Open Access Article
Open Access ArticleSyntheses, crystal structures, magnetic properties and ESI-MS studies of a series of trinuclear CuIIMIICuII compounds (M = Cu, Ni, Co, Fe, Mn, Zn)†
Nairita Haria,
Shuvankar Mandala,
Arpita Janaa,
Hazel A. Sparkesb and
Sasankasekhar Mohanta *a
*a
aDepartment of Chemistry, University of Calcutta, 92 A. P. C. Road, Kolkata 700 009, India. E-mail: sm_cu_chem@yahoo.co.in; Fax: +91-33-23519755
bSchool of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS, UK
First published on 14th February 2018
Abstract
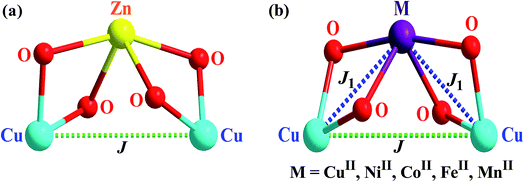
Six trinuclear CuIIMIICuII compounds (M = Cu, Ni, Co, Fe, Mn, Zn) derived from the Schiff base ligand, H2L (2 + 1 condensation product of salicylaldehyde and trans-1,2-diaminocyclohexane) are reported in this investigation. The composition of the metal complexes are [{CuIIL(ClO4)}2CuII(H2O)]·2H2O (1), [{CuIIL(ClO4)}{NiII(H2O)2}{CuIIL}]ClO4·CH3COCH3 (2), [{CuIIL(ClO4)}{CoII(CH3COCH3)(H2O)}{CuIIL(CH3COCH3)}]ClO4 (3) and isomorphic [{CuIIL(ClO4)}2MII(CH3OH)2] (4, M = Fe; 5, M = Mn; 6, M = Zn). Two copper(II) ions in 1–6 occupy N2O2 compartments of two L2− ligands, while the second metal ion occupies the O(phenoxo)4 site provided by the two ligands, i.e., the two metal ions in both CuIIMII pairs are diphenoxo-bridged. Positive ESI-MS of 1–6 reveals some interesting features. Variable-temperature and variable-field magnetic studies reveal moderate or weak antiferromagnetic interactions in 1–6 with the following values of magnetic exchange integrals (H = −2JS1S2 type): J1 = −136.50 cm−1 and J = 0.00 for the CuIICuIICuII compound 1; J1 = −22.16 cm−1 and J = −1.97 cm−1 for the CuIINiIICuII compound 2; J1 = −14.78 cm−1 and J = −1.86 cm−1 for the CuIICoIICuII compound 3; J1 = −6.35 cm−1 and J = −1.17 cm−1 for the CuIIFeIICuII compound 4; J1 = −6.02 cm−1 and J = −1.70 cm−1 for the CuIIMnIICuII compound 5; J = −2.25 cm−1 for the CuIIZnIICuII compound 6 (J is between two CuII in the N2O2 compartments; J1 is between CuII and MII through a diphenoxo bridge).
Introduction
A major goal in the research of magnetochemistry is the establishment of magneto-structural correlations1 so that magnetic properties of new compounds can be predicted and the intimate relationship of spin coupling can be better rationalized. Although the magnetic exchange interaction in discrete molecules was reported for the first time in the early 1950s,2 the first straightforward magneto-structural correlation was reported in 1976 when Hatfield, Hodgson and coworkers established a linear correlation between the magnetic exchange integral and the Cu–O–Cu hydroxo bridge angle in planar dihydroxo-bridged dicopper(II) compounds.3 Later, a number of experimental and theoretical correlations have been determined in varieties of systems. Most of the concerned systems are homometallic such as bis(μ-hydroxo)/bis(μ-alkoxo)/bis(μ-phenoxo)/bis(μ-halide)/bis(μ1,1-azide)/bis(μ1,3-azide)/μ-phenoxo dicopper(II) compounds,4 linear tricopper(II) compounds where the two metal ions in a copper(II)⋯copper(II) pair are diphenoxo-bridged,5 alkoxo-bridged tetracopper(II) systems,6 bis(μ-phenoxo)/bis(μ1,1-azide)/bis(μ1,3-azide)/bis(μ-oximato)/μ-phenoxo-μ1,1-azide dinickel(II) compounds,4e–g,7 end-to-end azide bridged tetranickel(II) compounds,8 μ-oxo/bis(μ-dialkoxo) diiron(III) compounds,9a,b hexairon(III) systems having μ-hydroxo-bis(μ-carboxylate)/bis(μ-alkoxo)-μ-carboxylate bridging moiety between two metal ions,9c bis(μ1,1-azide) dimanganese(II) compounds,4d bis(μ-oxo) dimanganese(IV) compounds,9d tris(μ-hydroxo)/tris(μ-oxo)tris(μ-ethoxide)/tris(μ-chloride)/tris(μ-bromide)/tris(μ-iodide)/μ-oxo-bis(μ-hydroxo)/bis(μ-oxo)-μ-hydroxo dimanganese(IV)/divanadium(II)/dichromium(III) compounds,9e radical bridged digadolinium(III) systems,10 etc. In comparison to those in homometallic systems, the correlations in heterometallic systems are only a few and those are the experimental correlations in diphenoxo-bridged copper(II)⋯gadolinium(III)11a/copper(II)⋯oxovanadium(IV)11b/iron(III)⋯nickel(II)11c compounds and theoretical correlations in diphenoxo-bridged copper(II)⋯gadolinium(III)11d/nickel(II)⋯gadolinium(III)11e/iron(III)⋯nickel(II)11f systems. Hence, syntheses, and magnetic studies of new heterometallic systems deserves attention so that magneto-structural correlations in heterometallic systems may be established.The Schiff base ligands obtained on the [2 + 1] condensation of salicylaldehyde/2-hydroxyacetophenone/2-hydroxypropiophenone/3-methoxysalicylaldehyde/3-ethoxysalicylaldehyde and a diamine (such as ethylenediamine, 1,3-diaminopropane, trans-1,2-diaminocyclohexane, o-phenylenediamine, 2,2-dimethyl-1,3-diaminopropane, etc.) belong to an excellent class of ligands to stabilize homo/heterometallic systems,12–24 most of which, in turn, are important in the research areas of exchange-coupled systems. The synthesis procedure involves the isolation of a 3d mononuclear metallo-ligand (such as a CuII metallo-ligand) in which the metal ion occupies the N(imine)2O(phenoxo)2 compartment. The treatment of the metallo-ligand with a second metal ion produces homo/heterometallic systems. The reason of the incorporation of two metal ions per ligand is the bridging ability of the phenoxo oxygen atoms and, from that perspective, all the above mentioned ligands may be considered as similar. It is also worth mentioning that a copper(II) metallo-ligand has been mostly utilized to derive homo/heterometallic systems following metallo-ligand + second metal ion approach. Although a lot of compounds have been reported from the above mentioned ligands, exploration of the metal complexes from a new or a less utilized ligand always deserves attention due to the possibility of getting new aspects in terms of structures and properties. To be noted that the particular Schiff base ligand (H2L; Scheme 1) containing salicylaldehyde as the aldehyde counterpart and trans-1,2-diaminocyclohexane as the diamine counterpart has only been rarely utilized to derive copper(II)–second metal ion compounds,12,24 although many copper(II)–second metal ion systems are known from closely similar ligands.12–23 Therefore, we thought to explore the copper(II)–second metal ion complexes from this ligand as a part of our continuous contribution to enrich the homo/heterometallic systems from the above mentioned types of ligands.11c,13i,14h,i,15a,b,19i–m,20a,22,23 Accordingly, we have reacted [CuIIL] with the perchlorate salts of copper(II), nickel(II), cobalt(II), iron(II), manganese(II) and zinc(II) and isolated six trinuclear CuIIMIICuII compounds. Herein, we report the syntheses, crystal structures, variable-temperature and variable-field magnetic properties and electrospray ionization mass spectra in positive mode (ESI-MS positive) of the derived six compounds.
Experimental section
Caution! Perchlorate complexes of metal ions with organic ligands are potentially explosive. Only a small amount of material should be prepared, and it should be handled with care.Materials and physical measurements
All the reagents and solvents were purchased from commercial sources and used as received. [CuIIL] was synthesized by a known procedure.24 Elemental (C, H and N) analyses were performed on a Perkin-Elmer 2400 II analyzer. IR spectra were recorded in the region 400–4000 cm−1 on a Bruker-Optics Alpha-T spectrophotometer with samples as KBr disks. The electrospray ionization mass spectra were recorded on a Waters Xevo G2 QTOF Mass Spectrometer. Magnetic data were collected with a SQUID-VSM (Quantum Design) instrument.Syntheses
Data for 1. Yield: 0.130 g (80%). Anal. calc. for C40H46N4O15Cl2Cu3: C, 44.31; H, 4.28; N, 5.17. Found: C, 44.72; H, 4.09; N, 5.35. FT-IR (cm−1, KBr): [ν(H2O)] 3441m; [ν(C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)] 1638vs; [ν(ClO4−)] 1099vs, 620s.
N)] 1638vs; [ν(ClO4−)] 1099vs, 620s.
Data for 2. Yield: 0.125 g (75%). Anal. calc. for C43H50N4O15Cl2Cu2Ni: C, 46.13; H, 4.50; N, 5.00. Found: C, 45.78; H, 4.39; N, 4.82. FT-IR (cm−1, KBr): [ν(H2O)] 3418m; [ν(C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)] 1637vs; [ν(ClO4−)] 1095vs, 626s.
N)] 1637vs; [ν(ClO4−)] 1095vs, 626s.
Data for 3. Yield: 0.140 g (80%). Anal. calc. for C46H54N4O15Cl2Cu2Co: C, 47.63; H, 4.69; N, 4.83. Found: C, 47.94; H, 4.78; N, 5.05. FT-IR (cm−1, KBr): [ν(H2O)] 3420m; [ν(C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)] 1636vs; [ν(ClO4−)] 1094vs, 625s.
N)] 1636vs; [ν(ClO4−)] 1094vs, 625s.
Data for 4. Yield: 0.105 g (65%). Anal. calc. for C42H48N4O14Cl2Cu2Fe: C, 46.42; H, 4.45; N, 5.16. Found: C, 46.26; H, 4.28; N, 5.30. FT-IR (cm−1, KBr): [ν(C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)] 1636vs; [ν(ClO4−)] 1095vs, 623s.
N)] 1636vs; [ν(ClO4−)] 1095vs, 623s.
Data for 5. Yield: 0.098 g (60%). Anal. calc. for C42H48N4O14Cl2Cu2Mn: C, 46.46; H, 4.46; N, 5.16. Found: C, 46.29; H, 4.40; N, 5.25. FT-IR (cm−1, KBr): [ν(C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)] 1636vs; [ν(ClO4−)] 1107vs, 624s.
N)] 1636vs; [ν(ClO4−)] 1107vs, 624s.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)] 1637vs; [ν(ClO4−)] 1093vs, 623s.
N)] 1637vs; [ν(ClO4−)] 1093vs, 623s.Crystal structure determination of 1–6
The crystallographic data for 1–6 are summarized in Table 1. X-ray diffraction data were collected on a Bruker-APEX II SMART CCD diffractometer at 296(2) K using graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). For data processing, the SAINT packages were used.25a All data were corrected for Lorentz-polarization effects. Multiscan absorption correction were made for all the six cases using the program SADABS.25b Structures were solved by direct and Fourier methods and refined by full-matrix least-squares based on F2 using SHELXL.25c,d All of the non-hydrogen atoms were refined anisotropically. While all of the hydrogen atoms were located geometrically and refined using a riding model with the exception of the water hydrogen atoms in 1 and 3 which were located in the difference map and refined with the O–H distance restrained. In the case of 3, the structure was refined as a racemic twin, giving a twin scale fraction of 0.20(2). In addition Squeeze within Platon25e,f was used to remove disordered solvent that could not be sensibly modelled from the lattices of 1 and 2. The electron counts per unit cell for the eliminated solvent molecules are 42 and 124 (31 per void) for 1 and 2, respectively, indicating the presence of two water molecules (Z = 2 in 1) and one acetone molecule (Z = 4 in 2) as solvent of crystallization in the molecular formula. In 2, 3, 4, 5 and 6 the perchlorate ions were disordered over two positions, the occupancies of the two orientations were refined with their sum set to equal 1. Restraints and constraints were applied to maintain sensible thermal and geometric parameters for the disordered atoms. In addition to the perchlorate disorder, 2, 4, 5 and 6 also showed full or partial disorder in the cyclohexane ring, the occupancies of the atoms were refined with their sum set to equal 1, restraints and constraints were also applied to maintain sensible thermal and geometric parameters for the disordered atoms.| CuIICuIICuII (1) | CuIINiIICuII (2) | CuIICoIICuII (3) | CuIIFeIICuII (4) | CuIIMnIICuII (5) | CuIIZnIICuII (6) | |
|---|---|---|---|---|---|---|
| a R1 = [∑||Fo| − |Fc||/∑|Fo|].b wR2 = [∑w(Fo2 − Fc2)2/∑wFo4]1/2. | ||||||
| Empirical formula | C40H42N4O13Cl2Cu3 | C40H44N4O14Cl2Cu2Ni | C46H54N4O15Cl2Cu2Co | C42H48N4O14Cl2Cu2Fe | C42H48N4O14Cl2Cu2Mn | C42H48N4O14Cl2Cu2Zn |
| Formula weight | 1048.29 | 1061.48 | 1159.84 | 1086.67 | 1085.76 | 1096.19 |
| Crystal color | Red | Red | Red | Red | Red | Red |
| Crystal system | Triclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/n | Cc | C2/c | C2/c | C2/c |
| a/Å | 10.021(2) | 10.9788(6) | 20.9449(9) | 16.0994(12) | 16.287(9) | 16.200(19) |
| b/Å | 13.993(3) | 23.9304(13) | 13.2515(6) | 12.1164(9) | 12.113(6) | 12.254(15) |
| c/Å | 15.997(4) | 18.3403(10) | 19.8004(9) | 24.2086(18) | 24.283(13) | 24.32(3) |
| α/° | 89.031(8) | 90.00 | 90.00 | 90.00 | 90.00 | 90.00 |
| β/° | 80.648(8) | 91.497(2) | 112.976(2) | 107.775(2) | 108.528(7) | 108.149(15) |
| γ/° | 80.267(8) | 90.00 | 90.00 | 90.00 | 90.00 | 90.00 |
| V/Å3 | 2181.3(8) | 4816.8(5) | 5059.6(4) | 4496.9(6) | 4542(4) | 4589(9) |
| Z | 2 | 4 | 4 | 4 | 4 | 4 |
| T/K | 296(2) | 296(2) | 296(2) | 296(2) | 296(2) | 296(2) |
| 2θ range for data collection/° | 2.58–50.052 | 2.798–51.348 | 3.73–51.43 | 3.534–51.388 | 4.274–51.1 | 4.248–50.046 |
| μ/mm−1 | 1.640 | 1.437 | 1.332 | 1.446 | 1.390 | 1.623 |
| ρcalcd/g cm−3 | 1.596 | 1.464 | 1.523 | 1.605 | 1.588 | 1.587 |
| F(000) | 1070 | 2176 | 2388 | 2232 | 2228 | 2248 |
| Crystal size/mm3 | 0.1 × 0.09 × 0.08 | 0.12 × 0.11 × 0.08 | 0.8 × 0.12 × 0.1 | 0.14 × 0.12 × 0.09 | 0.12 × 0.11 × 0.08 | 0.8 × 0.12 × 0.1 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| Absorption correction | Multi-scan | Multi-scan | Multi-scan | Multi-scan | Multi-scan | Multi-scan |
| Index ranges | −11 ≤ h ≤ 10 | −13 ≤ h ≤ 13 | −25 ≤ h ≤ 25 | −16 ≤ h ≤ 19 | −19 ≤ h ≤ 19 | −19 ≤ h ≤ 19 |
| −16 ≤ k ≤ 16 | −29 ≤ k ≤ 27 | −16 ≤ k ≤ 15 | −14 ≤ k ≤ 14 | −14 ≤ k ≤ 12 | −14 ≤ k ≤ 14 | |
| −19 ≤ l ≤ 19 | −22 ≤ l ≤ 22 | −24 ≤ l ≤ 23 | −29 ≤ l ≤ 29 | −28 ≤ l ≤ 29 | −28 ≤ l ≤ 28 | |
| Reflections collected | 25662 | 57816 | 34320 | 24823 | 16140 | 13690 |
| Independent reflections | 7658 | 9103 | 9017 | 4256 | 4230 | 4018 |
| Rint, Rsigma | 0.1060, 0.1171 | 0.0665, 0.0454 | 0.0278, 0.0291 | 0.0377, 0.0285 | 0.0348, 0.0351 | 0.1132, 0.1302 |
| Data/restraints/parameters | 7658/12/559 | 9103/608/711 | 9017/259/643 | 4256/84/309 | 4230/318/354 | 4018/318/354 |
| Goodness-of-fit on F2 | 0.979 | 1.027 | 1.040 | 1.087 | 1.040 | 0.977 |
| R1a, wR2b [I > 2σ(I)] | 0.0687, 0.1607 | 0.0448, 0.1116 | 0.0395, 0.1053 | 0.0427, 0.1108 | 0.0368, 0.0925 | 0.0943, 0.2252 |
| R1a, wR2b (for all data) | 0.1435, 0.2022 | 0.0781, 0.1291 | 0.0440, 0.1091 | 0.0529, 0.1187 | 0.0504, 0.1006 | 0.1387, 0.2693 |
| Largest diff. peak/hole/e Å−3 | 0.78/−0.90 | 0.65/−0.32 | 0.57/−1.12 | 0.77/−0.46 | 0.51/−0.33 | 1.48/−2.04 |
The final refinement converged at the R1 (I > 2σ(I)) values of 0.0687, 0.0448, 0.0395, 0.0427, 0.0368 and 0.0943 for 1–6, respectively.
Results and discussion
Syntheses and characterization of 1–6
The trinuclear CuIIMIICuII compounds 1–6 were readily prepared in good yield on treating [CuIIL] with M(ClO4)2·6H2O/M(ClO4)2·xH2O in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio in acetone-diethylether for 1 (M = Cu), 2 (M = Ni) and 3 (M = Co), in methanol-diethylether for 4 (M = Fe) and 5 (M = Mn) and in methanol for 6 (M = Zn). Reaction of [CuIIL] and M(ClO4)2·6H2O in 1
1 stoichiometric ratio in acetone-diethylether for 1 (M = Cu), 2 (M = Ni) and 3 (M = Co), in methanol-diethylether for 4 (M = Fe) and 5 (M = Mn) and in methanol for 6 (M = Zn). Reaction of [CuIIL] and M(ClO4)2·6H2O in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio also produced the compounds 1–6 but the yield is reduced, revealing that trinuclear CuIIMIICuII systems are more stable in this ligand ([L]2−) environment in comparison to systems of other nuclearity. It is also worth mentioning that solvent has a profound role in the isolation of these compounds, e.g., 1–3 and 4 and 5 could not isolated in crystalline form on evaporating the concerned reaction mixture in acetone and methanol, respectively, while crystalline compounds are isolated on diffusing the reaction solution with diethylether. It is also worth mentioning that 1–3 could not be isolated in methanol-diethylether and 4 and 5 could not be isolated from acetone-diethylether. Only the zinc(II) analogue 6 could be isolated in crystalline form on slow evaporation of the reaction solution (in methanol). Inspite of the variation required in solvent and technique (diffusion/evaporation), all the six compounds 1–6 are trinuclear in which a pair of CuIIMII metal ions are solely diphenoxo bridged. We would like to mention that isolation of the five compounds 1–5 in crystalline form was troublesome, which may be a reason that the copper(II)–second metal ion compounds derived from H2L is just two till date,24 while such compounds from closely related ligands are too many. It may be mentioned that although several salicylaldehyde/2-hydroxyacetophenone/2-hydroxypropiophenone/3-methoxysalicylaldehyde/3-ethoxysalicylaldehyde–diamine ligands were previously utilized to derive copper(II)–second metal ion compounds, compounds with all the six second metal ions from MnII to ZnII are rare; to the best of our knowledge, copper(II)–zinc(II)/copper(II)/nickel(II)/cobalt(II)/iron(II)/manganese(II) systems were reported from one ligand, 3-ethoxysalicylaldehyde–ethylenediamine,23a–d although the composition of all those six compounds are not similar – cocrystals of one dinuclear CuIIMII and two mononuclear CuII units when M = Zn/Cu/Co/Fe/Mn but either a trinuclear CuIINiIICuII or a cocrystal of one trinuclear CuIINiIICuII and two mononuclear CuII moieties when M = Ni. Hence, H2L herein is the second ligand of the above mentioned general class of Schiff base ligands where it has been possible to isolate/report all the six copper(II)–zinc(II)/copper(II)/nickel(II)/cobalt(II)/iron(II)/manganese(II) systems and, all the six are similar in composition, trinuclear CuIIMIICuII systems.
1 ratio also produced the compounds 1–6 but the yield is reduced, revealing that trinuclear CuIIMIICuII systems are more stable in this ligand ([L]2−) environment in comparison to systems of other nuclearity. It is also worth mentioning that solvent has a profound role in the isolation of these compounds, e.g., 1–3 and 4 and 5 could not isolated in crystalline form on evaporating the concerned reaction mixture in acetone and methanol, respectively, while crystalline compounds are isolated on diffusing the reaction solution with diethylether. It is also worth mentioning that 1–3 could not be isolated in methanol-diethylether and 4 and 5 could not be isolated from acetone-diethylether. Only the zinc(II) analogue 6 could be isolated in crystalline form on slow evaporation of the reaction solution (in methanol). Inspite of the variation required in solvent and technique (diffusion/evaporation), all the six compounds 1–6 are trinuclear in which a pair of CuIIMII metal ions are solely diphenoxo bridged. We would like to mention that isolation of the five compounds 1–5 in crystalline form was troublesome, which may be a reason that the copper(II)–second metal ion compounds derived from H2L is just two till date,24 while such compounds from closely related ligands are too many. It may be mentioned that although several salicylaldehyde/2-hydroxyacetophenone/2-hydroxypropiophenone/3-methoxysalicylaldehyde/3-ethoxysalicylaldehyde–diamine ligands were previously utilized to derive copper(II)–second metal ion compounds, compounds with all the six second metal ions from MnII to ZnII are rare; to the best of our knowledge, copper(II)–zinc(II)/copper(II)/nickel(II)/cobalt(II)/iron(II)/manganese(II) systems were reported from one ligand, 3-ethoxysalicylaldehyde–ethylenediamine,23a–d although the composition of all those six compounds are not similar – cocrystals of one dinuclear CuIIMII and two mononuclear CuII units when M = Zn/Cu/Co/Fe/Mn but either a trinuclear CuIINiIICuII or a cocrystal of one trinuclear CuIINiIICuII and two mononuclear CuII moieties when M = Ni. Hence, H2L herein is the second ligand of the above mentioned general class of Schiff base ligands where it has been possible to isolate/report all the six copper(II)–zinc(II)/copper(II)/nickel(II)/cobalt(II)/iron(II)/manganese(II) systems and, all the six are similar in composition, trinuclear CuIIMIICuII systems.
The characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching in 1–6 appears as a strong band at practically the same position, 1636–1638 cm−1. The appearance of one signal of strong intensity in the range 1093–1107 cm−1 and a medium intensity in the range 620–626 cm−1 indicates the presence of perchlorate. Compounds 1–3 exhibit a band of medium intensity in the range 3418–3441 cm−1, indicative of the presence of water molecules.
N stretching in 1–6 appears as a strong band at practically the same position, 1636–1638 cm−1. The appearance of one signal of strong intensity in the range 1093–1107 cm−1 and a medium intensity in the range 620–626 cm−1 indicates the presence of perchlorate. Compounds 1–3 exhibit a band of medium intensity in the range 3418–3441 cm−1, indicative of the presence of water molecules.
Description of crystal structures of 1–6
The crystal structures of 1–6 are shown in Fig. 1–3, S13, S2 and S3,† respectively. The structures reveal that all the six compounds are trinuclear CuIIMIICuII systems (M = Cu (1), Ni (2), Co (3), Fe (4), Mn (5), Zn (6)). In each structure, there are two L2− ligands and the N(imine)2O(phenoxo)2 compartment of each L2− is occupied by a copper(II) ion. The two [CuIIL] moieties in each structure are so disposed that a O(phenoxo)4 site is generated and that site is occupied by a second metal ion (Cu (1), Ni (2), Co (3), Fe (4), Mn (5), Zn (6)). Clearly, the metal ion in the O(phenoxo)4 site and each of the two copper(II) ions in the N(imine)2O(phenoxo)2 compartment are diphenoxo bridged. Except tetracoordinated Cu2 centre in the CuIINiIICuII compound 2, all other copper(II) centres in the N(imine)2O(phenoxo)2 compartment are pentacoordinated; the fifth coordination position is occupied by a perchlorate oxygen atom for Cu1 and Cu2 in 1, Cu1 in 2, Cu1 in 3, Cu1/Cu1D in 4, 5 and 6, and by the oxygen atom of an acetone molecule for Cu2 in 3. Regarding the second metal ion (in the O(phenoxo)4 site), copper(II) centre in 1 is pentacoordinated but other metal ions (NiII, CoII, FeII, MnII and ZnII) are hexacoordinated. The fifth or fifth and sixth coordination site (s) is/are occupied by the following: the oxygen atom of a water molecule for Cu3 in 1; two oxygen atoms of two water molecules for Ni1 in 2; the oxygen atom of a water molecule and the oxygen atom of an acetone molecule for Co1 in 3; two oxygen atoms of two methanol molecules for Fe1/Mn1/Zn1 in 4/5/6. There are two water molecules in 1 and one acetone molecule in 2 as solvent of crystallization, while there is no solvent of crystallization in the other four structures. It is also worth mentioning that each of the compounds 4–6 crystallizes in monoclinic space group and C2/c crystal system with very close unit cell parameters (Table 1) and so these four compounds are isomorphous. One half of the structure of each of 4–6 is symmetry related to the second half.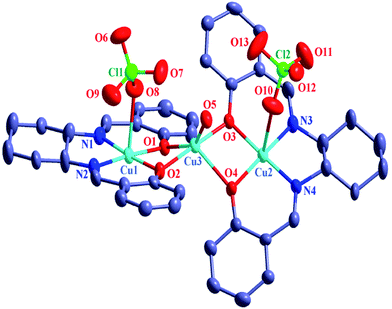 | ||
| Fig. 1 ORTEP drawing (ellipsoid probability at 30%) of the structure of [{CuIIL(ClO4)}2CuII(H2O)]·2H2O (1). All hydrogen atoms are omitted for clarity. | ||
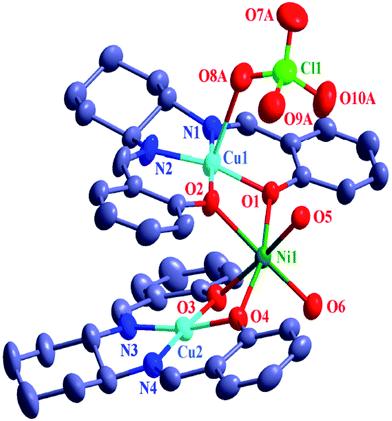 | ||
| Fig. 2 ORTEP drawing (ellipsoid probability at 30%) of the structure of [{CuIIL(ClO4)}{NiII(H2O)2}{CuIIL}]ClO4·CH3COCH3 (2). All hydrogen atoms and one perchlorate anion, are omitted for clarity. | ||
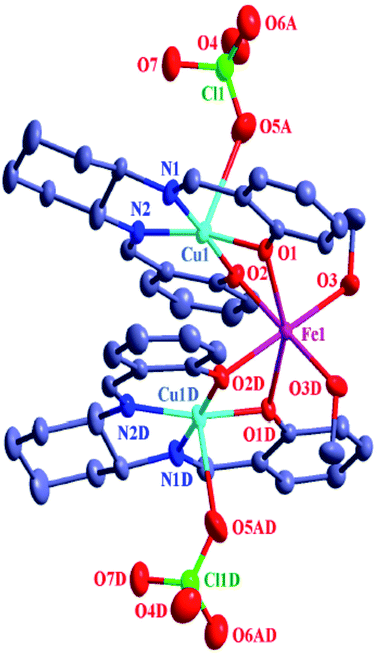 | ||
| Fig. 3 ORTEP drawing (ellipsoid probability at 30%) of the structure of [{CuIIL(ClO4)}2FeII(CH3OH)2] (4). All hydrogen atoms are omitted for clarity. Symmetry code: D, 1 − x, y, 0.5 − z. | ||
The arrangement of the three metal ions in all of 1–6 is triangular; isosceles in 4–6 and closely isosceles in 1–3 (Scheme S1†). The Cu1⋯Cu3⋯Cu2, Cu1⋯Ni1⋯Cu2, Cu1⋯Co1⋯Cu2, Cu1⋯Fe1⋯Cu2, Cu1⋯Mn1⋯Cu2, and Cu1⋯Zn1⋯Cu2 angles in 1–6 are, respectively, 155.42°, 94.48°, 93.00°, 84.67°, 83.14° and 83.14°, while the other two metal⋯metal⋯metal angles in the triangular arrangement in 1–6 are, respectively, 12.53/12.05°, 42.48/43.03°, 43.63/43.37°, 47.67/47.67°, 48.43/48.43° and 48.43/48.43°. The distances of the two copper(II) centres in the N(imine)2O(phenoxo)2 compartments (i.e., Cu1⋯Cu2) in 1–6 are, respectively, 5.802 Å, 4.365 Å, 4.383 Å, 4.105 Å, 4.102 Å and 4.047 Å, while the other two metal⋯metal distances in the triangular arrangement in 1–6 are, respectively, 2.919/3.027 Å, 2.988/2.957 Å, 3.014/3.028 Å, 3.048/3.048 Å, 3.091/3.091 Å and 3.050/3.050 Å.
The structural parameters around the copper(II) centres in the N(imine)2O(phenoxo)2 compartment in 1–6 are listed in Table S1.† The geometry of the coordination environment of the copper(II) centres in the N(imine)2O(phenoxo)2 compartment is distorted square planar for Cu2 in 2 and distorted square pyramidal (N(imine)2O(phenoxo)2 is the basal plane) for others in 1–6; the value of the discrimination parameter, τ (α − β/60, where α is the largest angle and β is the second largest angle in the coordination environment) for Cu1 and Cu2 in 1 are 0.005 and 0.012 and those for the copper(II) centres in 2–6 lie in the range 0.144–0.178. The Cu–O/N distances for the copper(II) centres in the N(imine)2O(phenoxo)2 compartment have close values; the overall ranges of the Cu–O and Cu–N distances in 1–6 are 1.895–1.928 Å and 1.889–1.935 Å, respectively. In comparison to the bond distances involving phenoxo/imine O/N atoms in the basal plane, the Cu–O(apical) distances involving an oxygen atom of perchlorate/acetone are significantly longer (the overall range in 1–6 is 2.596–2.868 Å), which is expected for copper(II) due to Jahn Teller distortion. The overall range of the transoid and cisoid angles in 1–6 are 166.4–178.6° and 81.3–105.51°, respectively. Notably, the values of the structural parameters around copper(II) in the N(imine)2O(phenoxo)2 compartment in 1–6 are in the ranges of those in the previously reported systems from related ligands.13,16,17,19,23a–d,24
The values of some structural parameters around the second metal ions (the metal ions in the O(phenoxo)4 site) are compared in Tables 2 and 3. Of such metal centres, only copper(II) (Cu3) in 1 is pentacoordinated and the concerned τ value is 0.52, which indicates that this coordination environment is intermediate between square pyramidal and trigonal bipyramidal. The copper(II)–O(phenoxo) bond distances lie in the wide range of 1.937–2.214 Å, while the copper(II)–O(water) bond distance is 2.00 Å. The coordination environment of nickel(II), cobalt(II), iron(II), manganese(II) and zinc(II) in 2–6 is highly distorted octahedral as evidenced by the ranges of transoid and cisoid angles – the ranges of the transoid angles are 165.35–173.81° for NiII, 163.17–170.2° for CoII, 153.40–175.86° for FeII, 150.24–174.56° for MnII and 152.0–176.4° for ZnII and the ranges of the cisoid angles are 75.52–99.22° for NiII, 72.96–100.8° for CoII, and 72.29–111.12° for FeII and 71.31–113.55° for MnII and 72.98–110.4° for ZnII. In all of 2–6, the MII–O(water/methanol/acetone) bond distances are smaller than the MII–O(phenoxo) bond distances – the average MII–O(phenoxo) bond distances are 2.082 Å for NiII, 2.118 Å for CoII, 2.152 Å for FeII, 2.201 Å for MnII and 2.149 Å for ZnII and the average MII–O(water/methanol/acetone) bond distances are 2.030 Å for NiII, 2.075 Å for CoII, 2.078 Å for FeII, 2.153 Å for MnII and 2.073 Å for ZnII.
| 1 (M = CuII) | 2 (M = NiII) | 3 (M = CoII) | 4 (M = FeII) | 5 (M = MnII) | 6 (M = ZnII) | |
|---|---|---|---|---|---|---|
| Number and type of ligands | 4 phenoxo, 1 water | 4 phenoxo, 2 water | 4 phenoxo, 1 water, 1 acetone | 4 phenoxo, 2 methanol | 4 phenoxo, 2 methanol | 4 phenoxo, 2 methanol |
| Coordination number | 5 | 6 | 6 | 6 | 6 | 6 |
| M–O(phenoxo) | 1.937, 1.947, 2.012, 2.214 | 2.060, 2.077, 2.087, 2.103 | 2.083, 2.123, 2.125, 2.141 | 2.134, 2.171 | 2.187, 2.215 | 2.129, 2.169 |
| M–O(water) | 2.000 | 2.024, 2.037 | 2.064 | — | — | — |
| M–O(MeOH) | — | — | — | 2.078 | 2.153 | 2.073 |
| M–O(acetone) | — | — | 2.086 | — | — | — |
| Cu⋯M | 2.9119 (with Cu1), 3.0266 (with Cu2) | 2.9878 (with Cu1), 2.9573 (with Cu2) | 3.0137 (with Cu1), 3.0282 (with Cu2) | 3.0480 (with Cu1) | 3.0908 (with Cu1) | 3.050 (with Cu1) |
| Transoid angle range | 145.268–176.5 | 165.35–173.81 | 163.17–170.2 | 153.40–175.86 | 150.24–174.56 | 152.0–176.4 |
| Cisoid angle range | 75.5–115.4 | 75.52–99.22 | 72.96–100.8 | 72.29–111.12 | 71.31–113.55 | 72.9–110.4 |
| Discrimination parameter | 0.5205 | — | — | — | — | — |
| dM | 0.2929 (sq py), 0.0155 (tbp) | 0.0049 | 0.0275 | 0.000 | 0.000 | 0.000 |
| dav | 0.2821 (sq py), 0.000 (tbp) | 0.1013 | 0.1384 | 0.0634 | 0.0927 | 0.0606 |
| 1 (M = CuII) | 2 (M = NiII) | 3 (M = CoII) | 4 (M = FeII) | 5 (M = MnII) | 6 (M = ZnII) | ||
|---|---|---|---|---|---|---|---|
| Cu–O(phenoxo)–M bridge angles (α) | Cu1–O1–M | 95.9 | 97.70 | 96.32 | 96.28 | 97.42 | 97.3 |
| Cu1–O2–M | 98.0 | 96.16 | 98.00 | 97.74 | 96.73 | 96.2 | |
| Cu2–O3–M | 103.5 | 95.63 | 97.30 | — | — | — | |
| Cu2–O4–M | 94.5 | 95.79 | 96.35 | — | — | — | |
| Out of plane shift (φ) | O2–O1–C1 | 5.74 | 12.46 | 7.3 | 7.37 | 7.8 | 7.78 |
| O1–O2–C20 | 5.51 | 6.11 | 6.08 | 7.8 | 7.13 | 7.12 | |
| O3–O4–C40 | 6.71 | 9.44 | 7.24 | — | — | — | |
| O4–O3–C21 | 9.56 | 6.34 | 6.81 | — | — | — | |
| Torsion angle (τ) | Cu1–O1–M–O2 | 19.55 | 19.02 | 19.48 | 23.15 | 23.09 | 23.17 |
| Cu1–O2–M–O1 | 19.61 | 18.92 | 19.64 | 23.32 | 23.16 | 23.23 | |
| Cu2–O3–M–O4 | 11.36 | 21.84 | 23.2 | — | — | — | |
| Cu2–O4–M–O3 | 11.20 | 21.78 | 23.11 | — | — | — |
The two Cu–O–Cu phenoxo bridge angles involving Cu1 and Cu3 are 95.9 and 98.0° and those involving Cu2 and Cu3 are 94.5° and 103.5°. In 2–6, the four Cu–O–M phenoxo bridge angles not very different (95.63–97.70° in 2, 96.32–98.00° in 3, 96.28–97.74° in 4, 96.73–97.42° in 5 and 96.2–97.3° in 6); the average values are 96.32°, 96.99°, 97.01°, 97.07° and 96.75° for 2–6, respectively.
Weak interactions in 1–6
There are three and two hydrogen bonds in, respectively, the CuIICuIICuII compound 1 (Fig. S4†) and CuIINiIICuII compound 2 (Fig. S5†). Of the three hydrogen bonds in 1, two are O–H⋯O interactions (O5–H5A⋯O7, O5–H5B⋯O10) involving the coordinated water molecule and two oxygen atoms of two coordinated perchlorates. The remaining hydrogen bond in 1 is a C–H⋯O interaction involving a CH (C32H32B) hydrogen atom of a cyclohexane moiety and a perchlorate oxygen atom (O10E). The later interaction interlinks two trinuclear units to form a dimer-of-trinuclear type self-assembly. The two hydrogen bonds in 2 are O–H⋯O interactions (O5–H5B⋯O13E, O6–H6A⋯O11E) involving two coordinated water molecules and two oxygen atoms of one perchlorate anion. However, no supramolecular cluster or polymer is generated in 2.It was realized that the perchlorate oxygen atoms in 3 are engaged in few hydrogen bonding interactions. However, it is not possible to analyze the interactions or the supramolecular structure resulted therefrom due to disorder in most of the perchlorate oxygen atoms.
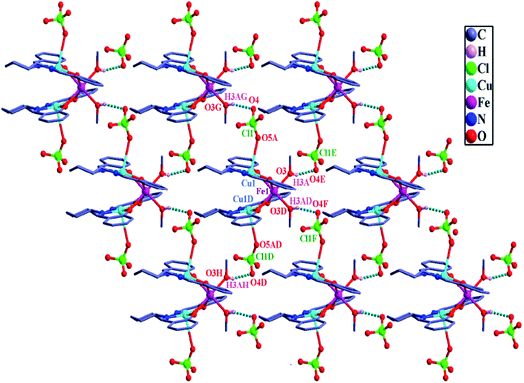
The OH (O3H3A) hydrogen atom of the crystallographically single type of coordinated methanol molecule in the CuIIFeIICuII compound 4 forms a hydrogen bond with one oxygen atom (O4E) of a perchlorate anion. One half of this compound is symmetry related to the second half in such a way that this O–H⋯O weak interaction generates a two-dimensional topology in the crystallographic ab plane (Fig. 4). The supramolecular topology of the CuIIMnIICuII compound 5 and CuIIZnIICuII compound 6, which are isomorphous with 4, are similar to that of 4 (corresponding illustrations for 5 and 6 are shown in Fig. S6 and S7,† respectively).
The values of the parameters of the hydrogen bonds in 1–6 are listed in Tables S2 and S3,† revealing that these interactions are moderately strong or weak.
ESI-MS positive studies of 1–6
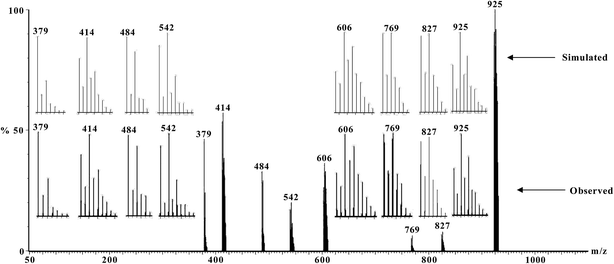
ESI-MS of the methanol solution of 1 and acetonitrile solutions of 2–6 have been recorded. The observed and simulated spectra of the CuIINiIICuII (2) and CuIIMnIICuII (5) compounds are shown in Fig. 5 and 6, respectively, while those of the CuIICuIICuII (1), CuIICoIICuII (3), CuIIFeIICuII (4) and CuIIZnIICuII (6) compounds are shown in Fig. S8–S11,† respectively. The species appeared in the spectra are listed in Table 4 (without drawing) and Table S4† (with drawing). The line-to-line separation in a peak for the dipositive and monopositive ions are 0.5 and 1.0, respectively.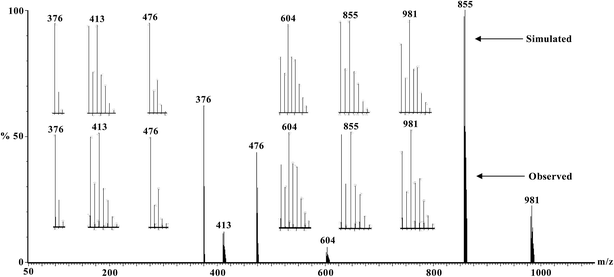 | ||
| Fig. 6 Electrospray ionization mass spectrum in positive mode (ESI-MS positive) of [{CuIIL(ClO4)}2MnII(CH3OH)2] (5) in acetonitrile, showing observed and simulated isotopic distribution patterns. | ||
| Ions in ESI-MS | Line-to-line m/z gap | 1 (M = CuII) | 2 (M = NiII) | 3 (M = CoII) | 4 (M = FeII) | 5 (M = MnII) | 6 (M = ZnII) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intensity | m/z | Intensity | m/z | Intensity | m/z | Intensity | m/z | Intensity | m/z | Intensity | m/z | ||
| [(CuIIL)3MII]2+, (I; C60H60N6O6CuII3MII) | 0.5 | — | — | 37% | 606 | 12% | 606 | 28% | 605 | 6% | 604 | 7% | 610 |
| [(CuIIL)MII(ClO4)(CuIIL)]+, (II; C40H40N4O8ClCuII2MII) | 1.0 | 100% | 930 | 100% | 925 | 100% | 926 | 100% | 923 | — | — | 100% | 931 |
| [(CuIIL)MI(CH3OH)(CuIIL)]+, (III; C41H44N4O5CuII2MI) | 1.0 | — | — | — | — | — | — | — | — | 100% | 855 | — | — |
| [(CuIIL)MII(CuIIL)]2+, (IV; C40H40N4O4CuII2MII) | 0.5 | — | — | 58% | 414 | 59% | 415 | 61% | 412 | 12% | 413 | 73% | 417 |
| [(CuIIL)MII(ClO4)(CH3OH)(CuIIL)]+, (V; C41H44N4O9ClCuII2MII) | 1.0 | — | — | — | — | — | — | 9% | 955 | — | — | — | — |
| [(CuIIL)(CH3CN)MII(ClO4)(CuIIL)(H2O)]+, (VI; C42H45N5O9ClCuII2MII) | 1.0 | — | — | — | — | — | — | — | — | 23% | 981 | — | — |
| [(CuIIL)MII(ClO4)]+, (VII; C20H20N2O6ClCuIIMII) | 1.0 | 9% | 547 | 21% | 542 | 8% | 542 | — | — | — | — | 24% | 548 |
| [(CuIIL)MI]+, (VIII; C20H20N2O2CuIIMI) | 1.0 | 9% | 446 | — | — | — | — | — | — | — | — | — | — |
| [{CuII(HL)}(CuIIL)]+, (IX; C40H41N4O4CuII2) | 1.0 | 12% | 769 | 7% | 769 | 5% | 769 | 32% | 769 | — | — | 7% | 769 |
| [{CuII(HL)}(CuIIL)(CH3COCH3)]+, (X; C43H47N4O5CuII2) | 1.0 | 83% | 827 | 9% | 827 | — | — | — | — | — | — | — | — |
| [CuII(HL)]+, (XI; C20H21N2O2CuII) | 1.0 | 62% | 384 | — | — | — | — | 43% | 384 | — | — | 19% | 384 |
| [CuII(H2L)(ClO4)]+, (XII; C20H22N2O6ClCuII) | 1.0 | 42% | 484 | 33% | 484 | 42% | 484 | 60% | 484 | — | — | — | — |
| [MII(HL)]+, (XIII; C20H21N2O2MII) | 1.0 | — | — | 47% | 379 | 46% | 380 | 61% | 377 | 63% | 376 | — | — |
| [MII(H2L)(ClO4)]+, (XIV; C20H22N2O6ClMII) | 1.0 | — | — | — | — | — | — | — | — | 44% | 476 | 42% | 485 |
A total of fourteen types of species (I–XIV), which are tetra/tri/di/mononuclear, are observed in the spectra of the six compounds 1–6, although no single type of ion corresponds to all the six compounds. The types of species involve a tetranuclear (possibly star) system of composition [(CuIIL)3MII]2+ (I), appeared in all the five heterometallic compounds 2–6. Five types of trinuclear systems are appeared and those are: (i) [(CuIIL)MII(ClO4)(CuIIL)]+ (II), which is the 100% intensity signal in all but the manganese(II) analogue; (ii) [(CuIIL)MI(CH3OH)(CuIIL)]+ (III), which is the 100% intense peak in the manganese(II) analogue; (iii) [(CuIIL)MII(CuIIL)]2+ (IV), which appears in the five compounds 2–6; (iv) [(CuIIL)MII(ClO4)(CH3OH)(CuIIL)]+ (V), which only appears in the iron(II) analogue; (v) [(CuIIL)(CH3CN)MII(ClO4)(CuIIL)(H2O)]+ (VI), which appears only in the manganese(II) analogue. Four dinuclear ions that are observed are: (i) [(CuIIL)MII(ClO4)]+ (VII) which appears in the spectra of all but the iron(II) and manganese(II) analogues; (ii) [(CuIIL)MI]+ (VIII) which appears in only the CuII3 compound 1; (iii) [{CuII(HL)}(CuIIL)]+ (IX) which appears in all but the manganese(II) analogue; (iv) [{CuII(HL)}(CuIIL)(CH3COCH3)]+ (X) which appears in 1 and 2 only. Four mononuclear ions are: (i) [CuII(HL)]+ (XI; in 1, 4 and 6); (ii) [CuII(H2L)(ClO4)]+ (XII; in all but the manganese(II) and zinc(II) analogues); (iii) [MII(HL)]+ (XIII; in the four compounds 2–5); (iv) [MII(H2L)(ClO4)]+ (XIV; in 5 and 6).
Although the heteronuclear compounds 2–6 are trinuclear CuIIMIICuII systems, ions of higher nuclearity, i.e., the tetranuclear [(CuIIL)3MII]2+ (I) species, are stabilized in their ESI-MS. Most probably, such tetranuclear ions are star systems (Table S4†). It is worth mentioning that similar heterometallic star systems containing one PbII and three CuII (i.e., PbIICuII3) are also stabilized in the ESI-MS of trinuclear CuIIPbIICuII systems derived from closely similar single compartment ligands.14i Notably, star systems in solid state are rarely observed in the homo/heterometallic family derived from the ligands similar to H2L.15a,b,17f From that perspective, the stabilization of star ions in the ESI-MS of all the five heterotrinuclear compounds 2–6 may be considered interesting. Another remarkable feature in the ESI-MS of 1–6 is the metal ion dependent stability of the 100% intense trinuclear ion. For the CuIIMIICuII compounds 1–4 and 6 (M = Cu, Ni, Co, Fe and Zn), the 100% intense species is the trinuclear CuIIMIICuII ion of composition [(CuIIL)MII(ClO4)(CuIIL)]+ (II), which is not appeared in the spectrum of the MnII analogue 5. On the other hand, the 100% intense ion in the spectrum of the CuIIMnIICuII compound 5 is a trinuclear CuIIMnICuII species of composition [(CuIIL)MnI(CH3OH)(CuIIL)]+ (III) and such a species is not appeared in the spectra of the other five compounds 1–4 and 6. This observation may be considered as interesting firstly due to the fact that II is the most intense ion in 1–4 and 6 and is not appeared in 5 while III is the most intense ion in 5 and is not appeared in 1–4 and 6 and secondly due to the fact that III is an unusual MnI species while II is an usual MII (M = Cu, Ni, Co, Fe, Zn) species.
Magnetic properties of 1–6
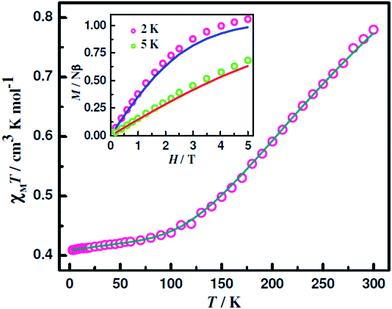
Variable-temperature (2–300 K) χMT versus T plots for the CuIIMIICuII compounds 1–6 are shown in Fig. 7 (for 1; M = Cu), Fig. 8 (for 2, 3, 4 and 5; M = Ni, Co, Fe, Mn) and Fig. S12† (for 6; M = Zn). The χMT values (in cm3 mol−1 K) at 300 K for the CuIICuIICuII (1), CuIINiIICuII (2), CuIICoIICuII (3), CuIIFeIICuII (4), CuIIMnIICuII (5) and CuIIZnIICuII (6) compounds are, respectively, 0.78, 1.87, 3.23, 3.7, 4.96 and 0.89 while the spin-only theoretical values (with g = 2.0) of noninteracting metal ions (S = 1/2, 1, 3/2, 2 and 5/2 for CuII, NiII, CoII, FeII and MnII, respectively) are, respectively, 1.12, 1.75, 2.62, 3.75, 5.12 and 0.75. So, while the observed and calculated values are not very different for the NiII (2), FeII (4), MnII (5) and ZnII (6) analogues, the observed value is smaller by 0.34 and greater by 0.61 in the case of the CuII (1) and CoII (3) analogues, respectively. On lowering of temperature, χMT values for all the six compounds 1–6 decrease throughout the temperature range. However, the pattern of decrease is different for the different compounds. For 1, χMT values decrease sharply in the temperature range 300–100 K and then decrease slowly to 0.41 at 2 K. For the four compounds 2–5, χMT values decrease slowly up to a certain temperature (ca. 100 K for 2 and 3 and ca. 80 K for 4 and 5) and then decrease rapidly to the values of 0.001, 0.36, 0.43 and 1.82 at 2 K for 2–5, respectively. For the ZnII analogue 6, χMT decreases very slowly in the temperature range 300–28 K (from 0.89 to 0.80) and then the values decrease sharply to 0.13 at 2 K. The profiles indicate that the metal centres are coupled by very weak antiferromagnetic interaction in 6, by weak antiferromagnetic interaction in 2–5 and by moderate antiferromagnetic interaction in 1.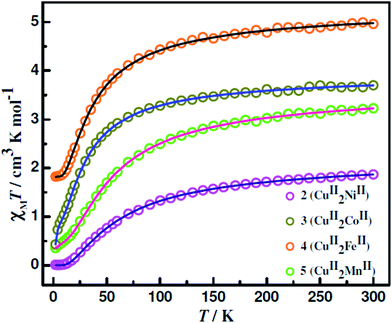 | ||
| Fig. 8 Fitting of χMT versus T of 2–5 between 2 and 300 K. The experimental data are shown in symbols and the lines correspond to the fitted values. | ||
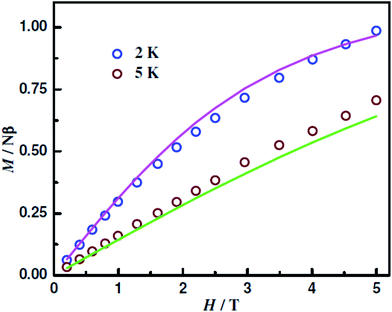
The magnetization (M) data up to 5 T of 1–5 were collected at 2/2.5 and 5 K. For the NiII analogue (2), the M values even at 5 T are very small, less than 0.2, indicating its nonmagnetic ground state. The data of the other four compounds 1, 3, 4 and 5 are shown in Fig. 7, 9, 10 and S13,† respectively. The M values at 2 K and 5 T of 1.05 Nβ of the CuIICuIICuII compound 1, 0.99 Nβ of the CuIICoIICuII compound 3 and 3.06 Nβ of the CuIIMnIICuII compound 5 indicate that their spin ground states are, respectively, ST = 1/2, ST = 1/2 and ST = 3/2. On the other hand, the M value at 2 K and 5 T of the CuIIFeIICuII compound 4 is 1.22 Nβ, which is significantly smaller than that (2.0 Nβ) of ST = 1 spin ground state. However, such a decrease in M values takes place due to single-ion zero-field effect (vide infra; DFe = 3.45 cm−1).
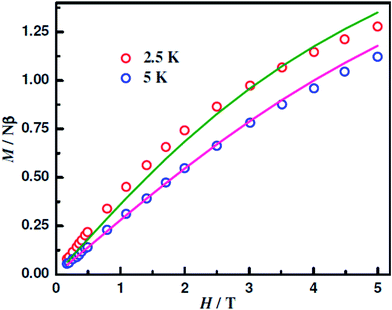 | ||
| Fig. 10 Magnetization of [{CuIIL(ClO4)}2FeII(CH3OH)2] (4) at the indicated temperatures. The symbols are the experimental data, while the solid lines represent the fitted curves. | ||
The CuII⋯CuII distance in the CuIIZnIICuII compound 6 is 4.00 Å, indicating that the two copper(II) centres can interact through space or through the bis(μ-phenoxo)⋯ZnII⋯bis(μ-phenoxo) long route and this is the only exchange interaction possible in 6. Thus the HDvV spin Hamiltonian for this case is H = −2JS1S3 (where S1 = S3 = 1/2; Scheme 2). The main exchange interactions in 1–5 should be the two CuII⋯MII interactions (M = Cu, Ni, Co, Fe, Mn) propagated via bis(μ-phenoxo) bridging moiety. In 1–5, the CuII (in N2O2 site)⋯CuII (in N2O2 site) interaction should also be taken into account as the CuII⋯CuII distance lie in the range 4.09–5.80 Å (less than 6 Å). The two CuII⋯MII interactions in 4 and 5 are the same as the two pairs are symmetry related. In each of 1–3, the comparison of the values of the parameters in the two CuII⋯MII pairs that can govern the magnetic exchange are as follows: (i) CuII⋯MII distances are close (vide supra, Scheme S1†); (ii) the phenoxo bridge angles in the two pairs are not very different (average in two pairs: 99.05 and 96.85° in 1, 96.99 and 95.62° in 2, 97.06 and 96.89° in 3; Table 3); (iii) Cu–O–M–O torsion angles (τ) are not very different (Table 3); (iv) Out-of-plane shift of the phenyl rings are not very different (Table 3). Hence, it is logical to assume the two CuII⋯MII interactions as the same. Single-ion zero-field parameter should be considered for NiII, CoII and FeII. So, the spin Hamiltonian for 1 (CuIICuIICuII) and 5 (CuIIMnIICuII) is given by H = −2J1(S1S2 + S3S2) − 2JS1S3 (where S1 = S3 = 1/2 and S2 is 1/2 for 1 and 5/2 for 5; Scheme 2) and for 2 (CuIINiIICuII), 3 (CuIICoIICuII) and 4 (CuIIFeIICuII) is given by eqn (1) (where S1 = S3 = 1/2 and S2 is 1, 3/2 and 2, respectively; Scheme 2).
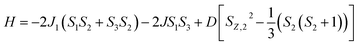 | (1) |
Taking into consideration of the above mentioned models and also temperature independent paramagnetism (TIP) and different g values for different metal ions, the magnetic data of 1–6 were simulated with PHI software,26 resulting in excellent fits with the following sets of converging parameters: J1 = −136.50 cm−1, J = 0.00, g = 2.09 and TIP = 230 × 10−6 cm3 mol−1 for the CuIICuIICuII compound 1; J1 = −22.16 cm−1, J = −1.97 cm−1, gCu = 2.10, gNi = 2.19, DNi = 1.64 cm−1 and TIP = 315 × 10−6 cm3 mol−1 for the CuIINiIICuII compound 2; J1 = −14.78 cm−1, J = −1.86 cm−1, gCu = 2.11, gCo = 2.39, DCo = 23.96 cm−1 and TIP = 213 × 10−6 cm3 mol−1 for the CuIICoIICuII compound 3; J1 = −6.35 cm−1, J = −1.17 cm−1, gCu = 2.09, gFe = 2.01, DFe = 3.45 cm−1 and TIP = 150 × 10−6 cm3 mol−1 for the CuIIFeIICuII compound 4; J1 = −6.02 cm−1, J = −1.70 cm−1, gCu = 2.07, gMn = 2.00 and TIP = 212 × 10−6 cm3 mol−1 for the CuIIMnIICuII compound 5; J = −2.25 cm−1, g = 2.12 and TIP = 165 × 10−6 cm3 mol−1 for the CuIIZnIICuII compound 6. Notably, both χMT versus T and M versus H data of 1, 3, 4 and 5 were simulated contemporaneously.
The order of the extent of antiferromagnetic interactions through bis(μ-phenoxo) route in 1–5 are as follows: CuIICuIICuII (1; J1 = −136.50 cm−1) > CuIINiIICuII (2; J1 = −22.16 cm−1) > CuIICoIICuII (3; J1 = −14.78 cm−1) > CuIIFeIICuII (4; J1 = −6.35 cm−1) ≈ CuIIMnIICuII (5; J1 = −6.02 cm−1). As the geometry of the copper(II) centre in the O(phenoxo)4 site of the two ligands in 1 is intermediate between square pyramidal and trigonal bipyramidal and that of other metal ions in the similar site in 2–4 is distorted octahedral, it is complicated to explain the overall trend in terms of orbital model without theoretical calculations. However, assuming dx2−y2 as the magnetic orbital of all the copper(II) centres in 1–5, the trend can be qualitatively explained on the basis of magnetic orbitals.1a,11b,27 While the magnetic orbital for copper(II) is only one (dx2−y2), the number of magnetic orbitals increases on going from copper(II) to manganese(II). Of the different types of orbital combinations, only dx2−y2 ↔ dx2−y2 is antiferromagnetic, whereas other combinations are ferromagnetic. As a result, the order of ferromagnetic contributions in the five compounds 1–5 should be CuIIMnIICuII > CuIIFeIICuII > CuIICoIICuII > CuIINiIICuII > CuIICuIICuII. Therefore, the order of the extent of antiferromagnetic interaction should be CuIICuIICuII > CuIINiIICuII > CuIICoIICuII > CuIIFeIICuII > CuIIMnIICuII, which is almost matched with the observed trend.
Previously, a number of magneto-structural correlations were established in bis(μ-hydroxo/alkoxo/phenoxo) dicopper(II) compounds.3,4a–c,5 Based on experimental magnetic properties in planar dihydroxo-bridged dicopper(II) systems, it was established that the magnetic exchange interaction should be ferromagnetic and antiferromagnetic, respectively, if the Cu–O–Cu bridge angle (α) is, respectively, smaller than and greater than a cross-over angle of around 97.5°.3 Later, based on density functional theoretical calculations in dihydroxo/dialkoxo/diphenoxo-bridged dicopper(II) systems, it has been established that the out-of-plane shift (φ) of the hydrogen atom/alkyl group/phenyl group is also a major factor and the hinge distortion (τ) of the Cu2O2 plane has also some role to govern the interaction.4a–c Some salient features of the DFT calculations may be summarized as follows: (i) if φ = 35° and τ = 20° in bis(μ-hydroxo) dicopper(II) systems and φ = 50° in bis(μ-phenoxo) dicopper(II) systems, the interaction is ferromagnetic and α has practically no role to switch the interaction to antiferromagnetic; (ii) if φ = 0° and τ = 0° in bis(μ-hydroxo) dicopper(II) systems and φ = 0° in bis(μ-phenoxo) dicopper(II) systems, J changes linearly with α where the more the α the more the antiferromagnetic interaction but the cross-over takes place at around 90° and 83° for the hydroxo and phenoxo systems, respectively. Hence, although larger α and smaller φ and τ favour antiferromagnetic interaction and vice versa, it is problematic to get a magneto-structural correlation based on experimental J values in compounds derived from different types of ligands as variation of one parameter on keeping two of the three parameters more or less similar is difficult. Moreover, the theoretical correlations were done on planar Cu2O2 complexes, where both copper(II) ions are square planar, which makes it more difficult to absolutely correlate the experimental J values with the theoretical correlations. However, the theoretical correlations are nice enough to qualitatively rationalize the magnetic exchange interactions of new systems. It is also worth mentioning that correlations established in diphenoxo-bridged dicopper(II) systems should be more or less similar in trinuclear CuII–bis(μ-phenoxo)–CuII–bis(μ-phenoxo)–CuII systems like 1. In 1, although the coordination environment of two CuII centres is distorted, that of one copper(II) centre (Cu3) is intermediate between square pyramidal and trigonal bipyramidal and hence this system is quite different from the models utilized in establishing theoretical correlations. However, average values of α (97.95°), φ (7.02°) and τ (15.35°) indicate that the interaction in 1 should not be ferromagnetic or strong antiferromagnetic, which is actually observed; the interaction is moderately strong in 1 with J = −136.5 cm−1. The J, α, φ and τ values of the structurally and magnetically characterized CuII–bis(μ-phenoxo)–CuII–bis(μ-phenoxo)–CuII systems5,14g,17c,20c,27b,28 are compared in Table S5† and J versus α, J versus φ and J versus τ plots are shown in Fig. S14–S16,† respectively, revealing that there is no correlation in experimental magnetic properties due to the reason already explained.
Till date, no magneto-structural correlation in CuIIMII compounds (M = Ni, Co, Fe, Mn) has been reported. However, it can be anticipated that above mentioned three parameters α, φ and τ should have roles in governing the nature and magnitude of magnetic exchange interaction in diphenoxo-bridged systems, such as in the CuIIMIICuII compounds 2–5. In all these four compounds, the out-of-plane shift of the phenyl group is small (average φ = 6.92–8.55°), favouring strong antiferromagnetic interaction. However, the hinge angle values are sufficiently large (average τ = 20.44–23.25°) and the phenoxo bridge angle values are sufficiently smaller (average α = 96.31–97.05°) to reduce the antiferromagnetic interaction. In balance, the magnetic exchange interaction is 2–5 is weak or moderate antiferromagnetic with J (CuII–MII) values of −22.16, −14.78, −6.35 and −6.02 cm−1, respectively.
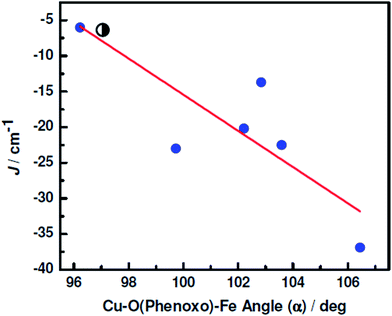
The J, α, φ and τ values of the known compounds having diphenoxo bridged CuIIMII species are listed in Tables S6† (for CuIINiII),13a,15a,29 Tables S7† (for CuIICoII),13g,16a,17a,b,g,19h,20e,23a,29d,30 Table 5 (for CuIIFeII)17b,20a,23b and Tables S8† (for CuIIMnII).13d,15a,17a,f,19h,m,20a,b,d,23a,e,29e,31 The nature of interaction in CuII–bis(μ-phenoxo)–MII moieties in all but one previously reported compounds is antiferromagnetic with J values lying in the ranges from −3.53 to −130.0, from −7.3 to −53.3, from −6 to −36.9 and from −6.35 to −36.8 cm−1, respectively, for the NiII, CoII, FeII and MnII analogues; the sole system exhibiting ferromagnetic interaction is a MnIICuII3 star with J value of 1.02 cm−1. Hence the J (CuII–MII) values of −22.16, −14.78, −6.35 and −6.02 cm−1 in 2–5, respectively, lie well in the ranges of the known compounds. However, the J versus α/φ/τ plots for the NiII (Fig. S17–S19†), CoII (Fig. S20–S22†) and MnII (Fig. S23–S25†) analogues show that it is not possible to establish a magneto-structural correlation; the graphs are significantly scattered. The same is the case for the J versus φ/τ plots (Fig. S26 and S27†) for the systems having diphenoxo bridged CuIIFeII species. On the other hand, a linear correlation (Fig. 11) can be established between the J and α values in diphenoxo bridged CuIIFeII compound and that is J = −2.54α + 238.61. According to this correlation the cross-over angle in diphenoxo-bridged CuIIFeII systems is 93.9°. However, the correlation should not be considered as strong as the number of data points is small in comparison to those in other CuIIMII systems (M = Ni, Co, Mn).
| Compound no. | CSD code | J (cm−1) | Average Cu–O(phenoxo)–Fe bridge angle (α) (in °) | Out-of-plane shift of phenyl group (φ) (in °) | Cu–O–Fe–O torsion angle (τ) (in °) | Reference |
|---|---|---|---|---|---|---|
| 1 | BICBEW | −20.2 | 102.21 | 6.57 | 8.40 | 20a |
| 2 | BICBOG | −36.9 | 106.45 | 2.91 | 5.45 | 20a |
| 3 | FIHFAD | −6 | 96.23 | 11.72 | 26.54 | 17b |
| 4 | FIHFEH | −23 | 99.72 | 24.55 | 27.56 | 17b |
| 5 | IVOVOF | −22.5 | 103.58 | 6.51 | 1.70 | 23b |
| 6 | IVOVUL | −13.7 | 102.84 | 6.27 | 3.83 | 23b |
| 7 | — | −6.35 | 97.01 | 7.58 | 23.23 | This work |
Conclusions
Six CuIIMIICuII compounds, 1 (M = Cu), 2 (M = Ni), 3 (M = Co), 4 (M = Fe), 5 (M = Mn) and 6 (M = Zn), are only the third examples of copper(II)–second metal ion systems, next to the previously reported a CuIIHgII and a CuII2GdIII2 compound,24 derived from salicylaldehyde-trans-1,2-diaminocyclohexane ligand, H2L. This aspect deserves attention as many copper(II)–second metal ion compounds are known from a number of closely similar ligands. The complications in isolating most of the compounds of 1–6 in crystalline form may be taken as a reason why this particular ligand has been remained so little explored to derive/report copper(II)–second metal ion systems. This is the second ligand system among salicylaldehyde/2-hydroxyacetophenone/2-hydroxypropiophenone/3-methoxysalicylaldehyde/3-ethoxysalicylaldehyde–diamine ligands in which all the six copper(II)–second metal ion compounds with MnII–ZnII as the second metal ions are reported. However, in comparison to the sole earlier case,23a–d all the six compounds herein have basically similar composition (trinuclear CuIIMIICuII).ESI-MS positive of 1–6 reveal some interesting features. For example, stabilization of heterometallic star ions for all the five heterometallic trinuclear compounds 2–6 may be mentioned. Again, although the most intense signal in all the six ESI-MS correspond to trinuclear ions, the nature is of two types; CuIIMIICuII for the CuII, NiII, CoII, FeII and ZnII analogues but CuIIMnICuII for the MnII analogue 5.
One or more O–H⋯O/C–H⋯O hydrogen bond (s) exist (s) in 1–6. The trinuclear units in 1, 3 and 4–6 are interlinked by hydrogen bonds to generate following types of supramolecular self-assemblies: dimeric in 1, one-dimensional in 3 and three-dimensional in 4–6.
Variable-temperature and variable-field magnetic studies reveal moderate or weak antiferromagnetic interaction between CuII and MII (M = Cu, Ni, Co, Fe, Mn) through diphenoxo bridge and very weak antiferromagnetic interaction between the two CuII in the N(imine)2O(phenoxo)2 compartments in 1–6. We have compared the magnetic exchange integrals and the key structural parameters of the previously reported homo/heterometallic systems having diphenoxo bridging moiety between the two metal ions. However, no correlation exists for the other systems except CuIIFeII. In the case of the latter, a linear correlation between J and CuII–O(phenoxo)–FeII bridge angle is established, although the relationship should not be considered as a strong one as the number of data points is rather limited in comparison to those of other systems.
We hope this particular ligand H2L henceforth will draw the attention of the coordination chemistry community, having particular interest in stabilizing heterometallic systems from salicylaldehyde/2-hydroxyacetophenone/2-hydroxypropiophenone/3-methoxysalicylaldehyde/3-ethoxysalicylaldehyde–diamine Schiff base ligands.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Financial support for this work has been received from Government of India through University Grants Commission (contingency from CAS-V project to S. Mohanta; Women Postdoctoral Fellowship to A. Jana; JRF/SRF to N. Hari) and Department of Science and Technology (INSPIRE Fellowship to Shuvankar Mandal). Crystallography and ESI-MS were performed at the DST-FIST-funded Single Crystal Diffractometer Facility and DST-PURSE-funded Mass Spectrometer facility, both at the Department of Chemistry, University of Calcutta. Centre for Research in Nanoscience and Nanotechnology, University of Calcutta, is acknowledged for magnetic data.References
- (a) O. Kahn, Molecular Magnetism, VCH Publications, New York, 1993 Search PubMed; (b) Magneto-Structural Correlations in Exchange Coupled Systems, ed. R. D. Willet, D. Gatteschi and O. Kahn, Reidel, Dordrecht, The Netherlands, 1985 Search PubMed.
- (a) B. C. Guha, Proc. Roy. Soc. Lond., 1951, A206, 353 CrossRef; (b) B. Bleaney and K. D. Bowers, Proc. Roy. Soc. Lond., 1952, A214, 451 CrossRef.
- V. H. Crawford, H. W. Richardson, J. R. Wasson, D. J. Hodgson and W. E. Hatfield, Inorg. Chem., 1976, 15, 2107 CrossRef CAS.
- (a) D. Venegas-Yazigi, D. Aravena, E. Spodine, E. Ruiz and S. Alvarez, Coord. Chem. Rev., 2010, 254, 2086 CrossRef CAS; (b) E. Ruiz, P. Alemany, S. Alvarez and J. Cano, Inorg. Chem., 1997, 36, 3683 CrossRef CAS PubMed; (c) E. Ruiz, C. de Graaf, P. Alemany and S. Alvarez, J. Phys. Chem. A, 2002, 106, 4938 CrossRef CAS; (d) A. Rodríguez-Fortea, P. Alemany, S. Alvarez and E. Ruiz, Inorg. Chem., 2002, 41, 3769 CrossRef; (e) E. Ruiz, J. Cano, S. Alvarez and P. Alemany, J. Am. Chem. Soc., 1998, 120, 11122 CrossRef CAS; (f) S. Triki, C. J. Gómez-García, E. Ruiz and J. Sala-Pala, Inorg. Chem., 2005, 44, 5501 CrossRef CAS PubMed; (g) F. F. de Biani, E. Ruiz, J. Cano, J. J. Novoa and S. Alvarez, Inorg. Chem., 2000, 39, 3221 CrossRef.
- L. Botana, J. Ruiz, J. M. Seco, A. J. Mota, A. Rodríguez-Diéguez, R. Sillanpää and E. Colacio, Dalton Trans., 2011, 40, 12462 RSC.
- L. Merz and W. Haase, J. Chem. Soc., Dalton Trans., 1980, 875 RSC.
- (a) K. K. Nanda, L. K. Thompson, J. N. Bridson and K. Nag, Chem. Commun., 1994, 1337 RSC; (b) M. A. Palacios, A. J. Mota, J. E. Perea-Buceta, F. J. White, E. K. Brechin and E. Colacio, Inorg. Chem., 2010, 49, 10156 CrossRef CAS PubMed; (c) S. Sasmal, S. Hazra, P. Kundu, S. Dutta, G. Rajaraman, E. C. Sañudo and S. Mohanta, Inorg. Chem., 2011, 50, 7257 CrossRef CAS PubMed.
- S. Sasmal, S. Hazra, P. Kundu, S. Majumder, N. Aliaga-Alcalde, E. Ruiz and S. Mohanta, Inorg. Chem., 2010, 49, 9517 CrossRef CAS PubMed.
- (a) D. M. Kurtz Jr, Chem. Rev., 1990, 90, 585 CrossRef; (b) F. L. Gall, F. F. de Biani, A. Caneschi, P. Cinelli, A. Cornia, A. C. Fabretti and D. Gatteschi, Inorg. Chim. Acta, 1997, 262, 123 CrossRef; (c) C. Cañada-Vilalta, T. A. O'Brien, E. K. Brechin, M. Pink, E. R. Davidson and G. Christou, Inorg. Chem., 2004, 43, 5505 CrossRef PubMed; (d) N. Berg, T. Rajeshkumar, S. M. Taylor, E. K. Brechin, G. Rajaraman and L. F. Jones, Chem.–Eur. J., 2012, 18, 5906 CrossRef CAS PubMed; (e) A. Niemann, U. Bossek, K. Wieghardt, C. Butzlaff, A. X. Trautwein and B. Nuber, Angew. Chem., Int. Ed. Engl., 1992, 31, 311 CrossRef.
- T. Rajeshkumar and G. Rajaraman, Chem. Commun., 2012, 48, 7856 RSC.
- (a) J.-P. Costes, F. Dahan and A. Dupuis, Inorg. Chem., 2000, 39, 165 CrossRef CAS PubMed; (b) S. Mohanta, K. K. Nanda, L. K. Thompson, U. Flörke and K. Nag, Inorg. Chem., 1998, 37, 1465 CrossRef CAS; (c) S. Sasmal, S. Roy, L. Carrella, A. Jana, E. Rentschler and S. Mohanta, Eur. J. Inorg. Chem., 2015, 680 CrossRef CAS; (d) G. Rajaraman, F. Totti, A. Bencini, A. Caneschi, R. Sessoli and D. Gatteschi, Dalton Trans., 2009, 3153 RSC; (e) S. K. Singh, N. K. Tibrewal and G. Rajaraman, Dalton Trans., 2011, 40, 10897 RSC; (f) S. Hazra, S. Bhattacharya, M. K. Singh, L. Carrella, E. Rentschler, T. Weyhermueller, G. Rajaraman and S. Mohanta, Inorg. Chem., 2013, 52, 12881 CrossRef CAS PubMed.
- The Cambridge Structural Database (CSD), version 5.37, The Cambridge Crystallographic Data Centre (CCDC), 2016 Search PubMed.
- (a) Y. Journaux, O. Kahn, I. Morgenstern-Badarau, J. Galy, J. Jaud, A. Bencini and D. Gatteschi, J. Am. Chem. Soc., 1985, 107, 6305 CrossRef CAS; (b) A. Bencini, C. Benelli, A. Caneschi, R. L. Carlin, A. Dei and D. Gatteschi, J. Am. Chem. Soc., 1985, 107, 8128 CrossRef CAS; (c) G. A. Brewer and E. Sinn, Inorg. Chim. Acta, 1987, 134, 13 CrossRef CAS; (d) R. Ruiz, F. Lloret, M. Julve and J. Faus, Inorg. Chim. Acta, 1993, 213, 261 CrossRef CAS; (e) I. Ramade, O. Kahn, Y. Jeannin and F. Robert, Inorg. Chem., 1997, 36, 930 CrossRef CAS; (f) M. Ryazanov, V. Nikiforov, F. Lloret, M. Julve, N. Kuzmina and A. Gleizes, Inorg. Chem., 2002, 41, 1816 CrossRef CAS PubMed; (g) A. Gutiérrez, M. F. Perpiñán, A. E. Sánchez, M. C. Torralba and M. R. Torres, Inorg. Chim. Acta, 2010, 363, 1837 CrossRef; (h) F. Pointillart and K. Bernot, Eur. J. Inorg. Chem., 2010, 952 CrossRef CAS; (i) A. Biswas, S. Mondal, L. Mandal, A. Jana, P. Chakraborty and S. Mohanta, Inorg. Chim. Acta, 2014, 414, 199 CrossRef CAS.
- (a) D. W. Harrison and J.-C. G. Bdnzli, Inorg. Chim. Acta, 1985, 109, 185 CrossRef CAS; (b) G. Novitchi, S. Shova, A. Caneschi, J.-P. Costes, M. Gdaniec and N. Stanica, Dalton Trans., 2004, 1194 RSC; (c) Q. Shi, L. Sheng, K. Ma, Y. Sun, X. Cai, R. Liu and S. Wang, Inorg. Chem. Commun., 2009, 12, 255 CrossRef CAS; (d) I. Gamba, P. Gamez, E. Monzani, L. Casella, I. Mutikainen and J. Reedijk, Eur. J. Inorg. Chem., 2011, 4360 CrossRef CAS; (e) Z.-L. You, Y. Lu, N. Zhang, B.-W. Ding, H. Sun, P. Hou and C. Wang, Polyhedron, 2011, 30, 2186 CrossRef CAS; (f) R. Vafazadeh, B. Khaledi, A. C. Willis and M. Namazian, Polyhedron, 2011, 30, 1815 CrossRef CAS; (g) S. Saha, A. Sasmal, C. Roy Choudhury, C. J. Gómez-Garcia, E. Garribba and S. Mitra, Polyhedron, 2014, 69, 262 CrossRef CAS; (h) L. Mandal, S. Bhattacharya and S. Mohanta, Inorg. Chim. Acta, 2013, 406, 87 CrossRef CAS; (i) P. Chakraborty and S. Mohanta, Inorg. Chim. Acta, 2017, 455, 70 CrossRef CAS.
- (a) S. Mondal, S. Mandal, L. Carrella, A. Jana, M. Fleck, A. Köhn, E. Rentschler and S. Mohanta, Inorg. Chem., 2015, 54, 117 CrossRef CAS PubMed; (b) S. Mondal, S. Mandal, A. Jana and S. Mohanta, Inorg. Chim. Acta, 2014, 415, 138 CrossRef CAS; (c) L. T. Yildirim, R. Kurtaran, H. Namli, A. D. Azaz and O. Atakol, Polyhedron, 2007, 26, 4187 CrossRef CAS.
- (a) R.-J. Tao, F.-A. Li, S.-Q. Zang, Y.-X. Cheng, Q.-L. Wang, J.-Y. Niu and D.-Z. Liao, J. Coord. Chem., 2006, 59, 901 CrossRef CAS; (b) S. Bandyopadhyay, J.-M. Lo, H.-H. Yao, F.-L. Liao and P. Chattopadhyay, J. Coord. Chem., 2006, 59, 2015 CrossRef CAS; (c) A. Bhunia, P. W. Roesky, Y. Lan, G. E. Kostakis and A. K. Powell, Inorg. Chem., 2009, 48, 10483 CrossRef CAS PubMed.
- (a) C. J. O'connor, D. P. Freyberg and E. Sinn, Inorg. Chem., 1979, 18, 1077 CrossRef; (b) G. A. Brewer and E. Sinn, Inorg. Chem., 1987, 26, 1529 CrossRef CAS; (c) P. Talukder, S. Shit, A. Sasmal, S. R. Batten, B. Moubaraki, K. S. Murray and S. Mitra, Polyhedron, 2011, 30, 1767 CrossRef CAS; (d) I. Morgenstern-Badarau, D. Laroque, E. Bill, H. Winkler, A. X. Trautwein, F. Robert and Y. Jeannin, Inorg. Chem., 1991, 30, 3180 CrossRef CAS; (e) C. Brewer, G. Brewer, W. R. Scheidt, M. Shang and E. E. Carpenter, Inorg. Chim. Acta, 2001, 313, 65 CrossRef CAS; (f) S. Biswas, S. Naiya, C. J. Gomez-Garcia and A. Ghosh, Dalton Trans., 2012, 41, 462 RSC; (g) S. Biswas, C. J. Gómez-García, J. M. Clemente-Juan, S. Benmansour and A. Ghosh, Inorg. Chem., 2014, 53, 2441 CrossRef CAS PubMed.
- M. Andruh, D. G. Branzea, R. Gheorghe and A. M. Madalan, CrystEngComm, 2009, 11, 2555 RSC.
- (a) J.-P. Costes, F. Dahan, A. Dupuis and J.-P. Laurent, Inorg. Chem., 1996, 35, 2400 CrossRef CAS PubMed; (b) J.-P. Costes, F. Dahan, A. Dupuis and J.-P. Laurent, Chem.–Eur. J., 1998, 4, 1616 CrossRef CAS; (c) O. Margeat, P. G. Lacroix, J. P. Costes, B. Donnadieu and C. Lepetit, Inorg. Chem., 2004, 43, 4743 CrossRef CAS PubMed; (d) J.-P. Costes, B. Donnadieu, R. Gheorghe, G. Novitchi, J.-P. Tuchagues and L. Vendier, Eur. J. Inorg. Chem., 2008, 5235 CrossRef CAS; (e) D. Visinescu, I.-R. Jeon, A. M. Madalan, M.-G. Alexandru, B. Jurca, C. Mathoniere, R. Clerac and M. Andruh, Dalton Trans., 2012, 41, 13578 RSC; (f) N. Bridonneau, L.-M. Chamoreau, P. P. Lainé, W. Wernsdorferc and V. Marvaud, Chem. Commun., 2013, 49, 9476 RSC; (g) J. Long, L.-M. Chamoreau and V. Marvaud, Dalton Trans., 2010, 39, 2188 RSC; (h) S. Osa, Y. Sunatsuki, Y. Yamamoto, M. Nakamura, T. Shimamoto, N. Matsumoto and N. Re, Inorg. Chem., 2003, 42, 5507 CrossRef CAS PubMed; (i) S. Bhattacharya, A. Jana and S. Mohanta, CrystEngComm, 2013, 15, 10374 RSC; (j) S. Majumder, R. Koner, P. Lemoine, M. Nayak, M. Ghosh, S. Hazra, C. R. Lucas and S. Mohanta, Eur. J. Inorg. Chem., 2009, 3447 CrossRef CAS; (k) A. Biswas, S. Mondal and S. Mohanta, J. Coord. Chem., 2013, 66, 152 CrossRef CAS; (l) S. Bhattacharya, A. Jana and S. Mohanta, Polyhedron, 2013, 62, 234 CrossRef CAS; (m) A. Biswas, M. Ghosh, P. Lemoine, S. Sarkar, S. Hazra and S. Mohanta, Eur. J. Inorg. Chem., 2010, 3125 CrossRef CAS.
- (a) A. Biswas, L. Mandal, S. Mondal, C. R. Lucas and S. Mohanta, CrystEngComm, 2013, 15, 5888 RSC; (b) D. G. Branzea, A. M. Madalan, S. Ciattini, N. Avarvari, A. Caneschi and M. Andruh, New J. Chem., 2010, 34, 2479 RSC; (c) S. Thakurta, C. Rizzoli, R. J. Butcher, C. J. Gómez-García, E. Garribba and S. Mitra, Inorg. Chim. Acta, 2010, 363, 1395 CrossRef CAS; (d) D. G. Branzea, A. Guerri, O. Fabelo, C. Ruiz-Pérez, L.-M. Chamoreau, C. Sangregorio, A. Caneschi and M. Andruh, Cryst. Growth Des., 2008, 8, 941 CrossRef CAS; (e) J.-P. Costes, R. Gheorghe, M. Andruh, S. Shova and J.-M. C. Juan, New J. Chem., 2006, 30, 572 RSC.
- (a) T. Kajiwara, M. Nakano, K. Takahashi, S. Takaishi and M. Yamashita, Chem.–Eur. J., 2011, 17, 196 CrossRef CAS PubMed; (b) R. Gheorghe, P. Cucos, M. Andruh, J.-P. Costes, B. Donnadieu and S. Shova, Chem.–Eur. J., 2006, 12, 187 CrossRef CAS PubMed; (c) X.-C. Huang, C. Zhou, H.-Y. Wei and X.-Y. Wang, Inorg. Chem., 2013, 52, 7314 CrossRef CAS PubMed.
- A. Jana and S. Mohanta, CrystEngComm, 2014, 16, 5494 RSC.
- (a) M. Nayak, R. Koner, H.-H. Lin, U. Flörke, H.-H. Wei and S. Mohanta, Inorg. Chem., 2006, 45, 10764 CrossRef CAS PubMed; (b) A. Jana, R. Koner, T. Weyhermueller, P. Lemoine, M. Ghosh and S. Mohanta, Inorg. Chim. Acta, 2011, 375, 263 CrossRef CAS; (c) A. Jana, R. Koner, M. Nayak, P. Lemoine, S. Dutta, M. Ghosh and S. Mohanta, Inorg. Chim. Acta, 2011, 365, 71 CrossRef CAS; (d) M. Nayak, S. Sarkar, S. Hazra, H. A. Sparkes, J. A. K. Howard and S. Mohanta, CrystEngComm, 2011, 13, 124 RSC; (e) M. Nayak, S. Hazra, P. Lemoine, R. Koner, C. R. Lucas and S. Mohanta, Polyhedron, 2008, 27, 1201 CrossRef CAS; (f) R. Koner, H.-H. Lin, H.-H. Wei and S. Mohanta, Inorg. Chem., 2005, 44, 3524 CrossRef CAS PubMed; (g) A. Jana, S. Majumder, L. Carrella, M. Nayak, T. Weyhermüeller, S. Dutta, D. Schollmeyer, E. Rentschler, R. Koner and S. Mohanta, Inorg. Chem., 2010, 49, 9012 CrossRef CAS PubMed; (h) M. Nayak, A. Jana, M. Fleck, S. Hazra and S. Mohanta, CrystEngComm, 2010, 12, 1416 RSC; (i) S. Sasmal, S. Majumder, S. Hazra, H. A. Sparkes, J. A. K. Howard, M. Nayak and S. Mohanta, CrystEngComm, 2010, 12, 4131 RSC.
- (a) M. L. Colon, S. Y. Qian, D. Vanderveer and X. R. Bu, Inorg. Chim. Acta, 2004, 357, 83 CrossRef CAS; (b) J.-P. Costes, C. Duhayon, S. Mallet-Ladeira, S. Shova and L. Vendier, Chem.–Eur. J., 2016, 22, 2171 CrossRef CAS PubMed.
- (a) Bruker-Nonius, APEX-II, SAINT-Plus and TWINABS, Bruker-Nonius AXS Inc., Madison, Wisconsin, USA, 2004 Search PubMed; (b) G. M. Sheldrick, SAINT (Version 6.02), SADABS (Version 2.03), Bruker AXS lnc., Madison, Wisconsin, 2002 Search PubMed; (c) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed; (d) G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3 CrossRef PubMed; (e) A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7 CrossRef CAS; (f) A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148 CrossRef CAS PubMed.
- N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini and K. S. Murray, J. Comput. Chem., 2013, 34, 1164 CrossRef CAS PubMed.
- (a) F. Birkelbach, M. Winter, U. Flörke, H.-J. Haupt, C. Butzlaff, M. Lengen, E. Bill, A. X. Trautwein, K. Wieghardt and P. Chaudhuri, Inorg. Chem., 1994, 33, 3990 CrossRef CAS; (b) M. Yonemura, K. Arimura, K. Inoue, N. Usuki, M. Ohba and H. Ohkawa, Inorg. Chem., 2002, 41, 582 CrossRef CAS PubMed.
- (a) Y.-F. Song, G. A. van Albada, J. Tang, I. Mutikainen, U. Turpeinen, C. Massera, O. Roubeau, J. S. Costa, P. Gamez and J. Reedijk, Inorg. Chem., 2007, 46, 4944 CrossRef CAS PubMed; (b) X.-H. Bu, M. Du, Z.-L. Shang, R.-H. Zhang, D.-Z. Liao, M. Shionoya and T. Clifford, Inorg. Chem., 2000, 39, 4190 CrossRef CAS PubMed; (c) Y. Song, P. Gamez, O. Roubeau, I. Mutikainen, U. Turpeinen and J. Reedijk, Inorg. Chim. Acta, 2005, 358, 109 CrossRef CAS; (d) M. Du, X.-J. Zhao, J.-H. Guo, X.-H. Bu and J. Ribas, Eur. J. Inorg. Chem., 2005, 294 CrossRef CAS; (e) Y. F. Song, G. A. van Albada, M. Quesada, I. Mutikainen, U. Turpeinen and J. Reedijk, Inorg. Chem. Commun., 2005, 8, 975 CrossRef CAS; (f) Y. Song, P. Gamez, O. Roubeau, M. Lutz, A. L. Spek and J. Reedijk, Eur. J. Inorg. Chem., 2003, 2924 CrossRef CAS.
- (a) C. N. Verani, E. Rentschler, T. Weyhermüller, E. Bill and P. Chaudhuri, Dalton Trans., 2000, 251 RSC; (b) C. N. Verani, T. Weyhermüller, E. Rentschler, E. Bill and P. Chaudhuri, Chem. Commun., 1998, 2475 RSC; (c) M. Yonemura, M. Ohba, K. Takahashi, H. Ōkawa and D. E. Fenton, Inorg. Chim. Acta, 1998, 283, 72 CrossRef CAS; (d) R.-J. Tao, C.-Z. Mei, S.-Q. Zang, Q.-L. Wang, J.-Y. Niu and D.-Z. Liao, Inorg. Chim. Acta, 2004, 357, 1985 CrossRef CAS; (e) A. Hori, Y. Mitsuka, M. Ohba and H. Ōkawa, Inorg. Chim. Acta, 2002, 337, 113 CrossRef CAS; (f) T. Aono, H. Wada, Y.-I. Aratake, N. Matsumoto, H. Ōkawa and Y. Matsuda, J. Chem. Soc., Dalton Trans., 1996, 25 RSC; (g) I. Morgenstern-Badarau, M. Rerat, O. Khan, J. Jaud and J. Galy, Inorg. Chem., 1982, 21, 3050 CrossRef CAS.
- (a) R.-J. Tao, F.-A. Li, S.-Q. Zang, Y.-X. Cheng, Q.-L. Wang, J.-Y. Niu and D.-Z. Liao, Polyhedron, 2006, 25, 2153 CrossRef CAS; (b) Y. Sunatsuki, T. Matsuo, M. Nakamura, F. Kai, N. Matsumoto and J.-P. Tuchagues, Bull. Chem. Soc. Jpn., 1998, 71, 2611 CrossRef CAS; (c) S. Ghosh, G. Aromí, P. Gamez and A. Ghosh, Eur. J. Inorg. Chem., 2014, 3341 CrossRef CAS.
- (a) L. Bo, Z. Hong, P. Zhi-Quan, S. You, W. Cheng-Gang, Z. Han-Ping, H. Jing-Dong and C. Ru-An, J. Coord. Chem., 2006, 59, 1271 CrossRef CAS; (b) D. Visinescu, J.-P. Sutter, C. Ruiz-Pérez and M. Andruh, Inorg. Chim. Acta, 2006, 359, 433 CrossRef CAS; (c) M. Pascu, F. Lloret, N. Avarvari, M. Julve and M. Andruh, Inorg. Chem., 2004, 43, 5189 CrossRef CAS PubMed; (d) H. Ōkawa, J. Nishio, M. Ohba, M. Tadokoro, N. Matsumoto, M. Koikawa, S. Kida and D. E. Fenton, Inorg. Chem., 1993, 32, 2949 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Structural, magnetic and ESI-MS schemes, figures and tables (Scheme S1, Fig. S1–S27, Tables S1–S8). CCDC 1584530–1584535 for 1–6, respectively. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra13763j |
| This journal is © The Royal Society of Chemistry 2018 |