Open Access Article
Open Access ArticleFabrication of a magnetite/diazonium functionalized-reduced graphene oxide hybrid as an easily regenerated adsorbent for efficient removal of chlorophenols from aqueous solution†
Xiaoqin Shena,
Xiaolei Chenb,
Dejun Sunb,
Tao Wu*b and
Yujiang Li *a
*a
aShandong Provincial Research Center for Water Pollution Control, School of Environmental Science & Engineering, Shandong University, Jinan, 250100, PR China. E-mail: yujiang@sdu.edu.cn; Fax: +86-531-88363358; Tel: +86-531-88363358
bKey Laboratory of Colloid & Interface Science of Education Ministry, Shandong University, Jinan, 250100, PR China. E-mail: wutao@sdu.edu.cn; Fax: +86-531-88365437; Tel: +86-531-88365437
First published on 15th February 2018
Abstract
A magnetic hybrid nanomaterial, which contains magnetite (Fe3O4) particles and diazonium functionalized-reduced graphene oxide (DF-RGO), was fabricated via a three-pot reaction. First, the reduced graphene oxide (RGO) was synthesized via a redox reaction. Second, diazonium functionalized-RGO was prepared via a feasible chemical reaction. Third, Fe3O4 particles were loaded onto the surface of DF-RGO by covalent bonding, fabricating the M-DF-RGO hybrid. The fabricated hybrid was characterized by SEM, TEM, AFM, XRD, XPS, FT-IR, TGA, Raman spectroscopy, and magnetometry. The resulting M-DF-RGO hybrid possessed unique magnetic properties and was applied to remove 4-chlorophenol (4-CP) and 2,4-dichlorophenol (2,4-DCP) from aqueous solution. The adsorption of 4-CP and 2,4-DCP on the M-DF-RGO hybrid was performed under various conditions, with respect to initial chlorophenol concentration, pH, and contact time. The results suggest that the adsorption of 4-CP and 2,4-DCP onto the M-DF-RGO hybrid is strongly dependent on pH and weakly dependent on contact time. In addition, the adsorption isotherm of 4-CP and 2,4-DCP on the M-DF-RGO hybrid fits the Freundlich model well and the adsorption capacities of 4-CP and 2,4-DCP on M-DF-RGO reached 55.09 and 127.33 mg g−1, respectively, at pH 6 and 25 °C. In this situation, intermolecular interactions including π–π interactions and hydrogen bonding are operative. The calculated results of density functional theory further demonstrate that 2,4-DCP molecules could be more easily absorbed than 4-CP molecules by the M-DF-RGO hybrid. Moreover, the M-DF-RGO hybrid could be easily separated by a magnetic separation process, and showed good recyclability of more than five cycles.
1. Introduction
Chlorophenols are important industrial and chemical raw materials widely used in the plastics, cosmetics, polymers, paints/dyes, pharmaceutical and pesticide industries.1–4 They are slightly acidic, appreciably soluble in water, and highly toxic to animals and human beings even at very low concentrations, and thus are considered one of the most major pollutants in wastewaters.5 Such wastewaters that contain chlorophenols are of increasing concern and represent a great potential hazard to both the environment and human health. Although chlorophenols can be biodegraded in the activated sludge process, the biodegradation rate of chlorophenols or phenolic compounds is very low. Furthermore, the presence of chlorophenols in wastewaters can increase the susceptibility of a biological system to becoming disturbed.1–5 Hence, chlorophenols or phenolic compounds must be removed from wastewater before it is discharged into receiving water bodies or the environment.To date, various techniques such as biological degradation, chemical oxidation, photodegradation, UV/ozone oxidation, and adsorption have been researched for the treatment of phenolic wastewater at high concentration.6–8 Of all of the known technologies, adsorption is considered to be the most effective, simplest, lowest-costing, and most frequently used method for the removal of chlorophenols or phenolic compounds. Various adsorbent materials such as clay minerals, metal hydroxides, resin, activated carbon and polymeric adsorbents have been applied for the removal of chlorophenols or phenolic compounds from aqueous solution.9–12 However, these traditional adsorbent materials still have certain problems that limit their practical applications, such as low selectivity, low adsorption capacity, poor dispersion stability, and difficulty in regeneration of the adsorbent material. As a result, the design and fabrication of new adsorbent materials to solve these problems are of significant importance.
Graphene is an excellent two-dimensional (2D) material, which possesses structural units that are similar to benzene rings that connect to each other, forming a 2D planar structure.13 Its attractive properties include high surface area, high electron mobility at room temperature, tunable optical properties, good thermal conductivity, and excellent mechanical strength.13,14 As a result, graphene is applied in diverse fields, such as nanoelectronics, optical ultrasensitive sensors, field-effect transistors, and energy storage and conversion.14–17 However, graphene has poor dispersivity and process stability. Graphene sheets tend to aggregate in the liquid phase due to van der Waals forces between graphene sheets, and this aggregation restricts the applications. Hence, covalent modification of graphene has been used to accomplish improvements of the physicochemical properties of graphene. Previous studies have demonstrated that graphene can be covalently functionalized by various direct or indirect functionalization methods.16–20 Either directly or indirectly covalently functionalized graphene is generated from graphene oxide (GO) as the precursor. GO, one of the most important derivatives of graphene, has a variety of oxygen-containing functional groups, such as hydroxyl, carbonyl, epoxide, and carboxyl groups, on its basal planes and edges. Taking into account these abundant oxygen-containing functional groups, GO can not only be well dispersed in water to form a stable colloidal dispersion, but also can potentially be used as nano-scale 2D building blocks for the fabrication of graphene-based composites.21,22 Among these oxygen-containing functional groups, the carboxyl groups are mainly located at the edges of the graphene sheets, and are excellent precursors for functionalizing various graphene-based composites without distorting the graphene plane.23 Therefore, the carboxyl group plays an important role in fabricating various new composites. However, other oxygen-containing functional groups (e.g. epoxide and carbonyl groups) on the planar surface of GO may disturb the covalent modification process. In this case, GO can be selectively reduced by thiourea dioxide, leaving the desired carboxyl groups and removing the undesired epoxide and carbonyl groups. This type of selectively reduced graphene is called carboxyl graphene or reduced graphene oxide (RGO). RGO can be used as a precursor and reacts with the functional groups of various organic molecules to introduce new functional groups for designing and fabricating new adsorbent materials. To the best of our knowledge, a magnetic diazonium functionalized-reduced graphene oxide (M-DF-RGO) hybrid has not been synthesized and applied in the removal of 4-CP and 2,4-DCP from aqueous solution.
In this work, the M-DF-RGO hybrid was synthesized via a three-pot reaction and used as an adsorbent to remove 4-CP and 2,4-DCP from aqueous solution. The fabricated M-DF-RGO hybrid was characterized using scanning electron microscopy, transmission electron microscopy, atomic force microscopy, X-ray diffraction, X-ray photoelectron spectroscopy, Fourier transform infrared spectroscopy, thermogravimetric analysis, Raman spectroscopy, and magnetometry. 4-CP and 2,4-DCP were chosen as model pollutants to describe the adsorption behavior of chlorophenol molecules on the M-DF-RGO hybrid, and to elucidate the interactions between chlorophenol molecules and the M-DF-RGO hybrid. The effects of initial chlorophenol concentration, pH, and contact time on the adsorption capacity were investigated. At the same time, the interactions between chlorophenol molecules and the M-DF-RGO hybrid were also analyzed using density functional theory. Finally, the reusability of the M-DF-RGO hybrid for 4-CP and 2,4-DCP was explored.
2. Materials and methods
2.1 Materials
Graphite powder (<45 μm, ≥99.99%) was purchased from Sigma-Aldrich Co., Ltd. (USA). Thiourea dioxide (TUD, 99%), hydrochloric acid (HCl, 36–38%), sodium nitrite (NaNO2, 98%), ferric chloride hexahydrate (FeCl3·6H2O, ≥98%), ferrous sulfate heptahydrate (FeSO4·7H2O, ≥99%), 4-chlorophenol (4-CP, >99%), 2,4-dichlorophenol (2,4-DCP, >99%), 4-aminobenzoic acid (99%), and sodium hydroxide (NaOH, 96%) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All of the other chemicals were analytically pure, and used without further purification. Milli-Q water (Millipore, USA) was used throughout the experimental process.2.2 Preparation of GO and RGO
2.3 Preparation of DF-RGO
The chemically functionalized RGO was prepared by covalently attaching organic groups on the planar surface of RGO. The aryl diazonium salt solution was first prepared according to the following method: 4.1 mmol L−1 NaNO2 and 1.2 mmol L−1 4-aminobenzoic acid were dissolved in 15 mL dilute NaOH solution (0.25%, w/v). The as-prepared solution was then added slowly to 0.1 mol L−1 HCl solution (20 mL) in an ice bath with continuous mechanical stirring.Before the aryl diazonium salt solution was added into the RGO colloidal dispersion, the pH value of the RGO colloidal dispersion was adjusted to 6 by the addition of dilute HCl solution in an ice bath. During the adjustment of the pH value from basic to acidic, the RGO colloidal dispersion (1 g L−1) precipitated and produced brown flocculent precipitates. Under continuous stirring, the aryl diazonium salt solution was immediately added dropwise to the RGO flocculent precipitates. Then, the mixture was kept in the ice bath without stirring for 2 h in order to adsorb sufficient aryl diazonium ions onto the RGO sheets. After 2 h, the mixture was heated to 80 °C for 10 h, and then cooled to room temperature. A homogeneous black dispersion of diazonium salt functionalized-RGO sheets was obtained. The resulting product was filtered and washed with Milli-Q water and ethanol. Finally, the DF-RGO sample was obtained by freeze-drying for 24 h.
2.4 Preparation of M-DF-RGO hybrid
To prepare the M-DF-RGO hybrid, DF-RGO (0.4 g) was first ultrasonicated in 200 mL Milli-Q water to form a homogeneous colloidal dispersion. Then, the colloidal dispersion was transferred to a three-neck flask (equipped with a reflux condenser) under a nitrogen atmosphere. A mixed aqueous solution of FeCl3·6H2O (0.5597 g) and FeSO4·7H2O (0.5592 g) in 20 mL Milli-Q water was added to the DF-RGO colloidal dispersion. The mixture was then heated to 80 °C and stirred under nitrogen protection. After that, 250 mL NH3·H2O solution (30%) was added to adjust the pH to 10, and the mixtures were stirred and maintained at 80 °C for 30 min. Finally, trisodium citrate (0.1 g) was added to the mixture and the temperature was raised to 95 °C, resulting in a black suspension. The obtained product was separated with a permanent magnet, washed several times with Milli-Q water, and dried under vacuum at 60 °C.2.5 Characterization
The surface morphologies and structural features of GO, RGO, DF-RGO, and M-DF-RGO were characterized using a JEOL-2010 transmission electron microscope (TEM, JEOL, Japan) and a JSM-6330 F scanning electron microscope (SEM, JEOL, Japan). Atomic force microscopy images of the samples were acquired using a Multimode Nano 4 microscope system (AFM, Bruker, USA) in the tapping mode. X-ray diffraction patterns of the samples were recorded by an X’Pert Super X-ray diffractometer (XRD, Philips, Holland) using Cu Kα (λ = 1.540598 Å) irradiation at 40 kV and 40 mA in the 2θ range of 5–70° at a scanning rate of 0.02° s−1. Fourier transform infrared spectra of the samples were recorded using a Nicolet Nexus 670 spectrometer (FI-IR, Thermo Fisher Scientific, USA) using KBr pellets in the range of 400–4000 cm−1, with a resolution of 2 cm−1. X-ray photoelectron spectroscopy was performed using a XSAM800 spectrometer (XPS, Kratos Company, UK). Raman spectra of the samples were obtained using a LabRAM electron energy spectrometer (HORIBA Company, France) with a 632.8 nm He–Ne excitation source. Thermogravimetric analysis was performed using TG209FI thermal analysis apparatus (TGA, NETZSCH, Germany) at a heating rate of 10 °C min−1 from 40 to 700 °C. Magnetic curves of the samples were measured using a 7410 vibrating sample magnetometer (VSM, Cryotronics Inc. United States, USA) at room temperature.2.6 Adsorption experiments and recycling tests
Batch experiments were performed to test the removal efficiency of 4-CP and 2,4-DCP from aqueous solution using the M-DF-RGO hybrid. The effects of initial chlorophenol concentration, pH, and contact time on the removal efficiency of 4-CP and 2,4-DCP were investigated. An initial experiment was conducted to determine the amount of adsorbent required. According to this experiment, 0.01 g of adsorbent was added to 10 mL phenolic aqueous solution. The initial pH of the aqueous solution was adjusted from 3 to 11 by adding either dilute HCl (0.1 mol L−1) or dilute NaOH (0.1 mol L−1). The initial chlorophenol concentration was in the range of 10–300 mg L−1 (100 mg L−1 for the adsorption study and 10–300 mg L−1 for the isotherm studies). The contact time ranged from 0 to 30 min. The mixtures were continuously shaken on a mechanical shaker (SHZ-82, Jintan, China) for 30 min at 298 K. After 30 min, the mixtures were easily separated by a magnetic separation process, and the concentration of the residual 4-CP and 2,4-DCP was analyzed using a UV-vis adsorption spectrometer (UV-1601, Shimadzu, Japan) at wavelengths of 279 and 286 nm for 4-CP and 2,4-DCP, respectively.Recycling tests were carried out to assess the recyclability of the M-DF-RGO hybrid. After adsorption experiments, the adsorbent particles were collected using a magnet. The 4-CP- and 2,4-DCP-adsorbed adsorbent particles were washed five times with 0.01 mol L−1 NaOH solution and ethanol, respectively, and vacuum-dried at 60 °C for 4 h. The recovered adsorbent particles were again used for subsequent adsorption experiments. Five cycles were performed to evaluate the reusability of the M-DF-RGO hybrid.
2.7 Computational models and methodology
All of the geometrical structures were optimized using the Density Functional Theory (DFT) method with the PBE0 functional25 in combination with the all-electron 6-31G* basis set.26,27 Grimme-type dispersion corrections (D3) were included throughout28 and the basis set superposition error (BSSE) was also considered for absorbing systems using the counterpoise method proposed by Boys and Bernardi.29 All calculations were carried out using the Gaussian09 package.30–32Fig. S4† shows the molecular models of DF-RGO, and the added p-carboxyphenyl groups have been attached to the surface of the graphene sheets. It should be noted that in the conditions of our calculations, we intentionally neglect Fe3O4 particles, which did not work for the adsorption of chlorophenols (Fig. S5†). The adsorption energy (Ead) was calculated as Ead = EG + EX − EG–X, where EG corresponds to the total energies of DF-RGO; EX1 corresponds to the total energies of the 4-CP system in isolated form; EX2 corresponds to the total energies of the 2,4-DCP system in isolated form; EG–X1 is the total energy of the DF-RGO and 4-CP complex system; and EG–X2 is the total energy of the DF-RGO and 2,4-DCP complex system. A positive value of Ead indicates that the adsorption is exothermic, and a higher positive value of Ead corresponds to a stronger adsorption capacity of the adsorbate onto the DF-RGO hybrid.
3. Results and discussion
3.1 Characterization
The morphologies and microstructures of GO, RGO, DF-RGO, and M-DF-RGO were studied using SEM and TEM techniques. As shown in Fig. 1a, the thin films of GO sheets with slight wrinkles and smooth surfaces were exfoliated. RGO (Fig. 1b) with a crumpled nano-sheet structure was transparent. The microstructure of DF-RGO (Fig. 1c) was similar to that of GO, which appeared transparent and was slightly folded. However, the contrast of DF-RGO was higher due to functionalization. As shown in Fig. 1d, magnetic nanoparticles were uniformly distributed and exhibited high-density coverage on the entire DF-RGO sheet surface, and no particle agglomerates were observed. The reason for this is that the diazonium functionalization of reduced graphene oxide significantly increased the number of carboxyl groups that can perfectly interact with Fe3O4 nanoparticles. From the SEM image of GO (Fig. 1e), it can be seen that the graphene sheets exhibit a wrinkled feature. Fig. 1f shows that RGO possesses a crumpled or wavy silk-like structure. Moreover, a flake-like structure can also be observed at the edge of the RGO sheets. From Fig. 1g, it can be seen that DF-RGO possesses a homogeneous and wrinkled structure. At the same time, crumpled wave-like carbon sheets can also be observed, which are characteristic of the single-layer graphene sheets.33 As shown in Fig. 1h, the Fe3O4 NPs appear as bright dots, and the large graphene flakes exhibit a slightly wrinkled surface.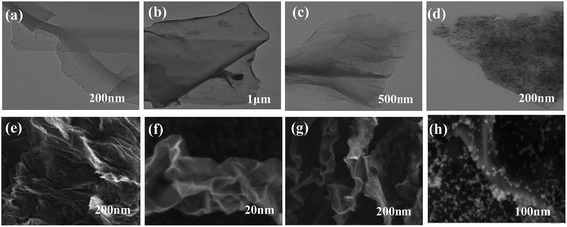 | ||
| Fig. 1 TEM images of (a) GO, (b) RGO, (c) DF-RGO, and (d) M-DF-RGO; and SEM images of (e) GO, (f) RGO, (g) DF-RGO, and (h) M-DF-RGO. | ||
AFM images confirmed that evaporated dispersions of GO, RGO, DF-RGO, and M-DF-RGO are composed of isolated graphitic sheets (Fig. S1a–d†). On average, the thickness of the GO sheets is ∼0.90 nm, while the thickness of RGO is ∼0.88 nm, which implies that the oxygenated groups are partially reduced to re-establish the conjugated graphite network. Considering the intrinsic monolayer ripples owing to thermal fluctuations, it can be concluded that the RGO sheets are mixtures of monolayers and bilayers. The thickness of single layer DF-RGO is measured to be 1.1–1.5 nm, which is slightly higher than that of RGO. The addition of thickness may be attributed to the perpendicular covalent attachment of the functional group moieties. The M-DF-RGO hybrid was redispersed to form a metastable dispersion and drop-dried on a mica substrate for the AFM study. We found that even after a long period of sonication during the preparation of the AFM specimen, the magnetic nanoparticles are still strongly anchored on the surface of the DF-RGO sheets, suggesting a strong interaction between the magnetic nanoparticles and the DF-RGO sheets. The thickness of the M-DF-RGO sheets was in the range of 10–12 nm. Since the size of the magnetic nanoparticles is about 10.12 nm (according to the XRD results) and the thickness of the DF-RGO is about 1.1–1.5 nm, it is reasonable to conclude that the hybrid consists of monolayer DF-RGO and magnetic nanoparticles.34
The powder X-ray diffraction (XRD) patterns are shown in Fig. 2a. Graphene oxide exhibited a diffraction peak at 2θ = 10.6°.35 As calculated from the Bragg equation, the interlayer spacing of GO (0.82 nm) was enhanced after graphite (0.34 nm) was oxidized, which may be due to the introduction of oxygen-containing functional groups and the adsorption of water molecules. For RGO, a weak and broad diffraction peak (002) was observed at 2θ = 22.8°, while the (001) diffraction peak of GO almost disappeared, which again proved that most of the oxygen-containing functional groups on the surface of GO had been removed. Concerning DF-RGO, a broad reflection peak (∼20°) exists that is associated with the (002) RGO planes. The interlayer distance of DF-RGO (0.90 nm) is larger than that of graphene oxide (0.82 nm) as a result of the presence of –C6H5–COOH groups on both sides of the RGO plane. The XRD pattern of M-DF-RGO exhibited weak reflections at 2θ = 35.84° (311), which is consistent with the existence of iron oxide either in Fe3O4 or γ-Fe2O3 phases.36 The adsorbed nanoparticles act as spacers that limit the restacking of graphene layers.37
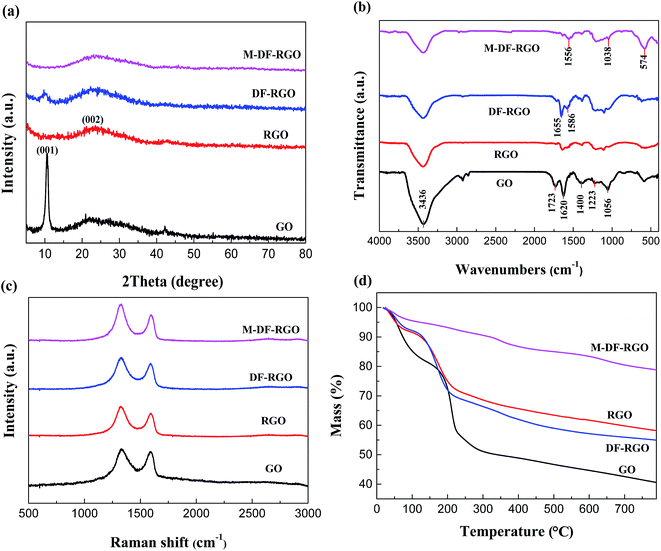 | ||
| Fig. 2 Characterization of samples. (a) XRD spectra; (b) FTIR spectra; (c) Raman spectra; (d) TGA curves. | ||
The FTIR spectra of the samples are shown in Fig. 2b. The characteristic absorption peaks of graphite oxide at 1723, 1620, 1400, 1223, and 1056 cm−1 are attributed to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, O–H, C–O–C, and C–O, respectively. An intense and broad peak at ∼3436 cm−1 could be assigned to the O–H stretching vibration.38 For RGO, the absorption peaks of the C
C, O–H, C–O–C, and C–O, respectively. An intense and broad peak at ∼3436 cm−1 could be assigned to the O–H stretching vibration.38 For RGO, the absorption peaks of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O–H, C–O, and C–O–C groups decreased remarkably or even disappeared, while the peaks of the carbonyl groups were retained. The absorption peak at 794 cm−1 of DF-RGO is associated with stretching of the C–H vibration of the benzoic acid group.39 Moreover, the peak at 1636 cm−1 can be assigned to the C
O, O–H, C–O, and C–O–C groups decreased remarkably or even disappeared, while the peaks of the carbonyl groups were retained. The absorption peak at 794 cm−1 of DF-RGO is associated with stretching of the C–H vibration of the benzoic acid group.39 Moreover, the peak at 1636 cm−1 can be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in a carboxylic acid and the intensity is evidently higher than that for bare GO, indicating that RGO has been successfully functionalized. The FTIR spectrum of M-DF-RGO differs from that of DF-RGO, as evidenced by the weakening of the C–O peak at 3430 cm−1. This suggests that heat treatment in an alkaline medium removed oxygen from the surface of DF-RGO. Two additional vibrational peaks appeared at approximately 1440 and 1380 cm−1. These derived from the formation of either a monodentate complex or a bidentate complex between the carboxyl group and Fe of the magnetic particles, indicating that the iron oxide nanoparticles were covalently bonded to the surface of DF-RGO by Fe–O–C bonds.36
O in a carboxylic acid and the intensity is evidently higher than that for bare GO, indicating that RGO has been successfully functionalized. The FTIR spectrum of M-DF-RGO differs from that of DF-RGO, as evidenced by the weakening of the C–O peak at 3430 cm−1. This suggests that heat treatment in an alkaline medium removed oxygen from the surface of DF-RGO. Two additional vibrational peaks appeared at approximately 1440 and 1380 cm−1. These derived from the formation of either a monodentate complex or a bidentate complex between the carboxyl group and Fe of the magnetic particles, indicating that the iron oxide nanoparticles were covalently bonded to the surface of DF-RGO by Fe–O–C bonds.36
Raman spectroscopy is a tool utilized in order to analyze ordered and disordered crystal structures of graphene based materials. Fig. 2c shows the Raman spectra of GO, RGO, DF-RGO, and the M-DF-RGO hybrid. The ratio of intensities between the D and G bands (ID/IG) of the samples in Raman spectroscopy is regarded as a measure of defects in samples.40 The ratio of the D to G band intensities (ID/IG) increased from 1.19 to 1.44 after GO was reduced by TUD. Meanwhile, a general characteristic of the spectrum of RGO is the shift to a lower wavenumber, suggesting that the average size of the in-plane sp2 domain had decreased, while the number of sp2 carbon atoms increased for RGO.41 This means that the electronic conjugation of RGO was restored, which is consistent with the XRD results. The ID/IG ratio for DF-RGO is approximately 1.52, indicating that the aryl diazonium treatment led to a higher degree of disorder and defect bond formation, and some functional groups were grafted onto the surface of RGO.42 The D band of the M-DF-RGO composite is shifted downward by approximately 10 cm−1 compared to DF-RGO, while the ID/IG ratio was calculated to be approximately 1.46 due to the reduction of DF-RGO during the co-precipitation reaction process.43
The TGA curves of the samples are presented in Fig. 2d. For GO powders, significant weight loss at 100–150 °C and 150–300 °C of ∼19.03 wt% and ∼30.87 wt%, respectively, can be observed, which is attributed to the decomposition of oxygen functional groups and carbon skeleton oxidation, respectively.44 In contrast, RGO possesses better thermal stability than GO, which is shown by a mass loss of just ∼15.22 wt% at 130–262 °C that arose from the residual carboxyl groups on the plane of RGO. TGA was employed to gauge the degree of functionalization of DF-RGO, and it enables comparison of the weight loss of the functionalized sample and RGO. The defunctionalization of DF-RGO is determined to occur at 200–700 °C.45 In addition, M-DF-RGO exhibited a lower thermal stability than DF-RGO. There was also a slow weight loss (∼9.8 wt%) at a low temperature (<100 °C), which can be attributed to the loss of the residual or absorbed solvent. The obvious weight loss occurring at and above 595 °C may be ascribed to the breakdown of the –COO group coordinated with Fe3O4 nanoparticles in the M-DF-RGO hybrid.46
The magnetization curves of Fe3O4 and M-DF-RGO were measured using VSM at room temperature, as shown in Fig. 3. The saturation magnetization of the M-DF-RGO hybrid was smaller than that of pure bulk Fe3O4 (79.20 emu g−1) due to the smaller size of Fe3O4 nanoparticles and the existence of DF-RGO.33 Meanwhile, the specific saturation magnetization of M-DF-RGO is 28.46 emu g−1, which can be attributed to the relatively high amount of Fe3O4 nanoparticles loaded on DF-RGO.47
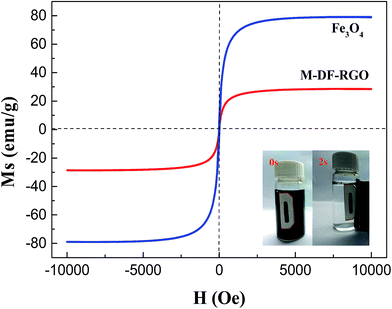 | ||
| Fig. 3 The magnetization curves of the samples, with the inset showing the separation efficiencies of M-DF-RGO. | ||
X-ray photoelectron spectroscopy (XPS) is a sensitive analytical technique used to determine the surface elemental compositions and types of functional groups available on carbon materials.15,21,34 Table 1 lists the elementary compositions of the surfaces of the samples.
| Sample | C 1s | O 1s | O 1s/C 1s | Fe 2p |
|---|---|---|---|---|
| GO | 63.88 | 36.12 | 0.56 | — |
| RGO | 78.00 | 36.12 | 0.28 | — |
| DF-RGO | 75.82 | 21.99 | 0.29 | — |
| M-DF-RGO | 64.50 | 26.98 | 0.42 | 8.52 |
The high resolution XPS C 1s spectrum is shown in Fig. 4. For GO, the raw peaks can be divided into five individual peaks at 284.6 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 285.6 eV (C–C), 286.6 eV (C–O), 287.4 eV (C
C), 285.6 eV (C–C), 286.6 eV (C–O), 287.4 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and 288.6 eV (O–C
O), and 288.6 eV (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).23,43 The high resolution XPS C 1s spectrum of RGO showed a significant decrease of signals at 286–288 eV, which can be ascribed to the reduction of C–O/C–O–C and C
O).23,43 The high resolution XPS C 1s spectrum of RGO showed a significant decrease of signals at 286–288 eV, which can be ascribed to the reduction of C–O/C–O–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionalities. The relative atomic percentage of oxygen obviously decreased from 36% for GO to 21% for RGO, while the relative atomic percentage of C
O functionalities. The relative atomic percentage of oxygen obviously decreased from 36% for GO to 21% for RGO, while the relative atomic percentage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups on RGO increased to some extent in comparison with that on GO. However, the actual amount of the carboxyl functional groups on RGO remained largely the same. Upon reaction with aryl diazonium salts, the ratio of the –COOH peak area increased rapidly to 4.2% for DF-RGO from 2.0% for GO. All of these data proved that the surface of RGO had been covalently modified by p-carboxyphenyl groups through aryl diazonium treatment. Compared with DF-RGO, the relative content of C
C groups on RGO increased to some extent in comparison with that on GO. However, the actual amount of the carboxyl functional groups on RGO remained largely the same. Upon reaction with aryl diazonium salts, the ratio of the –COOH peak area increased rapidly to 4.2% for DF-RGO from 2.0% for GO. All of these data proved that the surface of RGO had been covalently modified by p-carboxyphenyl groups through aryl diazonium treatment. Compared with DF-RGO, the relative content of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups on M-DF-RGO is higher, and the relative intensities of C–O/C–O–C and C
C groups on M-DF-RGO is higher, and the relative intensities of C–O/C–O–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionalities are lower, indicating the likelihood of DF-RGO to be reduced partially under alkaline conditions during the preparation of the M-DF-RGO hybrid. In the Fe 2p spectrum (Fig. S2†), the peaks at 711.8 and 725.1 eV are attributed to Fe 2p3/2 and Fe 2p1/2. The spin-orbital splitting of the Fe 2p peak is broad due to a small chemical shift between Fe2+ and Fe3+,47 both of which are present in Fe3O4.
O functionalities are lower, indicating the likelihood of DF-RGO to be reduced partially under alkaline conditions during the preparation of the M-DF-RGO hybrid. In the Fe 2p spectrum (Fig. S2†), the peaks at 711.8 and 725.1 eV are attributed to Fe 2p3/2 and Fe 2p1/2. The spin-orbital splitting of the Fe 2p peak is broad due to a small chemical shift between Fe2+ and Fe3+,47 both of which are present in Fe3O4.
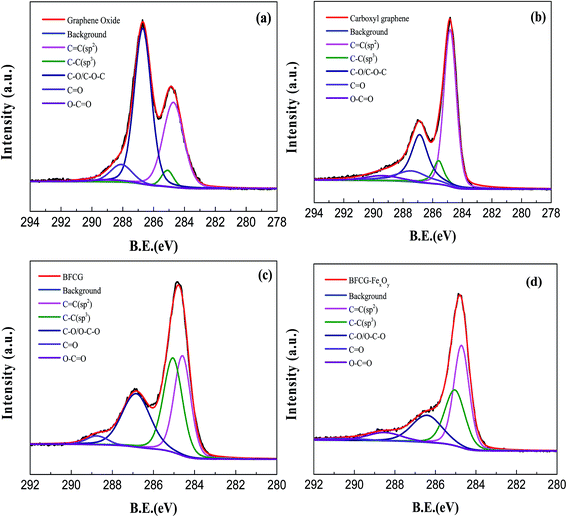 | ||
| Fig. 4 High resolution C 1s core level XPS spectra of (a) GO, (b) RGO, (c) DF-RGO, and (d) M-DF-RGO. | ||
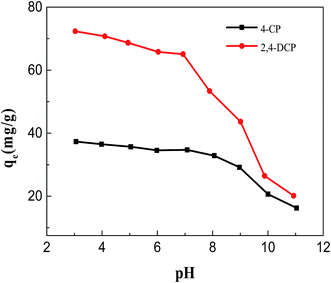
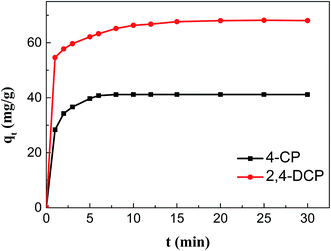
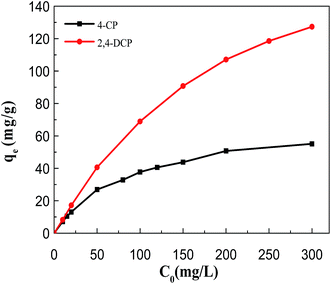
3.2 Chlorophenol removal studies
| qe = KFCe1/n | (1) |
Some researchers have proved that graphene based materials with π–electron-rich properties had a good adsorption effect on aromatic compounds through their π–electron-coupling ability.55,56 The reduction of GO with TUD removed some of the functional groups on the plane of GO, and the introduction of the p-carboxyphenyl group can restrain the agglomeration of RGO in water. Efforts were made to increase the π–π interaction between RGO and chlorophenols. Moreover, some remaining oxygen-containing functional groups for DF-RGO could form hydrogen bonds with the hydroxyl groups of phenol.57,58
It should also be noted that the adsorption capacities of chlorophenols on M-DF-RGO were remarkably distinguished from each other. The adsorbed amounts of 2,4-DCP with two –Cl on phenol was higher than that of 4-CP with one, suggesting that the number of substituents would significantly influence donor/acceptor strength,55 and chlorophenol with more substituted groups had a higher adsorption capacity.
3.3 DFT calculations
DFT calculations were applied to investigate the interaction of chlorophenols with DF-RGO. The optimized structures of two chlorophenols on DF-RGO are shown in Fig. 8, and their corresponding adsorption energies (Ead) are summarized in Table 2.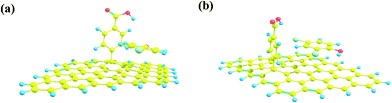 | ||
| Fig. 8 Optimized structures of the adsorption of (a) 4-CP and (b) 2,4-DCP on DF-RGO (carbon atom: yellow; chlorine atom: green; oxygen atom: red; hydrogen atom: blue). | ||
| System | Ead (kcal mol−1) |
|---|---|
| DF-RGO + 4-CP | 11.61 |
| DF-RGO + 2,4-DCP | 14.56 |
As shown in Fig. 8a and b, chlorophenol molecules were parallel to the basal plane of graphene, indicating that the π–π interaction plays an important role in the removal of chlorophenols. As shown in Table 2, the Ead value for 2,4-DCP on DF-RGO is 14.56 kcal mol−1, which is slightly higher than the 11.61 kcal mol−1 for 4-CP on DF-RGO, indicating that the adsorption of 2,4-DCP on the DF-RGO model surface is more stable and the interaction between DF-RGO and 2,4-DCP is stronger. This might be because the 2,4-DCP molecule has an extra –Cl group that could strengthen the π–π interaction between the phenol ring and the adsorbent.
Therefore, based on the results of the theoretical calculations, the higher Ead value indicated that the DF-RGO had a stronger affinity to 2,4-DCP than 4-CP, which is in good agreement with the experimental results.
3.4 Separation and reuse of M-DF-RGO
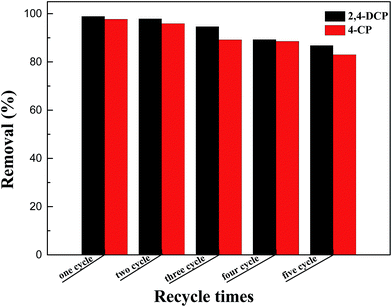
Separating the adsorbent from aqueous solution after adsorption was a major procedure in the practical application. From the inset of Fig. 3, it can be seen that M-DF-RGO can be easily separated from aqueous solution under an external magnetic field in a relatively short time, which evidently results from a high load of Fe3O4 on the surface of M-DF-RGO.The regeneration and reuse of adsorbents is also important in water treatment based on stringent ecological and economic demands for sustainability. We found that M-DF-RGO can go through multiple rounds of reuse by thorough washing with 0.01 mol L−1 NaOH solution and ethanol, separately. Fig. 9 showed that the adsorption capacities of chlorophenols slightly decreased with increasing times of reuse, and were still over 80% after reuse in five cycles, suggesting that M-DF-RGO has good reusability.
4. Conclusion
A magnetite/diazonium functionalized-reduced graphene oxide (M-DF-RGO) hybrid was successfully prepared. Benefiting from the combined advantages of DF-RGO and Fe3O4 nanoparticles, the M-DF-RGO composite not only possessed relatively high adsorption efficiency for chlorophenols, but could also be easily separated by an external magnetic field in a fairly short time, which could possibly reduce water treatment expenses. Furthermore, density functional theory calculations indicated that the π–π interaction played an important role in the removal of chlorophenols. The adsorption capacity of the regenerated M-DF-RGO was still over 80% after five cycles. All of these results indicated that M-DF-RGO can be utilized as an ideal adsorbent for the simple and rapid removal of phenolic pollutants from aqueous solution.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 21677087) and National Science and Technology Major Project of China “Environmental Protection Technology Integration and Key Equipment for Tight Reservoir Development” (2016ZX05040-005).Notes and references
- T. Matias, J. Marques, M. J. Quina, L. Gando-Ferreira, A. J. M. Valente, A. Portugal and L. Durães, Colloids Surf., A, 2015, 480, 260–269 CrossRef CAS.
- Q. Yang, M. Gao and W. Zang, Colloids Surf., A, 2017, 520, 805–816 CrossRef CAS.
- M. Ahmaruzzaman and S. L. Gayatri, J. Chem. Eng. Data, 2011, 56, 3004–3016 CrossRef CAS.
- M. H. Dehghani, M. Mostofi, M. Alimohammadi, G. McKay, K. Yetilmezsoy, A. B. Albadarin, B. Heibati, M. AlGhouti, N. M. Mubarak and J. N. Sahu, J. Ind. Eng. Chem., 2016, 35, 63–74 CrossRef CAS.
- P. Strachowski and M. Bystrzejewski, Colloids Surf., A, 2015, 467, 113–123 CrossRef CAS.
- J. Fan, J. Zhang, C. Zhang, L. Ren and Q. Shi, Desalination, 2011, 267, 139–146 CrossRef CAS.
- S. Zhang, X. Zhao, H. Niu, Y. Shi, Y. Cai and G. Jiang, J. Hazard. Mater., 2009, 167, 560–566 CrossRef CAS PubMed.
- Y. Wang, Y. N. Zhang, G. Zhao, H. Tian, H. Shi and T. Zhou, ACS Appl. Mater. Interfaces, 2012, 4, 3965–3972 CAS.
- X. Zhang, A. Li, Z. Jiang and Q. Zhang, J. Hazard. Mater., 2006, 137, 1115–1122 CrossRef CAS PubMed.
- J. S. Valente, F. Tzompantzi, J. Prince, J. G. H. Cortez and R. Gomez, Appl. Catal., B, 2009, 90, 330–338 CrossRef CAS.
- Z. W. Ming, C. J. Long, P. B. Cai, Z. Q. Xing and B. Zhang, J. Hazard. Mater., 2006, 128, 123–129 CrossRef CAS PubMed.
- G. Liao, W. Zhao, Q. Li, Q. Pang and Z. Xu, Chem. Lett., 2017, 46, 1631–1634 CrossRef CAS.
- Y. Shen and B. Chen, Environ. Sci. Technol., 2015, 49, 7364–7372 CrossRef CAS PubMed.
- C. K. Chua, A. Ambrosi and M. Pumera, J. Mater. Chem., 2012, 22, 11054 RSC.
- K. I. Bolotin, K. J. Sikes, Z. Jiang, M. Klima, G. Fudenberg, J. Hone, P. Kim and H. L. Stormer, Solid State Commun., 2008, 146, 351–355 CrossRef CAS.
- C.-M. Chen, Q. Zhang, M.-G. Yang, C.-H. Huang, Y.-G. Yang and M.-Z. Wang, Carbon, 2012, 50, 3572–3584 CrossRef CAS.
- L. S. Zhang, W. D. Wang, X. Q. Liang, W. S. Chu, W. G. Song, W. Wang and Z. Y. Wu, Nanoscale, 2011, 3, 2458–2460 RSC.
- V. Georgakilas, M. Otyepka, A. B. Bourlinos, V. Chandra, N. Kim, K. C. Kemp, P. Hobza, R. Zboril and K. S. Kim, Chem. Rev., 2012, 112, 6156–6214 CrossRef CAS PubMed.
- B. Jia and L. Zou, Carbon, 2012, 50, 2315–2321 CrossRef CAS.
- S. Chatterjee, R. K. Layek and A. K. Nandi, Carbon, 2013, 52, 509–519 CrossRef CAS.
- X. Huang, X. Qi, F. Boey and H. Zhang, Chem. Soc. Rev., 2012, 41, 666–686 RSC.
- S. Mao, H. Pu and J. Chen, RSC Adv., 2012, 2, 2643 RSC.
- N. Pan, D. Guan, Y. Yang, Z. Huang, R. Wang, Y. Jin and C. Xia, Chem. Eng. J., 2014, 236, 471–479 CrossRef CAS.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- W. J. Hehre, R. Ditchfield and J. A. Pople, J. Chem. Phys., 1972, 56, 2257–2261 CrossRef CAS.
- P. C. Hariharan and J. A. Pople, Theor. Chem. Acc., 1973, 28, 213–222 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- S. F. Boys and F. Bernardi, Mol. Phys., 2006, 19, 553–566 CrossRef.
- P. A. Denis, ChemPhysChem, 2013, 14, 3271–3277 CrossRef CAS PubMed.
- P. A. Denis, J. Phys. Chem. C, 2013, 117, 3895–3902 CAS.
- P. A. Denis, Chem. Phys. Lett., 2017, 672, 70–79 CrossRef CAS.
- Y. Fu, J. Wang, Q. Liu and H. Zeng, Carbon, 2014, 77, 710–721 CrossRef CAS.
- J. Shen, M. Shi, H. Ma, B. Yan, N. Li and M. Ye, Mater. Res. Bull., 2011, 46, 2077–2083 CrossRef CAS.
- Y. Gao, Y. Li, L. Zhang, H. Huang, J. Hu, S. M. Shah and X. Su, J. Colloid Interface Sci., 2012, 368, 540–546 CrossRef CAS PubMed.
- J. Shen, Y. Hu, M. Shi, N. Li, H. Ma and M. Ye, J. Phys. Chem. C, 2010, 114, 1498–1503 CAS.
- G. Liao, J. Chen, W. Zeng, C. Yu, C. Yi and Z. Xu, J. Phys. Chem. C, 2016, 120, 25935–25944 CAS.
- X. Mei and J. Ouyang, Carbon, 2011, 49, 5389–5397 CrossRef CAS.
- G. Wei, M. Yan, R. Dong, D. Wang, X. Zhou, J. Chen and J. Hao, Chemistry, 2012, 18, 14708–14716 CrossRef CAS PubMed.
- W. Zhao, M. Fang, F. Wu, H. Wu, L. Wang and G. Chen, J. Mater. Chem., 2010, 20, 5817 RSC.
- J. Li, H. Lin, Z. Yang and J. Li, Carbon, 2011, 49, 3024–3030 CrossRef CAS.
- Y. Wang, L. Meng, L. Fan, G. Wu, L. Ma, M. Zhao and Y. Huang, Appl. Surf. Sci., 2016, 362, 341–347 CrossRef CAS.
- N. A. Zubir, C. Yacou, J. Motuzas, X. Zhang and J. C. Diniz da Costa, Sci. Rep., 2014, 4, 4594 CrossRef PubMed.
- A.-L. Morel, S. I. Nikitenko, K. Gionnet, A. Wattiaux, J. Lai-Kee-Him, C. Labrugere, B. Chevalier, G. Deleris, C. Petibois and A. Brisson, ACS Nano, 2008, 2, 847–856 CrossRef CAS PubMed.
- Y. Zhu, A. L. Higginbotham and J. M. Tour, Chem. Mater., 2009, 21, 5284–5291 CrossRef CAS.
- X. Yang, X. Zhang, Y. Ma, Y. Huang, Y. Wang and Y. Chen, J. Mater. Chem., 2009, 19, 2710 RSC.
- G. Xie, P. Xi, H. Liu, F. Chen, L. Huang, Y. Shi, F. Hou, Z. Zeng, C. Shao and J. Wang, J. Mater. Chem., 2012, 22, 1033–1039 RSC.
- H. Yan, H. Li, X. Tao, K. Li, H. Yang, A. Li, S. Xiao and R. Cheng, ACS Appl. Mater. Interfaces, 2014, 6, 9871–9880 CAS.
- L. Zhang, B. Zhang, T. Wu, D. Sun and Y. Li, Colloids Surf., A, 2015, 484, 118–129 CrossRef CAS.
- Ihsanullah, H. A. Asmaly, T. A. Saleh, T. Laoui, V. K. Gupta and M. A. Atieh, J. Mol. Liq., 2015, 206, 176–182 CrossRef CAS.
- G. C. Chen, X. Q. Shan, Y. S. Wang, B. Wen, Z. G. Pei, Y. N. Xie, T. Liu and J. J. Pignatello, Water Res., 2009, 43, 2409–2418 CrossRef CAS PubMed.
- F. Li and M. J. Rosen, J. Colloid Interface Sci., 2000, 224, 265–271 CrossRef CAS PubMed.
- Z. Rawajfih and N. Nsour, J. Colloid Interface Sci., 2006, 298, 39–49 CrossRef CAS PubMed.
- A. Khenifi, B. Zohra, B. Kahina, H. Houari and D. Zoubir, Chem. Eng. J., 2009, 146, 345–354 CrossRef CAS.
- X. Wang, S. Huang, L. Zhu, X. Tian, S. Li and H. Tang, Carbon, 2014, 69, 101–112 CrossRef CAS.
- Z. Pei, L. Li, L. Sun, S. Zhang, X.-q. Shan, S. Yang and B. Wen, Carbon, 2013, 51, 156–163 CrossRef CAS.
- D. Cortés Arriagada, L. Sanhueza and K. Wrighton, Int. J. Quantum Chem., 2013, 113, 1931–1939 CrossRef.
- Z. Jin, X. Wang, Y. Sun, Y. Ai and X. Wang, Environ. Sci. Technol., 2015, 49, 9168–9175 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra00503f |
| This journal is © The Royal Society of Chemistry 2018 |