Open Access Article
Open Access ArticlePolymeric ionic liquid gels composed of hydrophilic and hydrophobic units for high adsorption selectivity of perrhenate†
Dong Han‡
a,
Xingxiao Li‡a,
Yu Cuia,
Xin Yanga,
Xibang Chena,
Ling Xubc,
Jing Peng*a,
Jiuqiang Lia and
Maolin Zhai *a
*a
aBeijing National Laboratory for Molecular Sciences, Radiochemistry and Radiation Chemistry Key Laboratory of Fundamental Science, The Key Laboratory of Polymer Chemistry and Physics of the Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China. E-mail: jpeng@pku.edu.cn; mlzhai@pku.edu.cn
bState Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, School of Public Health, Xiamen University, Xiamen, Fujian 161102, China
cPeking University Shenzhen Institute, Shenzhen 518057, China
First published on 5th March 2018
Abstract
The removal of TcO4− from aqueous solutions has attracted more and more attention recently, and ReO4− has been widely used as its natural analog. In this work, polymeric ionic liquid gel adsorbents, PC2-C12vimBr, with high adsorption capacity and selectivity towards ReO4− were synthesized by radiation-induced polymerization and crosslinking. PC2-C12vimBr was composed of two monomers: a hydrophobic unit, 1-vinyl-3-dodecylimidazolium bromide for high selectivity, and a hydrophilic unit, 1-vinyl-3-ethylimidazolium bromide for improved kinetics. A gel fraction up to 90% could be achieved under 40 kGy with varied monomer ratios. The adsorption of PC2-C12vimBr gels for ReO4− was evaluated by batch adsorption. The PC2-C12vimBr gel containing 20 mol% hydrophilic unit (named PC2-C12vimBr-A) could significantly improve the adsorption kinetics, which had an equilibrium time of ca. 24 h. The adsorption capacity obtained from the Langmuir model was 559 mg g−1 (Re/gel). The selective factor against NO3− was 33.4 ± 1.9, which was more than 10 times higher than that of PC2vimBr, and it could maintain ReO4− uptake as high as 100 mg g−1 in 0.5 mol kg−1 HNO3. The ΔHΘ and ΔSΘ of the NO3−/ReO4− ion-exchange reaction of PC2-C12vimNO3-A were −16.9 kJ mol−1 and 29 J mol−1 K−1, respectively, indicating physical adsorption. The adsorption mechanism of ReO4− onto PC2-C12vimBr-A gel was ion-exchange, and it could be recovered using 5.4 mol kg−1 HNO3.
1. Introduction
Technetium (Tc) is an artificial element, and all its isotopes are radioactive. A large amount of 99Tc is produced from nuclear plants nowadays, and exists in high-level liquid waste. Since Tc is hazardous and has high mobility in the environment, and it may affect the geological disposal of other radioactive nuclei, the removal of Tc from aqueous solutions has attracted more and more attention recently.1 And because of the radioactivity of Tc, it is not allowed to be handled in the normal lab, and rhenium (Re) has been extensively used as its analog owing to their similar chemistry properties.1–3Tc mainly exists in the form of TcO4− in the high-level waste.4 Many kinds of method to remove TcO4− and ReO4− have been investigated in recent years, including the reductive immobilization of TcO4− or ReO4− by Fe(0)5,6 and the direct adsorption by adsorbents.7–10 Comparing to the reductive method, the adsorption takes the advantage of high adsorption capacity and the reusability of the adsorbents.
Most adsorbents towards TcO4− and ReO4− depended on ion-exchange. MOFs,11–14 carbon materials,15–18 and organic adsorbents have been employed as the adsorbents. Organic adsorbents including resins,19,20 grafted materials21,22 and gels23,24 take the advantage of high adsorption capacities and easy design and synthesis. Quaternary ammonium19,23 including pyridinium25,26 and imidazolium3,21,27 have been used as the functional groups in many ion-exchange adsorbents.19 For the quaternary ammonium, alkyl has been widely used as the group on the quaternary N atom, including ethyl, propyl, butyl, pentyl, hexyl etc.28,29 And it was found that longer alkyl chain in quaternary ammonium may bring higher selectivity towards ReO4−.28
However, adsorbents depending on ion-exchange would be influenced by the other anions in the solution as competitors, and a large amount of NO3− exists in the high-level liquid waste. So it is very important to develop adsorbents with high selectivities towards TcO4− or ReO4−. Wang et al. reported a TcO4− adsorbent, NDTB-1, with an adsorption capacity of 162.2 mg g−1 and a maximum distribution coefficient Kd of 1.0534 × 104 mL g−1.10 Shu et al. developed a surface ion-imprinted magnetic microsphere ReO4− adsorbent containing imidazolium groups with an adsorption capacity of 62.8 mg g−1 and a Langmuir constant of 1.35 L mg−1.3
On the other hand, some of the reported excellent adsorbents contain metal elements, e.g. Ag in SCU-100,13 which may result in the increase of radioactive wastes. Adsorbents composed of only C, H, N, O may avoid the secondary radioactive waste since they follow the CHON principle.30,31 Organic adsorbents such as polymeric ionic liquids may meet this requirement.
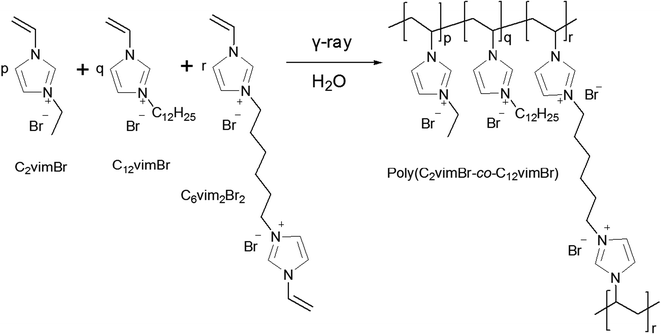
Polymeric ionic liquid is a kind of polymer which contains ionic liquid units, and they have attracted much attention in many fields, such as polyelectrolytes, polymeric surfactants, anion sensitive smart materials,32,33 gas separation membranes,34 and also ion-exchange adsorbents.35 Recently we reported a polymeric ionic liquid gel ReO4− adsorbent with high adsorption capacity of 865 mg g−1, but the selectivity is not high enough, and we proposed that hydrophobic polycations might have higher selectivity towards ReO4−.24 Polymers with both hydrophilic and hydrophobic segments have been reported with interesting self-assembling behaviors36 or as proton conductive membranes.37 Considering the advantages of combination of the hydrophilic and the hydrophobic segments in polymers, i.e. the hydrophobic one is favour for higher adsorption selectivity towards ReO4− and the hydrophilic one is better for adsorption kinetics, we herein developed a polymeric ionic liquid gel adsorbent, PC2-C12vimBr, with high adsorption capacity and selectivity, which was synthesized easily by radiation-induced polymerization and crosslinking of 1-vinyl-3-alkylimidazolium. Considering longer alkyl chain on the quaternary N atom can increase the hydrophobicity of gels, which may result in higher adsorption selectivity towards ReO4−, a hydrophobic unit, 1-vinyl-3-dodecylimidazolium bromide (C12vimBr) was chosen to improve the selectivity. On the other hand, a hydrophilic unit, 1-vinyl-3-ethylimidazolium bromide (C2vimBr), was chosen to improve the adsorption kinetics of ReO4− in aqueous solution. A series of PC2-C12vimBr gels with different molar ratio of hydrophobic and hydrophilic units were synthesized and characterized, and their adsorption kinetics towards ReO4− were investigated. Then the adsorption isotherm, selectivity and mechanism of PC2-C12vimBr gel were investigated in detail.
2. Experimental
2.1 Materials
C2vimBr and C12vimBr monomers were purchased from Lanzhou Greenchem ILs, LICP. CAS., dried in vacuum to remove the residual solvent. 3,3′-Divinyl-1,1′(1,6-hexanediyl) diimidazolium dibromide (C6vim2Br2) was easily synthesized as we reported before.24 1,6-Dibromohexane was purchased from J&K, and 1-vinylimidazole was provided by TCI. KReO4 was obtained from Alfa Aesar. Re standard solution was provided by NCS Testing Technology Co., Ltd. Some HNO3 mother solutions with certain concentrations were prepared from GR HNO3 (Xilong Chemical Co., Ltd.) and titrated. Other chemicals were analytical-grade reagents. Deionized water was used throughout the experiments.2.2 Preparation and characterization of PC2-C12vimBr gels
| Feed concentrations (M) | Names of the resulted gels | ||
|---|---|---|---|
| C2vimBr | C12vimBr | C6vim2Br2 | |
| 0 | 1 | 0.05 | PC12vimBr |
| 0.2 | 0.8 | 0.05 | PC2-C12vimBr-A |
| 0.4 | 0.6 | 0.05 | PC2-C12vimBr-B |
| 0.6 | 0.4 | 0.05 | PC2-C12vimBr-C |
| 0.8 | 0.2 | 0.05 | PC2-C12vimBr-D |
The resulted gels were cut into round pieces with a thickness of ca. 3 mm, and then every round piece was evenly cut into 4 sector pieces for further usage. The gels was immersed into excess ethanol for more than 6 times (at least 1 day for every time) to remove the sol part, and was subsequently put into excess water for more than 4 times. Then the gels were dried under 60 °C for more than 2 days to a constant weight. Gel fraction (GF) and equilibrium degree of swelling (EDS) were measured by gravimetry.
GF is defined as:
| GF (%) = wg/w0 × 100 | (1) |
EDS is defined as:
| EDS = we/wd | (2) |
2.3 Adsorption of ReO4− onto PC2-C12vimBr gels
The mother solution of Re was prepared from deionized water and KReO4. Inductively coupled plasma atomic emission spectroscopy (ICP-AES, Prodigy) was used to measure the Re concentration, and the relative error of Re concentration was estimated as 2%.Typically, an adsorption experiment was performed as following: a piece of gel was immersed into a Re solution, the mass ratio of the gel to the solution was 1 mg gel per 1 g solution. The system was shaked in a shaker at 25 °C for ca. 2 days to reach equilibrium, and the concentration of Re in the resulted solution was measured by ICP-AES.
The Re uptake, q, was calculated as following:
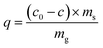 | (3) |
In the competitive adsorption against NO3−, the anion of the gel were exchanged into NO3− before the adsorption, forming PC2-C12vimNO3-A. In detail, PC2-C12vimBr-A was immersed into excess ca. 1.5 M HNO3 to equilibrium for 2 times, then into water for 3 times and dried. XPS and IR were performed to confirm the formation of PC2-C12vimNO3-A (Fig. S1†).
The selective factor (SF) was used to evaluate the selectivities toward ReO4− against NO3− and Br−. SF was defined as:36
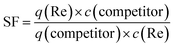 | (4) |
The SF of PC2vimBr, a Re adsorbent reported in our previous work,24 towards ReO4− against NO3− was measured by the same procedure.
For the selective adsorption in ethanol–water mix solvent, the concentration of HNO3 was 0.05 mol kg−1.
3. Result and discussion
3.1 Preparation and characterization of PC2-C12vimBr gels
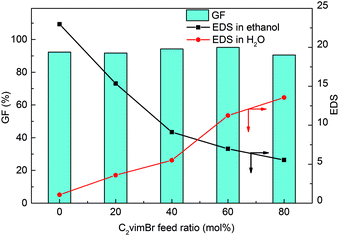
Radiation-induced polymerization and crosslinking is a green and clean method widely used to prepare gels,37 and it has been proved to be effective for the preparation of poly ionic liquid gels in our previous works.24 The synthesis of PC2-C12vimBr gels was illustrated in Scheme 1, and the GF and the EDS of PC2-C12vimBr gels were shown in Fig. 1.The GFs of all PC2-C12vimBr gels with different compositions synthesized under 40 kGy were above 90%, which indicated that γ-radiation-induced polymerization and crosslinking was suitable and effective for this system. IR, TGA, EA, and XPS were performed to confirm the composition and structure of the resultant gels, and the results were shown in the ESI (Fig. S2–S4 and Table S1†).
PC12vimBr, which had no C2vimBr units, nearly did not swell in water, but it swelled well in ethanol. The EDS of PC2-C12vimBr gels in water increased with the increasing C2vimBr content, while the EDS in ethanol decreased. Since all the five gels had the same crosslinker content, the differences of the EDS of the gels in water and in ethanol were due to the different contents of the short-chain C2vimBr units and the long-chain C12vimBr units, and the results reflected the hydrophilicities and hydrophobicities of the gels. The more C2vimBr units the gel contains, the more hydrophilic it was, while the C12vimBr units contributed to the hydrophobicity of the gel.
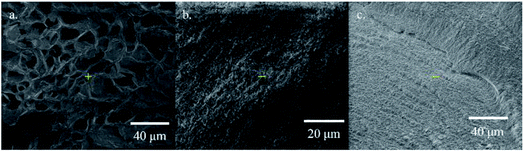
SEM images were taken from the xerogels prepared by freeze-drying of the corresponding gels swelled in water. SEM images of PC2-C12vimBr-A, PC2-C12vimBr-D and PC12vimBr gel were shown in Fig. 2. Among the three samples, PC2-C12vimBr-D had the largest holes, and it was agreed with its highest EDS in water. PC2-C12vimBr-A, which had least C2vimBr units among PC2-C12vimBr gels, also had small holes, while it could swell a little in water. In the image of PC12vimBr, the section was rough and no hole could be observed. PC12vimBr was relatively hydrophobic, and did not swell in water, while even a small amount of C2vimBr units could give the gels hydrophilicity and make them swell in water, which would improve their adsorption kinetics.
3.2 The adsorption kinetics of ReO4− onto PC2-C12vimBr gels
PC2-C12vimBr gel had a cationic polymer network with Br− as exchangeable counteranion, so anions such as ReO4− can be attracted easily by PC2-C12vimBr gel and then be adsorbed by anion-exchange. The adsorption kinetics of ReO4− onto four PC2-C12vimBr gels were shown in Fig. 3a, and that of PC12vimBr was shown in Fig. 3b.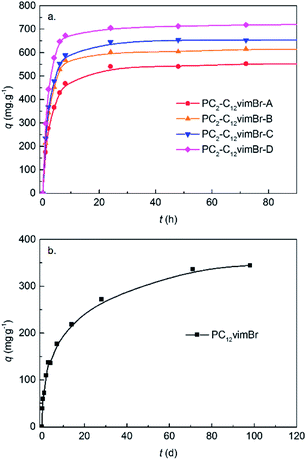 | ||
| Fig. 3 Adsorption kinetics of ReO4− onto (a) PC2-C12vimBr gels and (b) PC12vimBr. The c0 was 557 ppm. | ||
For all the four PC2-C12vimBr gels, the kinetics had no significant difference. The adsorption equilibriums were achieved at ca. 24 h, which was similar to the equilibrium time of poly(4-vinylpyridine) resins.29 However, PC12vimBr shown a quite slow adsorption kinetics, and could not achieve equilibrium in even 3 months. This was due to the high hydrophobicity of PC12vimBr, and as shown in Fig. 2c, it had no porous structure in water. On the other hand, as little as 20 mol% of the short-chain C2vimBr units could improve the hydrophilicity of the gel, and further efficiently improve the adsorption kinetics. Upon 20 mol%, more C2vimBr units did not show significant improvement to the adsorption kinetics. Since we introduced the long-chain C12vimBr units aiming at improving the adsorption selectivity towards ReO4− (the gels with lower C12vimBr contents had lower adsorption selectivity towards ReO4−, see Fig. S5†), PC2-C12vimBr-A, which had the highest content of C12vimBr units among the gels with suitable kinetics, was chosen for the further adsorption experiments.
3.3 The adsorption isotherms and selectivity of PC2-C12vimBr-A towards ReO4−
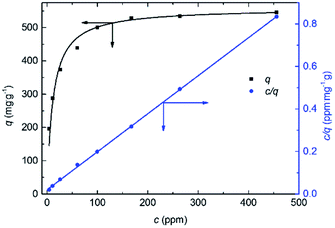
The adsorption isotherms of ReO4− onto PC2-C12vimBr-A agreed with the Langmuir model well:
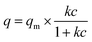 | (5) |
And it was fitted in the linear form:
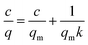 | (6) |
The parameters of fitting and the calculated qm and k were shown in Table 2:
| Intercept, 1/qmk | Slope, 1/qm | R2 | qm, (mg g−1) | K, (ppm−1) |
|---|---|---|---|---|
| 0.02036 | 0.00179 | 0.9997 | 559 | 0.0879 |
The qm of Re onto PC2-C12vimBr-A was 559 mg Re per g gel, which was equal to the theoretical value calculated from the N wt% in the EA of PC2-C12vimBr-A, assuming every imidazolium group could adsorb one ReO4− ion:
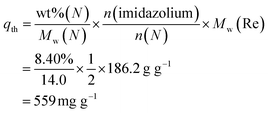 | (7) |
This result also supported that all imidazolium groups in PC2-C12vimBr-A were utilized with a ratio to ReO4− of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Since the long-chain unit C12vimBr has larger Mw than that of C2vimBr, the qm of PC2-C12vimBr-A was lower than that of the Re adsorbent PC2vimBr we reported before, which was 865 mg g−1.24 However, as a gel adsorbent, PC2-C12vimBr-A was fully composed of functional groups without matrix, its adsorption capacity was higher than most of the Re adsorbents reported.3,10,16
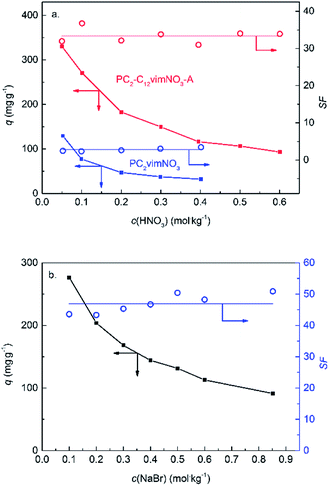
For the practical usage of Re/Tc adsorbents, the selectivity is more important than the adsorption capacities. Because in practical systems, TcO4− usually presences with competitors, e.g. HNO3 in the PUREX.4 The results of the competitive adsorption against Br− and NO3− were shown in Fig. 5.
As shown in Fig. 5, the q of Re decreased with the increasing concentration of the competitor in all the three curves, and it was due to the competition of NO3− or Br−. SF should be a constant according to the Langmuir model, and was fluctuating around the average value among the investigated conditions. The average values of SF were listed in Table 3.
| Adsorbent | Competitor | SF |
|---|---|---|
| PC2-C12vimNO3-A | NO3− | 33.4 ± 1.9 |
| PC2vimNO3 | NO3− | 2.8 ± 0.5 |
| PC2-C12vimBr-A | Br− | 46.9 ± 3.1 |
Comparing to PC2vimNO3, the adsorbent we previously reported,24 PC2-C12vimNO3-A had an SF against NO3− more than 10 times higher than that of the former one, which illustrated its excellent selectivity towards ReO4−. Since few literatures about Re adsorbents have reported the SF between NO3− and ReO4−, it is a little difficult to compare the selectivities widely among these adsorbents, considering some reported quantities, e.g. distribution coefficient Kd or removal rate, are not only decided by the nature of the materials, but also by the experiment parameters such as the phase ratio of adsorbent/solution and the c0 of Re. We estimated the SF towards ReO4− against NO3− of SCU-100, which is an excellent Re/Tc adsorbent reported recently with the highest Kd among inorganic Re adsorbents, from its adsorption capacity as 541 mg ReO4− per g SCU-100 and removal rate as 73% at 0.15 mM ReO4− and 15 mM NO3− with a phase ratio of 1 mL solution per 1 mg SCU-100:13
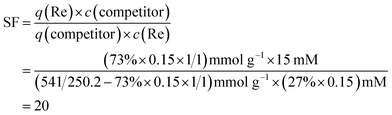 | (8) |
It was illustrated that the measured SF of PC2-C12vimNO3-A was higher than the estimated SF of SCU-100, i.e. PC2-C12vimNO3-A had better selectivity towards ReO4−. PC2-C12vimNO3-A could remain ca. 100 mg g−1 Re uptake under 0.5 mol kg−1 HNO3. It was even higher than the maximum adsorption capacities of some other reported adsorbents.3,38 The potential practical usage of PC2-C12vimBr-A for the treatment of radioactive liquid wastes was revealed by such high Re uptake under this condition. The SF of PC2vimBr against Br− was higher than that against NO3−, and it is agreed with the sequence of increasing hydration energies (ReO4− < NO3− < Br−).39
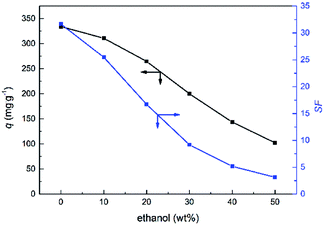
Varied contents of polar organic solvents in the mixed solvent could be used to investigate the effect of solvent on the adsorption.40 And the lower hydration energy of ReO4− than those of the competitors was widely used for the selective adsorption or extraction of ReO4−.1 Herein, ethanol was added in the solvent for illustrating the importance of water in the selective adsorption of ReO4−. As shown in Fig. 6, the q as well as the SF were decreased with the increasing ethanol content. With increasing ethanol content, the activity of water decreased, thus the contribution of the differences of hydration energies to the selective adsorption was decreased for the system. So it could be concluded that water as a solvent played an important role in the selective adsorption of ReO4−onto PC2-C12vimNO3-A gel.
3.4 The adsorption thermodynamics of PC2-C12vimBr-A towards ReO4−
The SF of PC2-C12vimNO3-A towards ReO4− against NO3− can be considered as the K of the following equation:| PC2-C12vimNO3-A + ReO4− = PC2-C12vimReO4-A + NO3− | (9) |
The K under different temperatures were measured, and the relation between ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K and 1000/T (Fig. 7) were fitted by the van't Hoff equation:
K and 1000/T (Fig. 7) were fitted by the van't Hoff equation:
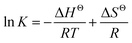 | (10) |
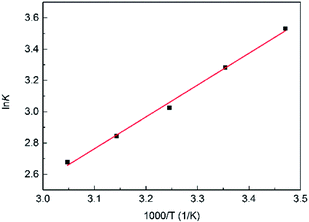 | ||
Fig. 7 Fitted curve of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K–1000/T of eqn (9). K–1000/T of eqn (9). | ||
The ΔHΘ and ΔSΘ of eqn (9) were calculated from the slope and the intercept, which were −16.9 kJ mol−1 and 29 J mol−1 K−1, respectively.
The negative ΔHΘ indicated that the adsorption process was exothermic, and lower temperature was beneficial to the adsorption process. The type of adsorption can be indicated by the magnitude of the enthalpy change value. Lower enthalpy change corresponds to physical adsorption, and higher one corresponds to chemical adsorption. The range of enthalpy change of physical adsorption is considered as 0–20 kJ mol−1 (ref. 41) or 0.5–5 kcal mol−1 (2.1–20.9 kJ mol−1).42 The absolute value of ΔHΘ of eqn (9) was in the range of enthalpy change of physical adsorption, so the adsorption process of PC2-C12vimNO3-A towards ReO4− was physical adsorption. This was agreed with the ion-exchange mechanism, where no covalent bond forms or breaks.
3.5 The adsorption cycles and the adsorption mechanism of PC2-C12vimBr-A towards ReO4−
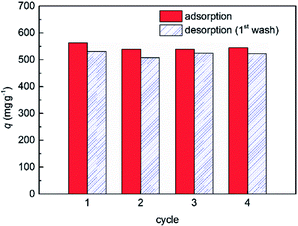
Though PC2-C12vimBr-A had high adsorption selectivity towards ReO4−, ReO4− can be exchanged into solution by high concentration NO3−. So the used adsorbent can be renewed by HNO3 with high concentration resulting in the formation of PC2-C12vimNO3-A. After washing off HNO3 by deionized water, PC2-C12vimNO3-A can adsorb ReO4− again by anion-exchanging. The adsorption cycles of PC2-C12vimBr-A towards ReO4− were shown in Fig. 8, where the Re uptake were marked by red and the Re desorbed in the first wash by 5.40 mol kg−1 HNO3 were marked by blue.The q in the second cycle was reduced to ca. 96% of that of the first cycle and kept well in the next two cycles. The decrease of q after the first cycle could be explained by the anion exchange from Br− to NO3− after the first cycle. PC2-C12vimReO4-A could be renewed into PC2-C12vimNO3-A by HNO3 and the adsorbent had excellent reusability. Most ReO4− could be desorbed in the first wash by 5.4 mol kg−1 NO3−, and the second wash could renew almost all the ion-exchange positions. The eluted Re in HNO3 solution exists without any other salts, and can be made into Re products for other usages easily, or disposed separately from other wastes.
IR and XPS were performed to characterize PC2-C12vimBr-A, PC2-C12vimReO4-A and the PC2-C12vimNO3-A after desorption (Fig. 9).
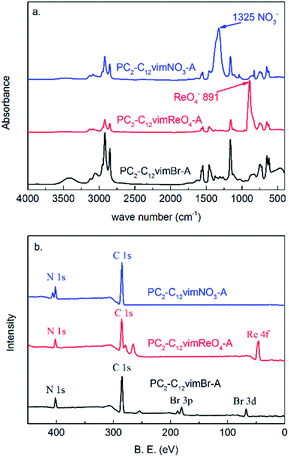 | ||
| Fig. 9 The (a) IR and (b) XPS pattern of PC2-C12vimBr-A, PC2-C12vimReO4-A and the PC2-C12vimNO3-A after desorption. | ||
As Br− has no IR absorbance, the peaks of the polymeric imidazolium cation were revealed clearly in the IR spectrum of PC2-C12vimBr-A. After the adsorption of ReO4−, in the IR spectrum of PC2-C12vimReO4-A, no change of the peaks of the polymeric imidazolium cation could be observed, and the peak of ReO4− at 891 cm−1 occurred. After the desorption by 5.4 mol kg−1 HNO3, the peak of ReO4− disappeared, and the strong peak of NO3− at 1325 cm−1 occurred, while the other peaks showed no change. It could be concluded from the IR spectra that the polymeric imidazolium cation did not change during the adsorption and the desorption process, and ReO4− and NO3− were exchanged onto the polycation without valence change. The XPS also confirmed the adsorption and the desorption was successful. The peak of Re occurred in PC2-C12vimReO4-A instead of that of Br in PC2-C12vimBr-A, and it disappeared in the XPS of PC2-C12vimNO3-A after desorption. The peak of N in PC2-C12vimNO3-A was a double peak, instead of the single peaks in PC2-C12vimBr-A and PC2-C12vimReO4-A, for the N in PC2-C12vimNO3-A could be divided into two kinds with different B.E., which belonged to NO3− and imidazolium respectively (see also Fig. S1†). So an ion-exchange mechanism could be concluded from the IR and the XPS results.
Furthermore, as an anion-exchange gel, PC2-C12vimBr may be used for the adsorption of other anions, such as Cr(VI) anions, anionic surfactants and anionic dyes in the future.
4. Conclusions
Polymeric ionic liquid gels, PC2-C12vimBr, was synthesized by radiation-induced polymerization and crosslinking, and its adsorption behavior towards ReO4− was investigated. The PC2-C12vimBr gel contained two kinds of imidazolium units, namely the hydrophilic units composed of ethylimidazolium and the hydrophobic units composed of dodecylimidazolium. Addition of small amount of hydrophilic units could effectively improve the adsorption kinetics of PC12vimBr gel, and the addition of hydrophobic units could improve the adsorption selectivity towards ReO4− of PC2vimBr gel. PC2-C12vimBr-A gel, with 20 mol% hydrophilic units and 80 mol% hydrophobic units, had an adsorption equilibrium time of ca. 24 h and adsorption capacity as high as 559 mg g−1 (mg Re per g gel). Its SF towards ReO4− against NO3− was 33.4 ± 1.9, which was more than 10 times of that of PC2vimBr, and it could keep a Re uptake as high as 100 mg g−1 in 0.5 mol kg−1 HNO3, which revealed its potential application in the treatment of Tc in radioactive liquid wastes. The adsorption mechanism was ion-exchange, and the selective adsorption was driven by the low hydration energy of ReO4− and the hydrophobicity of the polycation. The ΔHΘ and ΔSΘ of the NO3−/ReO4− ion-exchange reaction of PC2-C12vimNO3-A were −16.9 KJ mol−1 and 29 J mol−1 K−1, respectively. The adsorbent could be renewed by 5.4 mol kg−1 HNO3 for several times with high adsorption performance maintenance. The work provided a new approach to prepare novel ionic gel adsorbent by controlling the hydrophilicity and hydrophobicity of adsorbents which are related to the kinetics and selectivity towards adsorbates, respectively.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The National Natural Science Foundation of China (NNSFC, Project No. 21471161 and 11375019) and the Scientific and Technological Plan of Shenzhen City (JCYJ 20150616163111759) are acknowledged for supporting this research.Notes and references
- D. Banerjee, D. Kim, M. J. Schweiger, A. A. Kruger and P. K. Thallapally, Chem. Soc. Rev., 2016, 45, 2724–2739 RSC.
- S. Sarri, P. Misaelides, D. Zamboulis, X. Gaona, M. Altmaier and H. Geckeis, J. Radioanal. Nucl. Chem., 2016, 307, 681–689 CrossRef CAS.
- X. Shu, L. Shen, Y. Wei and D. Hua, J. Mol. Liq., 2015, 211, 621–627 CrossRef CAS.
- I. Yamagishi and M. Kubota, J. Nucl. Sci. Technol., 1993, 30, 717–719 CrossRef CAS.
- B. A. Lenell and Y. Arai, J. Hazard. Mater., 2017, 321, 335–343 CrossRef CAS PubMed.
- T. L. Yu, S. Q. Liu, M. Xu, J. Peng, J. Q. Li and M. L. Zhai, Radiat. Phys. Chem., 2016, 125, 94–101 CrossRef CAS.
- J. J. Neeway, R. M. Asmussen, A. R. Lawter, M. E. Bowden, W. W. Lukens, D. Sarma, B. J. Riley, M. G. Kanatzidis and N. P. Qafoku, Chem. Mater., 2016, 28, 3976–3983 CrossRef CAS.
- C. D. Williams and P. Carbone, Environ. Sci. Technol., 2016, 50, 3875–3881 CrossRef CAS PubMed.
- S. A. Wang, E. V. Alekseev, D. W. Juan, W. H. Casey, B. L. Phillips, W. Depmeier and T. E. Albrecht-Schmitt, Angew. Chem., Int. Ed., 2010, 49, 1057–1060 CrossRef CAS PubMed.
- S. A. Wang, P. Yu, B. A. Purse, M. J. Orta, J. Diwu, W. H. Casey, B. L. Phillips, E. V. Alekseev, W. Depmeier, D. T. Hobbs and T. E. Albrecht-Schmitt, Adv. Funct. Mater., 2012, 22, 2241–2250 CrossRef CAS.
- D. Banerjee, S. K. Elsaidi, B. Aguila, B. Li, D. Kim, M. J. Schweiger, A. A. Kruger, C. J. Doonan, S. Ma and P. K. Thallapally, Chem.–Eur. J., 2016, 22, 17581–17584 CrossRef CAS PubMed.
- D. Banerjee, W. Xu, Z. Nie, L. E. V. Johnson, C. Coghlan, M. L. Sushko, D. Kim, M. J. Schweiger, A. A. Kruger, C. J. Doonan and P. K. Thallapally, Inorg. Chem., 2016, 55, 8241–8243 CrossRef CAS PubMed.
- D. Sheng, L. Zhu, C. Xu, C. Xiao, Y. Wang, Y. Wang, L. Chen, J. Diwu, J. Chen, Z. Chai, T. E. Albrecht-Schmitt and S. Wang, Environ. Sci. Technol., 2017, 51, 3471–3479 CrossRef CAS PubMed.
- A. V. Desai, B. Manna, A. Karmakar, A. Sahu and S. K. Ghosh, Angew. Chem., Int. Ed., 2016, 55, 7811–7815 CrossRef CAS PubMed.
- H. Hu, B. Jiang, H. Wu, J. Zhang and X. Chen, J. Environ. Radioact., 2016, 165, 39–46 CrossRef CAS PubMed.
- H. Hu, B. Jiang, J. Zhang and X. Chen, RSC Adv., 2015, 5, 104769–104778 RSC.
- P. Rajec, O. Rosskopfova, M. Galambos, V. Fristak, G. Soja, A. Dafnomili, F. Noli, A. Dukic and L. Matovic, J. Radioanal. Nucl. Chem., 2016, 310, 253–261 CrossRef CAS.
- P. Rajec, M. Galambos, M. Dano, O. Rosskopfova, M. Caplovicova, P. Hudec, M. Hornacek, I. Novak, D. Berek and L. Caplovic, J. Radioanal. Nucl. Chem., 2015, 303, 277–286 CrossRef CAS.
- W. R. Wilmarth, G. J. Lumetta, M. E. Johnson, M. R. Poirier, M. C. Thompson, P. C. Suggs and N. P. Machara, Solvent Extr. Ion Exch., 2011, 29, 1–48 CrossRef CAS.
- C. Nash, B. Musall, M. Morse and D. McCabe, Sep. Sci. Technol., 2015, 50, 2881–2887 CAS.
- P. Xiao, D. Han, M. L. Zhai, L. Xu and H. B. Li, J. Hazard. Mater., 2017, 324, 711–723 CrossRef CAS PubMed.
- J.-H. Zu, Y.-Z. Wei, M.-S. Ye, F.-D. Tang, L.-F. He and R.-Q. Liu, Nucl. Sci. Tech., 2015, 26, 69–75 Search PubMed.
- B. Gierczyk, M. Ceglowski and M. Zalas, PLoS One, 2015, 10, e0122891 Search PubMed.
- D. Han, X. Li, J. Peng, L. Xu, J. Li, H. Li and M. Zhai, RSC Adv., 2016, 6, 69052–69059 RSC.
- J. Zu, M. Ye, P. Wang, F. Tang and L. He, RSC Adv., 2016, 6, 18868–18873 RSC.
- W. Luo, A. Inoue, T. Hirajima and K. Sasaki, Appl. Surf. Sci., 2017, 394, 431–439 CrossRef CAS.
- M. Petrova, M. Guigue, L. Venault, P. Moisy and P. Hesemann, Phys. Chem. Chem. Phys., 2015, 17, 10182–10188 RSC.
- B. H. Gu, G. M. Brown, P. V. Bonnesen, L. Y. Liang, B. A. Moyer, R. Ober and S. D. Alexandratos, Environ. Sci. Technol., 2000, 34, 1075–1080 CrossRef CAS.
- D. Jermakowicz-Bartkowiak and B. N. Kolarz, React. Funct. Polym., 2011, 71, 95–103 CrossRef CAS.
- D. Pan, G. Ye, F. Wang and J. Chen, Prog Chem., 2012, 24, 2167–2176 CAS.
- S. Tachimori, S. Suzuki and Y. Sasaki, J. At. Energy Soc. Jpn., 2001, 43, 1235–1241 CrossRef CAS.
- J. Y. Yuan, D. Mecerreyes and M. Antonietti, Prog. Polym. Sci., 2013, 38, 1009–1036 CrossRef CAS.
- J. Y. Yuan and M. Antonietti, Polymer, 2011, 52, 1469–1482 CrossRef CAS.
- L. C. Tome and I. M. Marrucho, Chem. Soc. Rev., 2016, 45, 2785–2824 RSC.
- Y. Jiang, F. Li, G. Ding, Y. Chen, Y. Liu, Y. Hong, P. Liu, X. Qi and L. Ni, J. Colloid Interface Sci., 2015, 455, 125–133 CrossRef CAS PubMed.
- Z. Dong, J. Z. Liu, W. J. Yuan, Y. P. Yi and L. Zhao, Chem. Eng. J., 2016, 283, 504–513 CrossRef CAS.
- J. Chen, Y. Y. Ao, T. R. Lin, X. Yang, J. Peng, W. Huang, J. Q. Li and M. L. Zhai, Polymer, 2016, 87, 73–80 CrossRef CAS.
- S. Milicevic, L. Matovic, D. Petrovic, A. Aukic, V. Milosevic, D. Dokic and K. Kumric, J. Radioanal. Nucl. Chem., 2016, 310, 805–815 CrossRef CAS.
- J. Behnsen and B. Riebe, Appl. Geochem., 2008, 23, 2746–2752 CrossRef CAS.
- R. Konasova, J. J. Dytrtova and V. Kasicka, J. Sep. Sci., 2016, 39, 4429–4438 CrossRef CAS PubMed.
- H. Demey, T. Vincent, M. Ruiz, A. M. Sastre and E. Guibal, Chem. Eng. J., 2014, 244, 576–586 CrossRef CAS.
- D. Liping, S. Yingying, S. Hua, W. Xinting and Z. Xiaobin, J. Hazard. Mater., 2007, 143, 220–225 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: IR, TGA, EA, and XPS of PC2-C12vimBr. See DOI: 10.1039/c8ra00838h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |