Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photocatalytic activity of BiFeO3/ZnFe2O4 nanocomposites under visible light irradiation†
B. Safizade,
S. M. Masoudpanah *,
M. Hasheminiasari and
A. Ghasemi
*,
M. Hasheminiasari and
A. Ghasemi
School of Metallurgy & Materials Engineering, Iran University of Science and Technology (IUST), Tehran, Iran. E-mail: masoodpanah@iust.ac.ir; Fax: +98 21 77240480; Tel: +98 21 77240540
First published on 13th February 2018
Abstract
Herein, BiFeO3/ZnFe2O4 nanocomposites were synthesized via a glyoxylate precursor method using a two-pot approach. Phase evolution is investigated by X-ray diffraction and Raman spectroscopy, which confirm that no impurity phases are formed between BiFeO3 and ZnFe2O4 following calcination at 600 °C. The specific surface area characterized by N2 adsorption–desorption isotherms decreases from 30.56 to 13.13 m2 g−1 with the addition of zinc ferrite. In contrast, the magnetization increases from 0.28 to 1.8 emu g−1 with an increase in the amount of ZnFe2O4. The composites show strong absorption in the visible region with the optical band gap calculated from the Tauc's plot in the range from 2.17 to 2.22 eV, as measured by diffuse reflectance spectroscopy. Furthermore, the maximum efficiency for the photodegradation of methylene blue under visible light is displayed by the composite containing 25 wt% ZnFe2O4 due to the synergic effect between BiFeO3 and ZnFe2O4, as confirmed by photoluminescence spectroscopy.
1. Introduction
In recent years, scientific research has been focused on new visible light photocatalysts based on semiconductors to address the increasing environmental pollution and energy demands by efficient utilization of solar energy.1,2 To date, various metal oxides (ZnO3 and TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and metal sulfides (ZnS5) have been studied to efficiently degrade harmful organic pollutants and for hydrogen production through water splitting under UV light irradiation.4 However, the UV region spans only 5% of the entire solar spectrum, restricting their applications. As a result of band gap engineering improvement, composites can be fabricated by coupling two narrow band gap semiconductors, which have attracted considerable attention for the development of efficient visible light photocatalysts.6–8
4) and metal sulfides (ZnS5) have been studied to efficiently degrade harmful organic pollutants and for hydrogen production through water splitting under UV light irradiation.4 However, the UV region spans only 5% of the entire solar spectrum, restricting their applications. As a result of band gap engineering improvement, composites can be fabricated by coupling two narrow band gap semiconductors, which have attracted considerable attention for the development of efficient visible light photocatalysts.6–8
Bismuth ferrite (BiFeO3), which has potential applications in sensors, actuators, and digital memory, is a well-known multiferroic material simultaneously possessing ferroelectric and ferromagnetic ordering at room temperature.9,10 Furthermore, BiFeO3 displays a distinct photovoltaic effect with an open circuit voltage of 0.8–0.9 V as a working solar device, which represents a new potential application.11,12 Due to its relatively narrow band gap of 2.2 eV, BiFeO3 has been considered as a possible visible light photocatalyst under solar light irradiation for the photodegradation of organic contaminants.13,14 However, its quantum yield is poor due to the rapid recombination of the photogenerated electron–hole pairs that limits its practical use in photocatalytic applications.15,16 Therefore, many strategies have been developed to enhance the photocatalytic efficiency of BiFeO3 by modifying the size and morphology of its particles, cation doping, and coupling with other semiconductors.17–19 For instance, several semiconductors such as g-C3N4, carbon nanofiber, graphene, CuO and ZnO have been coupled with BiFeO3 to improve its photogenerated electron–hole separation, thus enhancing its interfacial charge transfer the efficiency.6,20–27
Spinel magnetic zinc ferrite (ZnFe2O4) with a narrow band gap of 1.92 eV exhibits a significant photoresponse in the visible light region and has been utilized in gas sensors, catalysts and semiconductor photocatalysts.1 Furthermore, the magnetic properties of ZnFe2O4 can be used to recycle photocatalysts by the application of a magnetic field, making it an interesting product in the industrial photodegradation of organic pollutants.7,28 To the best of our knowledge, there are no reports on the synthesis and application of BiFeO3/ZnFe2O4 nanocomposites for pollutant degradation under visible light irradiation. Uniyal and Yadav only reported the dielectric and magnetic properties of BiFeO3/ZnFe2O4 composites synthesized via the sol–gel method as a function of annealing temperature.29
Herein, we report the structure, microstructure, magnetic properties and photocatalytic performances of BiFeO3/ZnFe2O4 composites synthesized via the glyoxylate precursor method. The optimum amount of ZnFe2O4 is determined to maximize the photocatalytic activity of BiFeO3 powder.
2. Experimental procedure
Starting materials of Fe(NO3)3·9H2O (>99%), Bi(NO3)2·5H2O (>99%), Zn(NO3)2·6H2O (>99%), 1,2-ethanediol (OH(CH2)2OH) and nitric acid (HNO3, 68 wt%) of analytical grade were provided by Merck & Co.BiFeO3 powder was prepared via the glyoxylate precursor method in which the required amount of Fe(NO3)3·9H2O was dissolved in 1,2-ethanediol (ethylene glycol) and then added to 15 mL of 3 mol L−1 nitric acid solution containing Bi(NO3)3·5H2O under magnetic stirring at 100 °C. The ethylene glycol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NO3− (EG/NO3) molar ratio was set to 2.5
NO3− (EG/NO3) molar ratio was set to 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Evolving bubbles of brown nitrogen oxide (NOx) indicated the initiation of the redox reaction between the NO3− anions and OH groups of diol. After drying at 130 °C, the precursor was calcined at 600 °C for 1 h in ambient air.30 ZnFe2O4 powder was produced by dissolving Zn(NO3)2·6H2O and Fe(NO3)3·9H2O in ethylene glycol under magnetic stirring at 100 °C. Once the NOx bubbles disappeared, the solution precursor was dried at 130 °C and then calcined at 600 °C for 1 h in air. BiFeO3/ZnFe2O4 composites were synthesized via a two-pot approach in which the required amount of previously synthesized BiFeO3 powder was added to the solution precursor of zinc ferrite, where the dried precursor was calcined at 600 °C for 1 hour.
1. Evolving bubbles of brown nitrogen oxide (NOx) indicated the initiation of the redox reaction between the NO3− anions and OH groups of diol. After drying at 130 °C, the precursor was calcined at 600 °C for 1 h in ambient air.30 ZnFe2O4 powder was produced by dissolving Zn(NO3)2·6H2O and Fe(NO3)3·9H2O in ethylene glycol under magnetic stirring at 100 °C. Once the NOx bubbles disappeared, the solution precursor was dried at 130 °C and then calcined at 600 °C for 1 h in air. BiFeO3/ZnFe2O4 composites were synthesized via a two-pot approach in which the required amount of previously synthesized BiFeO3 powder was added to the solution precursor of zinc ferrite, where the dried precursor was calcined at 600 °C for 1 hour.
Phase evolution was investigated using a PANalytical X'pert X-ray diffractometer (XRD) with monochromatic CuKα radiation. Raman analysis was performed on the powders using a WiTec Alpha 300R instrument (Nd:YAG laser source: λ = 532 nm and 0.7 MW power, and range: 100–900 cm−1). The morphology and microstructure of the powders were observed using a TESCAN Vega II scanning electron microscope (SEM). The specific surface areas of the as-prepared powders were determined according to the Brunauer–Emmett–Teller (BET) method with nitrogen adsorption at 77 K using a PHS-1020 instrument after degassing at 250 °C for 5 h. The Barrett–Joyner–Halenda (BJH) cumulative pore volume was calculated from the adsorption branch of the isotherms. The equivalent particle size was calculated based on the BET surface area as follows:
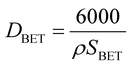 | (1) |
The photocatalytic activity of the BiFeO3/ZnFe2O4 nanocomposites was evaluated by the degradation of methylene blue (MB) in aqueous solution under visible light radiation. Two 100 W xenon lamps with a cutoff ultraviolet filter (λ = 420 nm) were introduced as the visible light source. In each experiment, 0.1 g of photocatalyst was added to 100 mL of methylene blue solution at a concentration of 15 mg L−1. In addition, the solution pH was adjusted to 2 by adding HCl to obtain the maximum MB adsorption on the catalyst surface,14 as shown in the ESI.† The suspension was stirred in the dark for 60 min to establish the adsorption/desorption equilibrium, then the solution was irradiated under visible light. At appropriate time intervals, about 5 mL of suspension was sampled, where the solid phase was separated from the solution via centrifugation at 4000 rpm for 20 min. The concentration of each degraded solution was monitored on a PG Instruments Ltd T80-UV/vis spectrophotometer.
3. Results and discussion
Fig. 1 shows the XRD patterns of the pure BiFeO3, pure ZnFe2O4 and the BiFeO3–xZnFe2O4 composites. The indexed diffraction peaks of ZnFe2O4 are (220), (311), (400), (422), (511), (440) and (533) which match well with the cubic spinel structure having the Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group and are in good agreement with the standard JCPDS card no. 22-1012. Pure BiFeO3 shows indexed diffraction peaks corresponding to a rhombohedral phase with the R3c space group (JCPDS no. 86-1518), which indicates well crystallized BiFeO3 nanoparticles were produced by the glyoxylate precursor method. However, some impurity Bi2Fe4O9 phases (JCPDS card no. 42-0181) were also observed with BiFeO3. The chemical synthesis of BiFeO3 typically leads to the formation of impurities, may be due to its chemical kinetics.31 After compositing with 25 wt% ZnFe2O4, a weak diffraction peak at 2θ = 35.32° corresponding to the (311) reflection peak of ZnFe2O4 appeared. With an increase in the zinc ferrite content, the diffraction peaks of ZnFe2O4 became clearer and stronger, and the impurity peak disappeared. Furthermore, no impurity species were formed between BiFeO3 and ZnFe2O4 during the calcination process, which indicates that ZnFe2O4 was successfully loaded on the BiFeO3 particles without destroying its crystal structure. The amount of BiFeO3 and ZnFe2O4 phases in the composites was calculated by Rietveld refinement, which is in agreement with the nominal values, as typically shown in the ESI.†
m space group and are in good agreement with the standard JCPDS card no. 22-1012. Pure BiFeO3 shows indexed diffraction peaks corresponding to a rhombohedral phase with the R3c space group (JCPDS no. 86-1518), which indicates well crystallized BiFeO3 nanoparticles were produced by the glyoxylate precursor method. However, some impurity Bi2Fe4O9 phases (JCPDS card no. 42-0181) were also observed with BiFeO3. The chemical synthesis of BiFeO3 typically leads to the formation of impurities, may be due to its chemical kinetics.31 After compositing with 25 wt% ZnFe2O4, a weak diffraction peak at 2θ = 35.32° corresponding to the (311) reflection peak of ZnFe2O4 appeared. With an increase in the zinc ferrite content, the diffraction peaks of ZnFe2O4 became clearer and stronger, and the impurity peak disappeared. Furthermore, no impurity species were formed between BiFeO3 and ZnFe2O4 during the calcination process, which indicates that ZnFe2O4 was successfully loaded on the BiFeO3 particles without destroying its crystal structure. The amount of BiFeO3 and ZnFe2O4 phases in the composites was calculated by Rietveld refinement, which is in agreement with the nominal values, as typically shown in the ESI.†
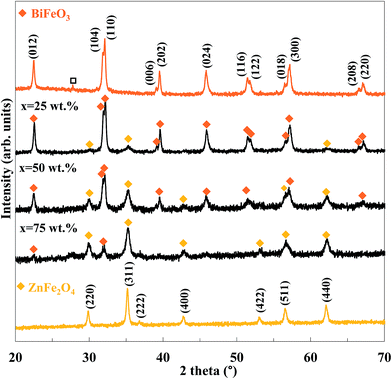 | ||
| Fig. 1 XRD patterns of the BiFeO3–xZnFe2O4 composites as a function of ZnFe2O4 content (x) (Bi2Fe4O9). | ||
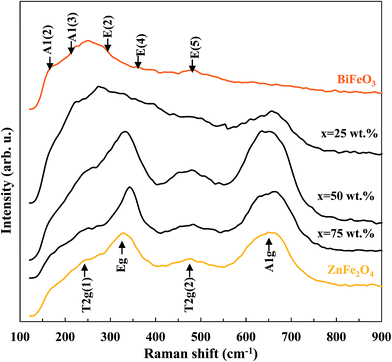
The Raman spectra of pure BiFeO3, pure ZnFe2O4 and BiFeO3–xZnFe2O4 composites are presented in Fig. 2. In the spectrum of pure BiFeO3, the Raman active modes with A1 and E symmetry can be summarized using the following irreducible representation Γ = 4A1 + 9E.32 The two peaks at 173 and 220 cm−1 are assigned as A1 modes, and the peaks at 286, 361 and 481 cm−1 correspond to the E modes. Pure ZnFe2O4 exhibited four peaks at 246, 327, 471 and 648 cm−1, which are assigned as the T2g(1), Eg, T2g(2) and A1g modes for a cubic spinel structure, respectively.33 The A1g mode of ZnFe2O4 appears after 25 wt% ZnFe2O4 was loaded, while the other modes were dominant at higher zinc ferrite contents. Moreover, the purity of the BiFeO3–xZnFe2O4 composites is confirmed by the absence of Raman modes of impurity phases.
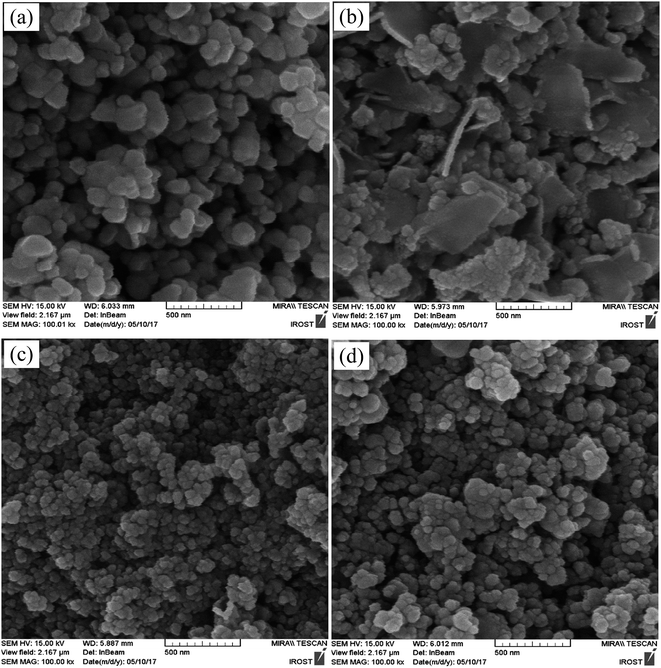 | ||
| Fig. 3 SEM images of (a) pure BiFeO3, (b) BiFeO3-25 wt% ZnFe2O4, (c) BiFeO3-75 wt% ZnFe2O4, and (d) pure ZnFe2O4 powders. | ||
The SEM images of pure BiFeO3, BiFeO3-25 wt% ZnFe2O4, BiFeO3-75 wt% ZnFe2O4 and pure ZnFe2O4 powders are displayed in Fig. 3. The quasi-spherical particles of BiFeO3 (210 nm) are larger than the ZnFe2O4 particles (80 nm). However, the BiFeO3-25 wt% ZnFe2O4 composite is composed of plate-like particles. Furthermore, the average particle size decreases while the particle size distribution becomes rather uniform with an increase in ZnFe2O4 content.
The N2 adsorption–desorption isotherms of the BiFeO3-50 wt% ZnFe2O4 composite are shown in Fig. 4. Table 1 also presents the specific surface area (SBET), equivalent particle size (DBET) and pore volume. The particle agglomerations show a typical type II isotherm according to the International Union of Pure and Applied Chemistry (IUPAC) classification.34 The surface area of pure BiFeO3 is 30.56 m2 g−1 and 13.13 m2 g−1 for pure ZnFe2O4. The higher specific surface area of pure BiFeO3 is attributed to more gaseous products being formed during its synthesis,35 as confirmed by its higher pore volume (0.089 cm3 g−1). The BJH pore size distribution is also depicted in the inset of Fig. 4. The pore size distribution of the BiFeO3-50 wt% ZnFe2O4 composite powder exhibits a mesopore spreading of about 3–4 nm.
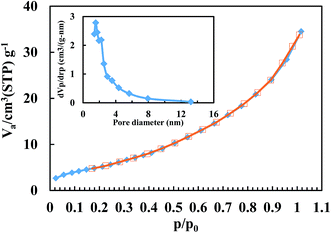 | ||
| Fig. 4 Adsorption (filled symbol)–desorption (open symbol) isotherms of the BiFeO3-50 wt% ZnFe2O4 composite (the inset shows the pore size distribution). | ||
| x | SBET (m2 g−1) | Pore volume (cm3 g−1) | DBET (nm) |
|---|---|---|---|
| BiFeO3 | 30.56 | 0.089 | 23.6 |
| 25 wt% | 28.42 | 0.086 | 27.9 |
| 50 wt% | 19.75 | 0.072 | 44.8 |
| 75 wt% | 18.97 | 0.069 | 52.6 |
| ZnFe2O4 | 13.13 | 0.053 | 87.1 |
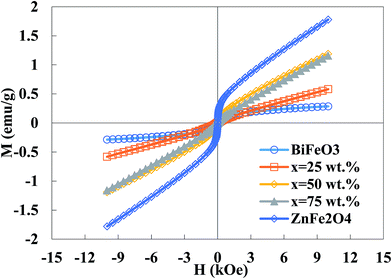
Fig. 5 illustrates the magnetization curves of the BiFeO3–xZnFe2O4 composites as well as the pure BiFeO3 and ZnFe2O4 powders. The pure BiFeO3 nanoparticles exhibit a ferrimagnetic response with the magnetization of 0.28 emu g−1 at 10 kOe. However, the magnetization increases with an increase in zinc ferrite content since pure ZnFe2O4 has a magnetization of 1.8 emu g−1. Bulk BiFeO3 is known to show a G-type antiferromagnetic ordering with a linear field-dependence of magnetization, while the BiFeO3 nanoparticles exhibit weak ferrimagnetism due to the interruption of the long-range antiferromagnetic order at the particle surface.36 The bulk ZnFe2O4 also has a normal spinel structure with antiferromagnetic behavior, while the ZnFe2O4 nanoparticles exhibit a partially inverse spinel structure with some magnetic moment at room temperature.37 A high surface-to-volume ratio in nanoparticles leads to more uncompensated spins from the surface, inducing an enhancement in magnetization. The BiFeO3–xZnFe2O4 composites show higher saturation magnetization than pure bismuth ferrite as a result of the higher magnetization in the zinc ferrite phase. This ferrimagnetism behavior can be exploited for the magnetic recovery of the photocatalyst after degradation.
The optical properties of the BiFeO3–xZnFe2O4 composites, as well as the pure BiFeO3 and ZnFe2O4 powders were investigated via UV-vis diffuse reflectance spectroscopy, which are presented in Fig. 6. The absorption spectra show that the samples absorb a considerable amount of visible light. The direct optical band gap, Eg, was determined using the equation (αhν)2 = A(hν − Eg), where, hν is the photon energy in eV, α is the absorption coefficient and A is a material constant,38 as shown in the inset of Fig. 6. According to the Tauc plots, the band gaps for x = 0, 25, 50, 75 and 100 wt% were calculated to be 2.17, 2.03, 2.14, 2.15 and 2.22 eV, respectively. The absorption band of BiFeO3 and ZnFe2O4 is attributed to the electronic transition from the valence band (O 2p orbital) to the conduction band (Fe 3d orbital) (O2p2− → Fe3d3+).39,40 Clearly, the band gap of the BiFeO3–xZnFe2O4 photocatalysts gradually decreases with an increase in BiFeO3. In other words, by introducing ZnFe2O4 into BiFeO3, the photocatalyst could absorb more visible light for the production of electron–hole pairs, which are favorable for photocatalytic reactions.
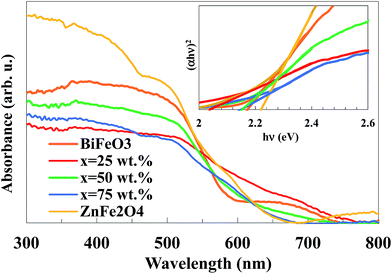 | ||
| Fig. 6 UV-vis absorption spectra of the BiFeO3–xZnFe2O4 composites (the inset shows the Tauc plots). | ||
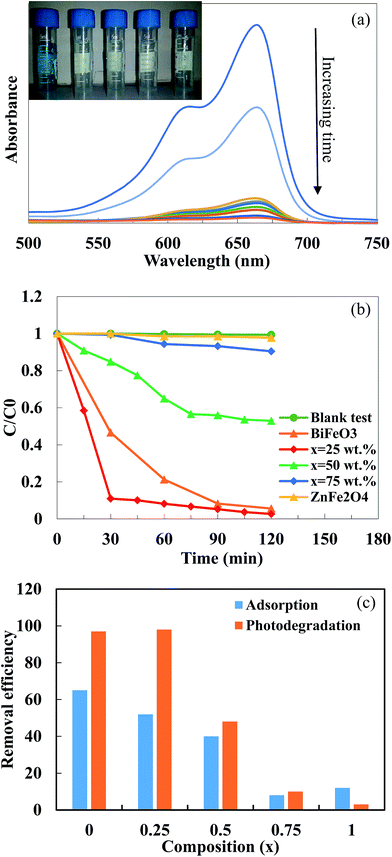
Fig. 7a shows the UV-vis spectra of the MB solution after different irradiation times in the presence of the BiFeO3-25 wt% ZnFe2O4 composite. The main absorption peaks of MB molecules at 664 nm almost completely disappeared after about 120 min, which suggests the excellent photocatalytic activity of the BiFeO3-25 wt% ZnFe2O4 composite. The photodegradation efficiency of MB dye by pure BiFeO3, pure ZnFe2O4 and BiFeO3–xZnFe2O4 composites as a function of irradiation time are summarized in Fig. 7b.
Methylene blue was hardly degraded (∼3%) by pure ZnFe2O4 which exhibited very limited photolysis of MB under visible light irradiation. The low photocatalytic efficiency of pure ZnFe2O4 can be attributed to its low valence band potential and poor photoelectric conversion.7,41 However, pure BiFeO3 can degrade 94.5% of MB after 2 hours of irradiation. The maximum MB photodegradation of ∼97% was observed for the BiFeO3-25 wt% ZnFe2O4 composite after 30 minutes of irradiation. The extraordinary photocatalytic efficiency of the BiFeO3-25 wt% ZnFe2O4 composite may be attributed to the formation of BiFeO3–ZnFe2O4 heterojunctions, which promote the separation of photogenerated electron–hole pairs, thus enhancing the photocatalytic activity. However, the number of effective heterojunctions and thus separation efficiency strongly depend on the content of the two components in the composite.20–22,42 For the optimal content of 25 wt% ZnFe2O4, the most appropriate BiFeO3/ZnFe2O4 heterojunctions might be formed, which benefit the transfer and separation of photogenerated electrons and holes, as can be inferred from the PL spectra.
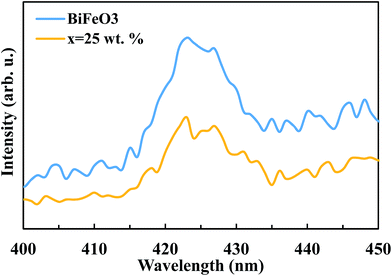
The suppression of charge recombination in BiFeO3 by pairing with ZnFe2O4 can be confirmed by photoluminescence (PL) emission spectra, as presented in Fig. 8. As is known, the recombination of excited electrons and holes leads to PL emission, where a lower emission intensity indicates a decrease in recombination probability. Fig. 8 shows the PL emission spectra of the pure BiFeO3 and BiFeO3-25 wt% ZnFe2O4 photocatalysts at an excitation wavelength of 210 nm. The irradiative recombination process of self-trapped excitations results in an emission band at about 423 nm for pure BiFeO3.43 Clearly, the PL emission intensity decreases when zinc ferrite was added, which confirms that the coupling of BiFeO3 with ZnFe2O4 results in an enhanced ability to capture photoinduced electrons in comparison with pure BiFeO3 and pure ZnFe2O4. The lower PL emission intensity of the BiFeO3-25 wt% ZnFe2O4 photocatalyst benefits a delay in the recombination rate and, thus, higher photocatalytic activity.44–47 In addition to the lower recombination rate of electron–hole pairs in the BiFeO3-25 wt% ZnFe2O4 catalyst, its higher specific surface area can also adsorb more MB dye on the exterior of its particles, as shown in Fig. 7c, hence facilitating the photodegradation of MB dye.
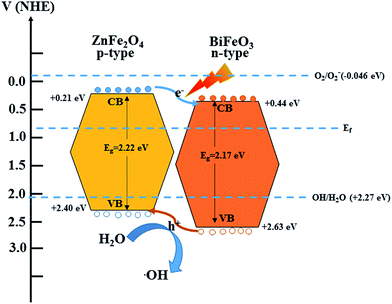
Based on the above structural characterizations and visible light photocatalytic tests, a possible mechanism for the photodegradation of MB by the BiFeO3/ZnFe2O4 photocatalyst under visible light irradiation is proposed. Fig. 9 shows the band positions and transfer path of the photogenerated electron–hole pairs between BiFeO3 and ZnFe2O4. The conduction (CB) and valence (VB) band positions of BiFeO3 and ZnFe2O4 at the point of zero charge were obtained from previous reports.15,48 According to the general p–n heterojunction formation process,8 the entire energy band of BiFeO3 increases while that of ZnFe2O4 decreases to achieve an equilibrium state of the Fermi energy level of BiFeO3 and ZnFe2O4. In this case, the conduction band and valence band of ZnFe2O4 become higher than that of BiFeO3.
Under visible light irradiation, a high energy photon excites an electron from the valence band (VB) to the conduction band (CB) of BiFeO3 and ZnFe2O4. The photoinduced electrons in ZnFe2O4 can easily transfer to BiFeO3, while the holes can transfer to the VB of ZnFe2O4 from the VB of BiFeO3 conveniently with the help of the internal electric field formed at the interface between BiFeO3 and ZnFe2O4.20 Therefore, the photogenerated electrons and holes are efficiently separated between BiFeO3 and ZnFe2O4 reducing the electron–hole recombination in the composite photocatalyst, thus improving the photo-oxidation efficiency. The separated holes when moving to the surface of the BiFeO3/ZnFe2O4 composite could react with H2O to form hydroxyl radicals, ˙OH, which are powerful oxidative species for the direct oxidation of MB, leading to its decomposition.49,50 However, the single electron reduction potential of O2 (E0(O2/O2−) = −0.046 eV) is less negative than the CB potentials, which confirms that the photoinduced electrons on the surfaces of BiFeO3/ZnFe2O4 could not reduce O2 to yield O2− and could not take part in the photodegradation process.50,51 The suitable ZnFe2O4 content causes good dispersion in the catalyst, which benefits the formation of heterojunctions between the BiFeO3 and ZnFe2O4 particles. Therefore, the high separation of charge carriers leads to the high photocatalytic activity of the BiFeO3-25 wt% ZnFe2O4 photocatalyst.
4. Conclusions
A two-pot approach was used for the synthesis of BiFeO3/ZnFe2O4 composites without any impurity species formed between BiFeO3 and ZnFe2O4. The particle size decreased from 210 nm for pure BiFeO3 to 80 nm for pure ZnFe2O4. The pure BiFeO3 nanoparticles exhibited a higher specific surface area than the pure ZnFe2O4 nanoparticles, which may be due to the greater amount of released gaseous products. The magnetization of the BiFeO3/ZnFe2O4 composites increased from 0.28 to 1.8 emu g−1 with an increase in the ZnFe2O4 content. The optical band gaps of composites initially decreased from 2.17 to 2.03 eV and then increased to 2.22 eV as a function of the amount of zinc ferrite. The maximum efficiency (∼97%) for the photodegradation of methylene blue under visible light was exhibited for BiFeO3-25 wt% ZnFe2O4 after 30 minutes irradiation due to the synergic effect between BiFeO3 and ZnFe2O4.Conflicts of interest
There are no conflicts to declare.References
- E. Casbeer, V. K. Sharma and X.-Z. Li, Synthesis and photocatalytic activity of ferrites under visible light: a review, Sep. Purif. Technol., 2012, 87, 1–14 CrossRef CAS.
- Z. Jian, H. Ru and L. Xiaoheng, Efficient visible light driven photocatalytic hydrogen production from water using attapulgite clay sensitized by CdS nanoparticles, Nanotechnology, 2013, 24, 505401 CrossRef PubMed.
- I. Udom, M. K. Ram, E. K. Stefanakos, A. F. Hepp and D. Y. Goswami, One dimensional-ZnO nanostructures: synthesis, properties and environmental applications, Mater. Sci. Semicond. Process., 2013, 16, 2070–2083 CrossRef CAS.
- H. Wang, X. Fei, L. Wang, Y. Li, S. Xu, M. Sun, L. Sun, C. Zhang, Y. Li, Q. Yang and Y. Wei, Magnetically separable iron oxide nanostructures-TiO2 nanofibers hierarchical heterostructures: controlled fabrication and photocatalytic activity, New J. Chem., 2011, 35, 1795–1802 RSC.
- W. Chen, H. Ruan, Y. Hu, D. Li, Z. Chen, J. Xian, J. Chen, X. Fu, Y. Shao and Y. Zheng, One-step preparation of hollow ZnO core/ZnS shell structures with enhanced photocatalytic properties, CrystEngComm, 2012, 14, 6295–6305 RSC.
- C. M. Raghavan, J. W. Kim, J. Y. Choi, T. K. Song and S. S. Kim, Microstructures and electrical properties of a Li–ZnO/BiFeO3 double-layered thin film fabricated by a chemical solution deposition method, Ceram. Int., 2015, 41(suppl. 1), S303–S307 CrossRef CAS.
- J. Hu, Y. Xie, X. Zhou and J. Yang, Solid-state synthesis of ZnO and ZnFe2O4 to form p–n junction composite in the use of dye sensitized solar cells, J. Alloys Compd., 2016, 676, 320–325 CrossRef CAS.
- S. J. A. Moniz, S. A. Shevlin, D. J. Martin, Z.-X. Guo and J. Tang, Visible-light driven heterojunction photocatalysts for water splitting – a critical review, Energy Environ. Sci., 2015, 8, 731–759 CAS.
- S. Zhang, M. Lu, D. Wu, Y. Chen and N. Ming, Larger polarization and weak ferromagnetism in quenched BiFeO3 ceramics with a distorted rhombohedral crystal structure, Appl. Phys. Lett., 2005, 87, 262907 CrossRef.
- J. Kong, Z. Rui, X. Wang, H. Ji and Y. Tong, Visible-light decomposition of gaseous toluene over BiFeO3–(Bi/Fe)2O3 heterojunctions with enhanced performance, Chem. Eng. J., 2016, 302, 552–559 CrossRef CAS.
- T. Choi, S. Lee, Y. Choi, V. Kiryukhin and S.-W. Cheong, Switchable ferroelectric diode and photovoltaic effect in BiFeO3, Science, 2009, 324, 63–66 CrossRef CAS PubMed.
- H. Yi, T. Choi, S. Choi, Y. S. Oh and S. W. Cheong, Mechanism of the switchable photovoltaic effect in ferroelectric BiFeO3, Adv. Mater., 2011, 23, 3403–3407 CrossRef CAS PubMed.
- S. M. Masoudpanah, S. M. Mirkazemi, R. Bagheriyeh, F. Jabbari and F. Bayat, Structural, magnetic and photocatalytic characterization of Bi1−xLaxFeO3 nanoparticles synthesized by thermal decomposition method, Bull. Mater. Sci., 2017, 40, 93–100 CrossRef CAS.
- T. Soltani and M. H. Entezari, Photolysis and photocatalysis of methylene blue by ferrite bismuth nanoparticles under sunlight irradiation, J. Mol. Catal. A: Chem., 2013, 377, 197–203 CrossRef CAS.
- S.-M. Lam, J.-C. Sin and A. R. Mohamed, A newly emerging visible light-responsive BiFeO3 perovskite for photocatalytic applications: a mini review, Mater. Res. Bull., 2017, 90, 15–30 CrossRef CAS.
- Z. Li, Y. Shen, Y. Guan, Y. Hu, Y. Lin and C.-W. Nan, Bandgap engineering and enhanced interface coupling of graphene–BiFeO3 nanocomposites as efficient photocatalysts under visible light, J. Mater. Chem. A, 2014, 2, 1967–1973 CAS.
- T. Soltani and M. H. Entezari, Solar photocatalytic degradation of RB5 by ferrite bismuth nanoparticles synthesized via ultrasound, Ultrason. Sonochem., 2013, 20, 1245–1253 CrossRef CAS PubMed.
- S. M. Masoudpanah, S. M. Mirkazemi, S. Shabani and P. T. Dolat Abadi, The effect of the ethylene glycol to metal nitrate molar ratio on the phase evolution, morphology and magnetic properties of single phase BiFeO3 nanoparticles, Ceram. Int., 2015, 41, 9642–9646 CrossRef CAS.
- P. Reddy Vanga, R. V. Mangalaraja and M. Ashok, Structural, magnetic and photocatalytic properties of La and alkaline co-doped BiFeO3 nanoparticles, Mater. Sci. Semicond. Process., 2015, 40, 796–802 CrossRef CAS.
- T. Fan, C. Chen and Z. Tang, Hydrothermal synthesis of novel BiFeO3/BiVO4 heterojunctions with enhanced photocatalytic activities under visible light irradiation, RSC Adv., 2016, 6, 9994–10000 RSC.
- F. Niu, D. Chen, L. Qin, N. Zhang, J. Wang, Z. Chen and Y. Huang, Facile Synthesis of Highly Efficient p–n Heterojunction CuO/BiFeO3 Composite Photocatalysts with Enhanced Visible-Light Photocatalytic Activity, ChemCatChem, 2015, 7, 3279–3289 CrossRef CAS.
- X. Wang, W. Mao, J. Zhang, Y. Han, C. Quan, Q. Zhang, T. Yang, J. Yang, X. A. Li and W. Huang, Facile fabrication of highly efficient g-C3N4/BiFeO3 nanocomposites with enhanced visible light photocatalytic activities, J. Colloid Interface Sci., 2015, 448, 17–23 CrossRef CAS PubMed.
- T. Soltani and B.-K. Lee, Sono-synthesis of nanocrystallized BiFeO3/reduced graphene oxide composites for visible photocatalytic degradation improvement of bisphenol A, Chem. Eng. J., 2016, 306, 204–213 CrossRef CAS.
- F. Chen, W. An, L. Liu, Y. Liang and W. Cui, Highly efficient removal of bisphenol A by a three-dimensional graphene hydrogel-AgBr@rGO exhibiting adsorption/photocatalysis synergy, Appl. Catal., B, 2017, 217, 65–80 CrossRef CAS.
- X. Wang, Y. Liang, W. An, J. Hu, Y. Zhu and W. Cui, Removal of chromium(VI) by a self-regenerating and metal free g-C3N4/graphene hydrogel system via the synergy of adsorption and photo-catalysis under visible light, Appl. Catal., B, 2017, 219, 53–62 CrossRef CAS.
- Y. Li, W. Cui, L. Liu, R. Zong, W. Yao, Y. Liang and Y. Zhu, Removal of Cr(VI) by 3D TiO2-graphene hydrogel via adsorption enriched with photocatalytic reduction, Appl. Catal., B, 2016, 199, 412–423 CrossRef CAS.
- L. Liu, Y. Qi, J. Lu, S. Lin, W. An, Y. Liang and W. Cui, A stable Ag3PO4@g-C3N4 hybrid core@shell composite with enhanced visible light photocatalytic degradation, Appl. Catal., B, 2016, 183, 133–141 CrossRef CAS.
- S. M. Masoudpanah, M. Hasheminisari and A. Ghasemi, Magnetic properties and photocatalytic activity of ZnFe2−xLaxO4 nanoparticles synthesized by sol–gel autocombustion method, J. Sol-Gel Sci. Technol., 2016, 80, 487–494 CrossRef CAS.
- P. Uniyal and K. L. Yadav, Observation of the room temperature magnetoelectric effect in Dy doped BiFeO3, J. Phys.: Condens. Matter, 2009, 21, 012205 CrossRef CAS PubMed.
- S. Shabani, S. M. Mirkazemi, S. M. Masoudpanah and P. T. Dolat Abadi, Synthesis and Characterization of Pure Single Phase BiFeO3 Nanoparticles by the Glyoxylate Precursor Method, J. Supercond. Novel Magn., 2014, 27, 2795–2801 CrossRef CAS.
- T. Gao, Z. Chen, Y. Zhu, F. Niu, Q. Huang, L. Qin, X. Sun and Y. Huang, Synthesis of BiFeO3 nanoparticles for the visible-light induced photocatalytic property, Mater. Res. Bull., 2014, 59, 6–12 CrossRef CAS.
- M. Tyagi, M. Kumari, R. Chatterjee and P. Sharma, Raman scattering spectra, magnetic and ferroelectric properties of BiFeO3–CoFe2O4 nanocomposite thin films structure, Phys. B, 2014, 448, 128–131 CrossRef CAS.
- Z. Wang, D. Schiferl, Y. Zhao and H. S. C. O'Neill, High pressure Raman spectroscopy of spinel-type ferrite ZnFe2O4, J. Phys. Chem. Solids, 2003, 64, 2517–2523 CrossRef CAS.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Reporting Physisorption Data for Gas/Solid Systems, Handbook of Heterogeneous Catalysis, Wiley-VCH Verlag GmbH & Co. KGaA, 2008 Search PubMed.
- B. Pourgolmohammad, S. M. Masoudpanah and M. R. Aboutalebi, Synthesis of CoFe2O4 powders with high surface area by solution combustion method: effect of fuel content and cobalt precursor, Ceram. Int., 2017, 43, 3797–3803 CrossRef CAS.
- T.-J. Park, G. C. Papaefthymiou, A. J. Viescas, A. R. Moodenbaugh and S. S. Wong, Size-Dependent Magnetic Properties of Single-Crystalline Multiferroic BiFeO3 Nanoparticles, Nano Lett., 2007, 7, 766–772 CrossRef CAS PubMed.
- V. Blanco-Gutierrez, E. Climent-Pascual, M. J. Torralvo-Fernandez, R. Saez-Puche and M. T. Fernandez-Diaz, Neutron diffraction study and superparamagnetic behavior of ZnFe2O4 nanoparticles obtained with different conditions, J. Solid State Chem., 2011, 184, 1608–1613 CrossRef CAS.
- M. A. Butler, Photoelectrolysis and physical properties of the semiconducting electrode WO2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) , J. Appl. Phys., 1977, 48, 1914–1920 CrossRef CAS.
, J. Appl. Phys., 1977, 48, 1914–1920 CrossRef CAS. - H. Wang, Y. Zheng, M.-Q. Cai, H. Huang and H. L. W. Chan, First-principles study on the electronic and optical properties of BiFeO3, Solid State Commun., 2009, 149, 641–644 CrossRef CAS.
- J. Feng, L. Su, Y. Ma, C. Ren, Q. Guo and X. Chen, CuFe2O4 magnetic nanoparticles: a simple and efficient catalyst for the reduction of nitrophenol, Chem. Eng. J., 2013, 221, 16–24 CrossRef CAS.
- J. Li, Z. Liu and Z. Zhu, Magnetically separable ZnFe2O4, Fe2O3/ZnFe2O4 and ZnO/ZnFe2O4 hollow nanospheres with enhanced visible photocatalytic properties, RSC Adv., 2014, 4, 51302–51308 RSC.
- L. Zhang, Y. He, P. Ye, Y. Wu and T. Wu, Visible light photocatalytic activities of ZnFe2O4 loaded by Ag3VO4 heterojunction composites, J. Alloys Compd., 2013, 549, 105–113 CrossRef CAS.
- Y. Chen, Y. Tang, S. Luo, C. Liu and Y. Li, TiO2 nanotube arrays co-loaded with Au nanoparticles and reduced graphene oxide: facile synthesis and promising photocatalytic application, J. Alloys Compd., 2013, 578, 242–248 CrossRef CAS.
- Y. Liang, S. Lin, L. Liu, J. Hu and W. Cui, Oil-in-water self-assembled Ag@AgCl QDs sensitized Bi2WO6: enhanced photocatalytic degradation under visible light irradiation, Appl. Catal., B, 2015, 164, 192–203 CrossRef CAS.
- Y. Zhang, W. Cui, W. An, L. Liu, Y. Liang and Y. Zhu, Combination of photoelectrocatalysis and adsorption for removal of bisphenol A over TiO2-graphene hydrogel with 3D network structure, Appl. Catal., B, 2018, 221, 36–46 CrossRef CAS.
- H. Wang, Y. Liang, L. Liu, J. Hu and W. Cui, Highly ordered TiO2 nanotube arrays wrapped with g-C3N4 nanoparticles for efficient charge separation and increased photoelectrocatalytic degradation of phenol, J. Hazard. Mater., 2018, 344, 369–380 CrossRef CAS PubMed.
- H. Wang, Y. Liang, L. Liu, J. Hu and W. Cui, Reduced graphene oxide wrapped Bi2WO6 hybrid with ultrafast charge separation and improved photoelectrocatalytic performance, Appl. Surf. Sci., 2017, 392, 51–60 CrossRef CAS.
- J. He, R. Guo, L. Fang, W. Dong, F. Zheng and M. Shen, Characterization and visible light photocatalytic mechanism of size-controlled BiFeO3 nanoparticles, Mater. Res. Bull., 2013, 48, 3017–3024 CrossRef CAS.
- H. Cheng, B. Huang, Y. Dai, X. Qin and X. Zhang, One-Step Synthesis of the Nanostructured AgI/BiOI Composites with Highly Enhanced Visible-Light Photocatalytic Performances, Langmuir, 2010, 26, 6618–6624 CrossRef CAS PubMed.
- D. Wang, T. Kako and J. Ye, Efficient Photocatalytic Decomposition of Acetaldehyde over a Solid-Solution Perovskite (Ag0.75Sr0.25)(Nb0.75Ti0.25)O3 under Visible-Light Irradiation, J. Am. Chem. Soc., 2008, 130, 2724–2725 CrossRef CAS PubMed.
- L. Liu, L. Ding, Y. Liu, W. An, S. Lin, Y. Liang and W. Cui, A stable Ag3PO4@PANI core@shell hybrid: enrichment photocatalytic degradation with π–π conjugation, Appl. Catal., B, 2017, 201, 92–104 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The UV-vis spectra and refined XRD patterns. See DOI: 10.1039/c7ra13380d |
| This journal is © The Royal Society of Chemistry 2018 |