCs were obtained with TEM. TEM image in Fig. 1a shows that the as-prepared Mn-doped CsPb(Cl/Br)3 NCs have a cubic morphology with a uniform average size of 11.5 nm. X-ray diffraction (XRD) was used to elucidate the phase structure of the Mn-doped CsPb(Cl/Br)3 NCs. As shown in Fig. 1d, all of the diffraction peaks of Mn-doped CsPb(Cl/Br)3 NCs moved slightly to lower angles compared to the undoped cubic CsPbCl3 nanocrystals (space group Pm3m, PDF#73-0692). This could be attributed to the incorporation of Br− ions into CsPbCl3 NCs, which leads to the expansion of the cell, so all the peaks move to lower angles. The XRD also shows that the as-prepared Mn-doped CsPb(Cl/Br)3 NCs could be ascribed to the same cubic phase with undoped CsPbCl3 NCs, which means the doping process do not affect the cubic perovskite crystal structure as a result of the rigidity of its cationic framework. High-resolution TEM (HRTEM) image (Fig. 1b) reveals an interplanar spacing of 5.69 Å for its (100) plane set, which is larger than the 5.6 Å of CsPbCl3. This also confirms the host of the as-prepared Mn-doped NC is CsPb(Cl/Br)3. The width of the XRD peaks and the HRTEM image reflects the high crystalline nature of the Mn-doped CsPb(Cl/Br)3 NCs.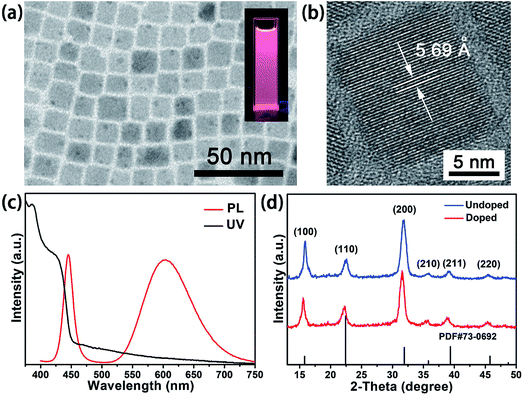 | ||
| Fig. 1 TEM image (a), HRTEM image (b), PL emission and UV-vis absorption spectra (c) and XRD pattern of Mn-doped CsPb(Cl/Br)3 NCs (d). Inset in (a): PL image excited by 365 nm UV light. | ||
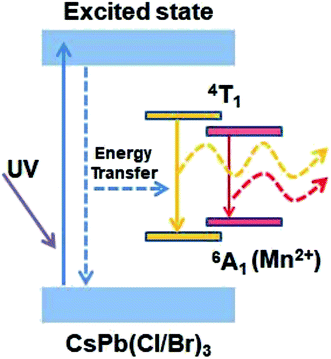
The optical properties of the as-synthesized Mn-doped CsPb(Cl/Br)3 NCs were studied by measuring ultraviolet and visible absorption (UV-vis) spectra and photoluminescence (PL) spectra of the material dispersed in cyclohexane (Fig. 1c). The inset of Fig. 1a shows that the solution of Mn-doped CsPb(Cl/Br)3 NCs exhibits a deep pink color emission under a 365 nm UV-lamp. The absorption peak of the Mn-doped CsPb(Cl/Br)3 NCs is around 424 nm. The PL spectra shows distinctive dual color emissions. The narrow band emission centered at 445 nm with FWHM 18 nm is assigned to the CsPb(Cl/Br)3 host. And, the broad emission centered at 603 nm with FWHM 87.5 nm is assigned to the Mn2+ ions doped in the CsPb(Cl/Br)3 NCs, which can be attribute to a transition between 4T1 and 6A1 energy levels of the Mn2+ 3d states. The energy levels and fluorescent mechanism of Mn-doped CsPb(Cl/Br)3 NCs is shown in Fig. 2. The intense Mn2+ luminescence indicates that the doped Mn2+ ions could be served as an efficient acceptor of the energy from the excited CsPb(Cl/Br)3 host. Although no evidence from microscopy that Mn2+ ions is incorporated into the lattice of the nanocrystal, the PL spectrum sufficiently confirms the existence of Mn2+ ions in the as-prepared CsPb(Cl/Br)3 NCs.
The optical properties of the Mn-doped CsPb(Cl/Br)3 NCs can be tailored by tuning the MnCl2-to-PbBr2 molar feed ration. By fixing the reaction temperature at 180 °C, the PL spectrum of the Mn-doped CsPb(Cl/Br)3 NCs with different MnCl2-to-PbBr2 molar feed ratio (from 1.05 to 2.25) is shown in Fig. 3a. As mentioned above, there are two emissions of the Mn-doped CsPb(Cl/Br)3 NCs, the emission of CsPb(Cl/Br)3 host and Mn2+ ions respectively. By increasing the MnCl2-to-PbBr2 molar feed ratio, the narrow PL emission of the CsPb(Cl/Br)3 host shows a blue shift from 457 to 422 nm, which is attribute to the increase of the Cl-to-Br molar ratio in the CsPb(Cl/Br)3 host. The change of the Br-to-Cl molar ratio also leads to a slight blue shift of the UV-vis absorption (Fig. 3b). At the same time, the broad emission of the Mn2+ ions shows a red shift from 597 nm to 613 nm. This is attributed to the formation of more Mn-to-Mn pairs with the increasing Mn percentage,46,47 which decreased the energy gap of 4T1–6A1 (Fig. 2) and resulted the red shifting of Mn2+ ions emission. With relatively low MnCl2-to-PbBr2 molar feed ratio, the Mn2+ substitution ratio in the CsPb(Cl/Br)3 host is not high. So the CsPb(Cl/Br)3 host would compete with the energy transfer of Mn2+ ions and quench its emission. However, by increasing the MnCl2-to-PbBr2 molar feed ratio, the Mn2+ substitution ratio in the CsPb(Cl/Br)3 host increases, which lead to the enhancement of exciton-to-Mn2+ energy transfer. The elemental ratio was determined with inductive coupled plasma (ICP). When the MnCl2-to-PbBr2 molar feed ratio is increased from 1.05 to 2.25, the Mn2+ substitution ratio increases from 1.50 to 2.33%. So, the emission of Mn2+ ions is greatly enhanced and the emission of the CsPb(Cl/Br)3 host gradually faded, which result in the increase of the orange emission. And, with increasing PL intensity of Mn2+ ions, the PLQYs of the Mn-doped CsPb(Cl/Br)3 NCs prepared with the 1.05 to 1.65 MnCl2-to-PbBr2 molar feed ratio also increase from 14.3 to 38.2%. The PLQYs of the prepared NCs with 2.25 MnCl2-to-PbBr2 molar feed ratio is slightly lowed to 37.1%, which is much attributed to the decrease of the host PLQY. The PLQYs of the Mn-doped CsPb(Cl/Br)3 NCs prepared with different MnCl2-to-PbBr2 molar feed ratio are summarized in Table 1. The above results show that by tuning MnCl2-to-PbBr2 molar feed ration in the precursor solution, we can precisely control the optical properties of the Mn-doped CsPb(Cl/Br)3 NCs.
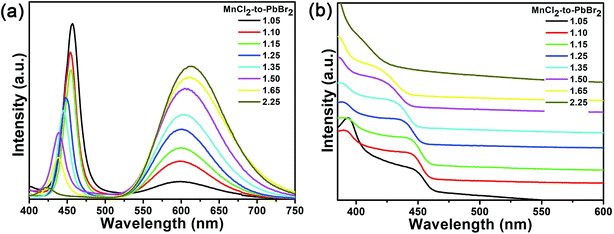 | ||
| Fig. 3 PL emission spectra (a) and absorption spectra (b) of the Mn-doped CsPb(Cl/Br)3 NCs prepared with different MnCl2-to-PbBr2 molar feed ratio at 180 °C. | ||
| MnCl2-to-PbBr2 molar feed ratio | Reaction temperature (°C) | PLQY (%) |
|---|---|---|
| 1.05 | 180 | 14.3 |
| 1.10 | 180 | 15.6 |
| 1.15 | 180 | 16.8 |
| 1.25 | 180 | 22.1 |
| 1.35 | 180 | 25.7 |
| 1.50 | 180 | 34.6 |
| 1.65 | 180 | 38.2 |
| 2.25 | 180 | 37.1 |
| 1.35 | 160 | 15.0 |
| 1.35 | 200 | 33.9 |
Fig. 4 shows the XRD patterns of the Mn-doped CsPb(Cl/Br)3 NCs with different MnCl2-to-PbBr2 molar feed ratio (from 1.05 to 2.25). All the patterns of the Mn-doped NCs could be ascribed to the same cubic phase, which further proves that the Mn-doped CsPb(Cl/Br)3 NCs prepared at different MnCl2-to-PbBr2 molar feed ratio retain their stable structure and possess the same crystalline structure of cubic CsPbCl3 (PDF#73-0692). We can also observe that all of the diffraction peaks of the XRD patterns shift to higher angles with the increase of the MnCl2-to-PbBr2 molar feed ratio. Because, the combination of Cl− ions leads to the shrink of the cell and all the peaks move to higher angles. These results are similar with the anion exchange reactions using Pb-based halide precursors or oleylammonium halide precursors in the previous reports.27
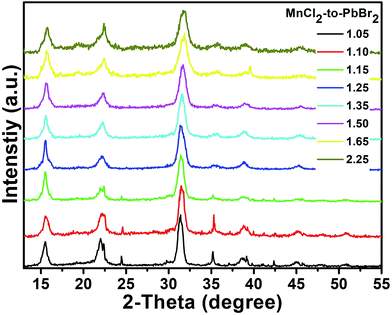 | ||
| Fig. 4 XRD patterns of the Mn-doped CsPb(Cl/Br)3 NCs with different MnCl2-to-PbBr2 molar feed ratio. | ||
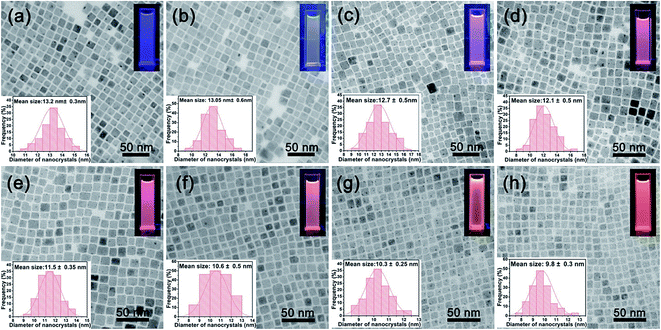
The nanostructures of the as-prepared Mn-doped CsPb(Cl/Br)3 NCs were obtained with TEM. TEM images in Fig. 5 show that the samples prepared at different MnCl2-to-PbBr2 molar feed ratio exhibit similar cubic morphology and slight size variation. With the increase of the MnCl2-to-PbBr2 molar feed ratio from 1.05 to 2.25, the size of the as-prepared Mn-doped CsPb(Cl/Br)3 NCs gradually decreases. The insets of the TEM images are the corresponding particle size distributution and PL images of the nanocrystal cyclohexane solution excited by 365 nm UV light.
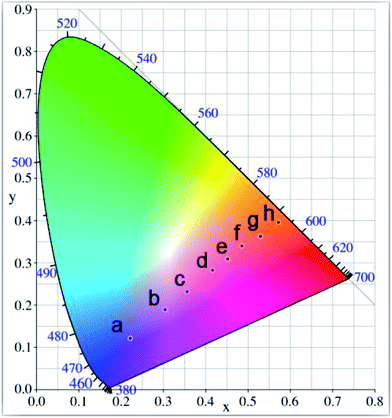
To evaluate the Mn-doped CsPb(Cl/Br)3 NCs performance on color luminescent emission with different MnCl2-to-PbBr2 molar feed ratio, CIE (Commission Intermationale de I'Eclairage 1931 chromaticity) chromaticity coordinates were concluded and presented in Fig. 6. The graph indicates that the color hue of the Mn-doped CsPb(Cl/Br)3 NCs can be tuned precisely, which can be clearly seen from the corresponding PL images of the nanocrystal cyclohexane solution excited by 365 nm UV light (insets of Fig. 5a–h).
Similar to the doping of Mn2+ ions into CdSe or ZnSe NCs, the doping of Mn2+ ions into CsPb(Cl/Br)3 NCs is also a thermodynamics-controlled process.48,49 By fixing the MnCl2-to-PbBr2 molar feed ratio at 1.35, the PL spectrum of the Mn-doped CsPb(Cl/Br)3 NCs with different reaction temperature is shown in Fig. 7a. The PL spectrum of the Mn-doped CsPb(Cl/Br)3 NCs that are prepared at 160, 180 and 200 °C also show two emission peaks, one of the CsPb(Cl/Br)3 host at about 445 nm and the other of the Mn2+ ions at about 600 nm. With the increase of the reaction temperature (160 to 200 °C), the emission of Mn2+ ions shows a red shift from 598 to 605 nm, which is attributed to the formation of more Mn-to-Mn pairs with the increasing Mn percentage. When the reaction temperature is increased from 160 to 200 °C, the Mn2+ substitution ratio increases from 1.25 to 2.46%. The increase of the Mn2+ substitution ratio lead to the enhancement of exciton-to-Mn2+ energy transfer, which strengthened the Mn2+ ions emission. So, the PLQYs of the Mn-doped CsPb(Cl/Br)3 NCs also increase from 15.0 to 33.9% (Table 1). The doping of Mn2+ ions into the CsPb(Cl/Br)3 host also causes the absorption spectra blue shift to 426 nm at higher reaction temperature (Fig. 7b), which is similar to that observed in Mn-doped CdSe NCs.50 The Mn-doped CsPb(Cl/Br)3 NCs prepared at different reaction temperatures retain their stable structure and possess the same crystalline structure of cubic CsPbCl3. As the Mn2+ substitution ratio do not increase obvious, we can hardly see any shifts of the diffraction peaks during this process.
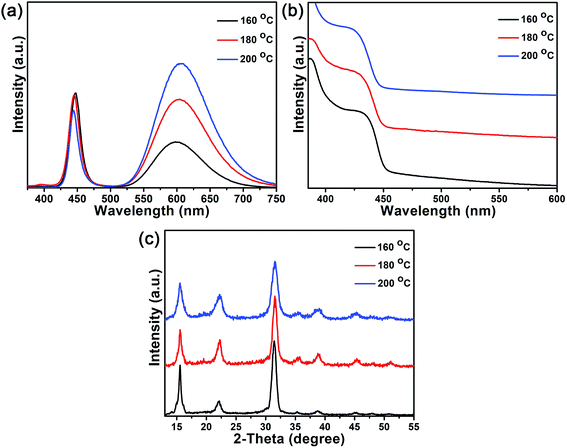 | ||
| Fig. 7 PL emission spectra (a) absorption spectra (b) and XRD patterns (c) of the Mn-doped CsPb(Cl/Br)3 NCs that are prepared at 160, 180 and 200 °C with the MnCl2-to-PbBr2 molar feed ratio of 1.35. | ||
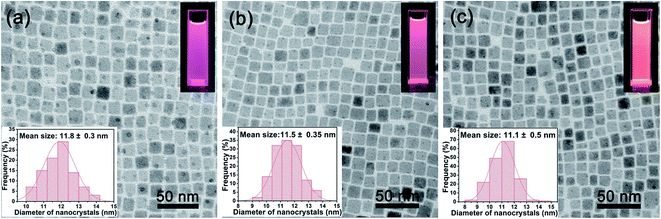
TEM images in Fig. 8 show that the Mn-doped CsPb(Cl/Br)3 NCs prepared at different reaction temperature with the same molar feed ratio of 1.35 exhibit similar monodispersed cubic morphology and little size variation. This means that the reaction temperature does not influence the basic growth process of the CsPb(Cl/Br)3 host. The insets of the TEM images are the corresponding particle size distributution and PL images of the nanocrystal cyclohexane solution excited by 365 nm UV light.
To evaluate the influence of the reaction temperature on color luminescent emission, CIE chromaticity coordinates were concluded and presented in Fig. 9. The corresponding PL images of the nanocrystal cyclohexane solution excited by 365 nm UV light (insets of Fig. 8a–c). Although the reaction temperature has an influence on the optical properties of the Mn-doped CsPb(Cl/Br)3 NCs, such an effect is less obvious than the MnCl2-to-PbBr2 molar feed ratio influences.
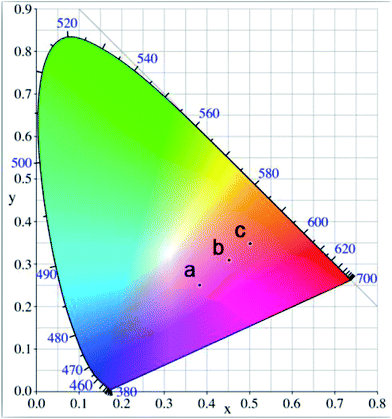 | ||
| Fig. 9 CIE chromaticity diagram of the Mn-doped CsPb(Cl/Br)3 NCs prepared at different reaction temperature: (a) 160 °C, (b) 180 °C, (c) 200 °C. | ||
In conclusion, in this work, we report a phosphine-free strategy to synthesize Mn-doped CsPb(Cl/Br)3 NCs to tune their optical properties in a wide color gamut. The as-prepared CsPb(Cl/Br)3 NCs are highly crystalline and uniform sized nanocubes with two emission peaks, including the host emission around 450 nm and the Mn2+ dopant emission around 600 nm, which are sensitive to the MnCl2-to-PbBr2 molar feed ratio or the reaction temperature. By varying the MnCl2-to-PbBr2 molar feed ratio or the reaction temperature, the relative PL intensities of dual color emission can be well manipulated to a wide color gamut, indicating their potential in light emitting devices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank National Natural Science Foundation of China (Grants 51372234, and 21301187) for financial support. B. Dong thanks Fundamental Research Funds for the Central Universities (Grant 201564001), project funding by China Postdoctoral Science Foundation (Grant 2015M582132 and 2016T60652), and the Qingdao Science and Technology Plan (Grant 15-9-1-60-jch).References
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- K. Wu, G. Liang, Q. Shang, Y. Ren, D. Kong and T. Lian, J. Am. Chem. Soc., 2015, 137, 12792–12795 CrossRef CAS PubMed.
- G. Rainò, G. Nedelcu, L. Protesescu, M. I. Bodnarchuk, M. V. Kovalenko, R. F. Mahrt and T. Stöferle, ACS Nano, 2016, 10, 2485–2490 CrossRef PubMed.
- M. Kulbak, D. Cahen and G. Hodes, J. Phys. Chem. Lett., 2015, 6, 2452–2456 CrossRef CAS PubMed.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643–647 CrossRef CAS PubMed.
- W. S. Yang, J. H. Noh, N. J. Jeon, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Science, 2015, 348, 1234–1237 CrossRef CAS PubMed.
- N. J. Jeon, J. H. Noh, W. S. Yang, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Nature, 2015, 517, 476–480 CrossRef CAS PubMed.
- M. Grätzel, Nat. Mater., 2014, 13, 838–842 CrossRef PubMed.
- S. D. Stranks and H. J. Snaith, Nat. Nanotechnol., 2015, 10, 391–402 CrossRef CAS PubMed.
- H. Zhu, Y. Fu, F. Meng, X. Wu, Z. Gong, Q. Ding, M. V. Gustafsson, M. T. Trinh, S. Jin and X. Zhu, Nat. Mater., 2015, 14, 636–642 CrossRef CAS PubMed.
- P. Ramasamy, D. H. Lim, B. Kim, S. H. Lee, M. S. Lee and J. S. Lee, Chem. Commun., 2016, 52, 2067–2070 RSC.
- Y. Xu, Q. Chen, C. Zhang, R. Wang, H. Wu, X. Zhang, G. Xing, W. W. Yu, X. Wang, Y. Zhang and M. Xiao, J. Am. Chem. Soc., 2016, 138, 3761–3768 CrossRef CAS PubMed.
- S. Yakunin, L. Protesescu, F. Krieg, M. I. Bodnarchuk, G. Nedelcu, M. Humer, G. De Luca, M. Fiebig, W. Heiss and M. V. Kovalenko, Nat. Commun., 2015, 6, 8056 CrossRef CAS PubMed.
- N. Yantara, S. Bhaumik, F. Yan, D. Sabba, H. A. Dewi, N. Mathews, P. P. Boix, H. V. Demir and S. Mhaisalkar, J. Phys. Chem. Lett., 2015, 6, 4360–4364 CrossRef CAS PubMed.
- Q. Zhou, Z. Bai, W. G. Lu, Y. Wang, B. Zou and H. Zhong, Adv. Mater., 2016, 28, 9163–9168 CrossRef CAS PubMed.
- G. Li, Z. K. Tan, D. Di, M. L. Lai, L. Jiang, J. H. Lim, R. H. Friend and N. C. Greenham, Nano Lett., 2015, 15, 2640–2644 CrossRef CAS PubMed.
- J. Liang, Y. Zhang, X. Guo, Z. Gan, J. Lin, Y. Fan and X. Liu, RSC Adv., 2016, 6, 71070–71075 RSC.
- X. J. Ma, Z. Q. Wang, Z. Y. Xiong, Y. Zhang, F. X. Yu, P. Chen, Z. H. Xiong and C. H. Gao, RSC Adv., 2017, 7, 50571–50577 RSC.
- X. Du, G. Wu, J. Cheng, H. Dang, K. Ma, Y. W. Zhang, P. F. Tan and S. Chen, RSC Adv., 2017, 7, 10391–10396 RSC.
- M. B. Teunis, K. N. Lawrence, P. Dutta, A. P. Siegel and R. Sardar, Nanoscale, 2016, 8, 17433–17439 RSC.
- S. Gonzalez-Carrero, R. E. Galian and J. Pérez-Prieto, J. Mater. Chem. A, 2015, 3, 9187–9193 CAS.
- H. Huang, A. S. Susha, S. V. Kershaw, T. F. Hung and A. L. Rogach, Adv. Sci., 2015, 2, 1500194 CrossRef PubMed.
- J. Song, J. Li, X. Li, L. Xu, Y. Dong and H. Zeng, Adv. Mater., 2015, 27, 7162–7167 CrossRef CAS PubMed.
- L. Polavarapu, B. Nickel, J. Feldmann and A. S. Urban, Adv. Energy Mater., 2017, 7, 1700267 CrossRef.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- D. Zhang, S. W. Eaton, Y. Yu, L. Dou and P. Yang, J. Am. Chem. Soc., 2015, 137, 9230–9233 CrossRef CAS PubMed.
- Q. A. Akkerman, V. D'Innocenzo, S. Accornero, A. Scarpellini, A. Petrozza, M. Prato and L. Manna, J. Am. Chem. Soc., 2015, 137, 10276–10281 CrossRef CAS PubMed.
- C. Guhrenz, A. Benad, C. Ziegler, D. Haubold, N. Gaponik and A. Eychmüller, Chem. Mater., 2016, 28, 9033–9040 CrossRef CAS.
- G. Nedelcu, L. Protesescu, S. Yakunin, M. I. Bodnarchuk, M. J. Grotevent and M. V. Kovalenko, Nano Lett., 2015, 15, 5635–5640 CrossRef CAS PubMed.
- D. Zhang, Y. Yang, Y. Bekenstein, Y. Yu, N. A. Gibson, A. B. Wong, S. W. Eaton, N. Kornienko, Q. Kong, M. Lai, A. P. Alivisatos, S. R. Leone and P. Yang, J. Am. Chem. Soc., 2016, 138, 7236–7239 CrossRef CAS PubMed.
- P. Wang, B. Dong, Z. Cui, R. Gao, G. Su, W. Wang and L. Cao, Inorg. Chim. Acta, 2017, 467, 251–255 CrossRef CAS.
- X. Li, Y. Wu, S. Zhang, B. Cai, Y. Gu, J. Song and H. Zeng, Adv. Funct. Mater., 2016, 26, 2435–2445 CrossRef CAS.
- Y. Tong, E. Bladt, M. F. Aygüler, A. Manzi, K. Z. Milowska, V. A. Hintermayr, P. Docampo, S. Bals, A. S. Urban and L. Polavarapu, Angew. Chem., Int. Ed., 2016, 55, 13887–13892 CrossRef CAS PubMed.
- V. A. Hintermayr, A. F. Richter, F. Ehrat, M. Doblinger, W. Vanderlinden, J. A. Sichert, Y. Tong, L. Polavarapu, J. Feldmann and A. S. Urban, Adv. Mater., 2016, 28, 9478–9485 CrossRef CAS PubMed.
- I. Lignos, S. Stavrakis, G. Nedelcu, L. Protesescu, A. J. deMello and M. V. Kovalenko, Nano Lett., 2016, 16, 1869–1877 CrossRef CAS PubMed.
- M. C. Weidman, M. Seitz, S. D. Stranks and W. A. Tisdale, ACS Nano, 2016, 10, 7830–7839 CrossRef CAS PubMed.
- Q. A. Akkerman, S. G. Motti, A. R. Srimath Kandada, E. Mosconi, V. D'Innocenzo, G. Bertoni, S. Marras, B. A. Kamino, L. Miranda, F. De Angelis, A. Petrozza, M. Prato and L. Manna, J. Am. Chem. Soc., 2016, 138, 1010–1016 CrossRef CAS PubMed.
- J. A. Sichert, Y. Tong, N. Mutz, M. Vollmer, S. Fischer, K. Z. Milowska, R. Garcia Cortadella, B. Nickel, C. Cardenas-Daw, J. K. Stolarczyk, A. S. Urban and J. Feldmann, Nano Lett., 2015, 15, 6521–6527 CrossRef CAS PubMed.
- Y. Tong, B. J. Bohn, E. Bladt, K. Wang and P. Muller-Buschbaum, Angew. Chem., Int. Ed., 2017, 56, 13887–13892 CrossRef CAS PubMed.
- D. A. Schwartz, N. S. Norberg, Q. P. Nguyen, J. M. Parker and D. R. Gamelin, J. Am. Chem. Soc., 2003, 125, 13205–13218 CrossRef CAS PubMed.
- K. E. Knowles, H. D. Nelson, T. B. Kilburn and D. R. Gamelin, J. Am. Chem. Soc., 2015, 137, 13138–13147 CrossRef CAS PubMed.
- F. Meinardi, A. Colombo, K. A. Velizhanin, R. Simonutti, M. Lorenzon, L. Beverina, R. Viswanatha, V. I. Klimov and S. Brovelli, Nat. Photonics, 2014, 8, 392–399 CrossRef CAS.
- R. Beaulac, P. I. Archer, S. T. Ochsenbein and D. R. Gamelin, Adv. Funct. Mater., 2008, 18, 3873–3891 CrossRef CAS.
- H. Liu, Z. Wu, J. Shao, D. Yao, H. Gao and Y. Liu, ACS Nano, 2017, 11, 2239–2247 CrossRef CAS PubMed.
- D. Parobek, B. J. Roman, Y. Dong, H. Jin, E. Lee, M. Sheldon and D. H. Son, Nano Lett., 2016, 16, 7376–7380 CrossRef CAS PubMed.
- H. Y. Chen, S. Maiti and D. H. Son, ACS Nano, 2012, 6, 583–591 CrossRef CAS PubMed.
- V. K. Sharma, S. Gokyar, Y. Kelestemur, T. Erdem, E. Unal and H. V. Demir, Small, 2014, 10, 4961–4966 CrossRef CAS PubMed.
- D. Magana, S. C. Perera, A. G. Harter, N. S. Dalal and G. F. Strouse, J. Am. Chem. Soc., 2006, 128, 2931–2939 CrossRef CAS PubMed.
- N. Y. D. J. Norris, F. T. Charnock and T. A. Kennedy, Nano Lett., 2001, 1, 3–7 CrossRef.
- V. A. Vlaskin, C. J. Barrows, C. S. Erickson and D. R. Gamelin, J. Am. Chem. Soc., 2013, 135, 14380–14389 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |