Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Discovery and preliminary SAR of 14-aryloxy-andrographolide derivatives as antibacterial agents with immunosuppressant activity†
Feng
Li‡
a,
Xiao-Min
Li‡
b,
Dekuan
Sheng
a,
Shao-Ru
Chen
b,
Xin
Nie
a,
Zhuyun
Liu§
a,
Decai
Wang
*a,
Qi
Zhao
 c,
Yitao
Wang
b,
Ying
Wang
*b and
Guo-Chun
Zhou
c,
Yitao
Wang
b,
Ying
Wang
*b and
Guo-Chun
Zhou
 *a
*a
aSchool of Pharmaceutical Sciences, Nanjing Tech University, Nanjing 211816, PR China. E-mail: gczhou@njtech.edu.cn; dcwang@njtech.edu.cn; Tel: +86-25-58139415
bState Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Avenida da Universidade, Taipa, Macao SAR, PR China. E-mail: emilyywang@umac.mo
cFaculty of Health Sciences, University of Macau, Avenida da Universidade, Taipa, Macao SAR, PR China
First published on 6th March 2018
Abstract
Antibacterials (which restore gut flora balance) and immunosuppressants (which correct immune defects) are two important and effective therapeutic agents for the treatment of inflammatory bowel disease (IBD) in clinical use today. Since the structural skeleton of andrographolide, isolated from Andrographis paniculata, has become known as a natural antibiotic with anti-inflammation and heat-clearing and detoxifying properties, 14-aryloxy andrographolide derivatives have been designed, synthesized, and tested for their antibacterial effects on E. coli, S. aureus, and E. faecalis, which are related to IBD. It has been discovered in this study that the andrographolide skeleton is more selective against E. faecalis, the 14-aryloxy group with basicity is important for antibacterial functions, and the 14-(8′-quinolinyloxy) group is a good pharmacophore with antibacterial activity. In addition, we found that 7b1 and 8b1 are good and selective inhibitors of E. faecalis; two 14β-(8′-quinolinyloxy) andrographolide derivatives, 6b17 and 9b, exhibit good activity against E. coli, S. aureus, and E. faecalis. Likewise and importantly, further exploration of immunosuppressant activity for IBD shows that compound 7b1 is a selective inhibitor of the TNF-α/NF-κB signaling pathway, whereas 8b1 is selectively active against the TLR4/NF-κB signaling pathway; moreover, the compounds 6b17 and 9b are active in inhibiting the IL-6/STAT3, TLR4/NF-κB, and TNF-α/NF-κB signaling pathways. Based on these results, we have further focused on the development of dual function inhibitors of IBD as antibacterial and immunosuppressant agents by structural modification of andrographolide.
1. Introduction
Inflammatory bowel disease (IBD)1 is an autoimmune disease that is characterized by relapsing and remitting chronic inflammation of the gastrointestinal tract; it is believed to be caused by unbalanced host-commensal microbiota and a common immune defect in addition to genetic predisposition.2 Immune responses have been recognized to be related to IBD pathogenesis,3 and a recent study demonstrated that the innate immune system is a major determinant of the serum and tissue profiles of IBD,4 further explaining the existence of regulatory cytokines that are implicated in immune responses related to IBD.5 Especially, overexpression of proinflammatory cytokines, such as interleukin-6 (IL-6), toll-like receptor 4 (TLR4), and tumor necrosis factor-α (TNF-α), is crucial for IBD onset and progression; these proinflammatory cytokines, as inflammatory mediators, are connected with the activation of nuclear factor of κ light polypeptide gene enhancer in B-cells (NF-κB) and signal transducer and activator of transcription 3 (STAT3).5 As NF-κB and STAT3 are known to play central roles in regulating inflammatory responses in patients with IBD, they are recognized as important targets for therapeutic intervention of IBD.6 Gastrointestinal microbiota are believed to be commensal and mutualistic to humans and animals and to have health benefits for humans and animals in some aspects.7 Significant imbalances of gastrointestinal flora are observed in patients with IBD as compared to the case of healthy people; for example, proteobacteria and actinobacteria appear to dominate in people with ulcerative colitis (UC), whereas Enterococcus (E.) faecium and several proteobacteria over-inhabit the gastrointestinal tracts of people with Crohn's disease (CD).8 Although the etiology and pathogenesis of IBD are not fully understood, E. coli and E. faecalis are recognized as pathogens of IBD.9 It is controversial whether S. aureus infection is involved in early lesions or established lesions of IBD; however, S. aureus infection occasionally may occur and complicate IBD during the course of the disease.10 Metabolites from certain members of the gut flora may have causal contributions to disorders such as obesity and colon cancer by changing host signaling pathways.11 In addition, the intrusion of gut flora components into other host compartments can lead to sepsis.11 Therefore, immunosuppressants (which correct immune defects) and antibacterials (which restore flora balance) are two important and effective therapeutic agents to treat IBD in clinical use. Considering this, we envisage that it is a meaningful exploration to discover single compounds with dual functions of antibacterial and immunosuppressant activities.Andrographis paniculata [Burm. F.] Nees, an herb known as a “natural antibiotic”, is commonly used in China, India and Southeast Asia for the treatment of a large variety of illnesses, especially infectious diseases, by reducing inflammation and “heat-clearing and detoxifying”. Andrographolide (1, Scheme 1), a bicyclic diterpenoid lactone, is a major active component isolated from Andrographis paniculata [Burm. F.] Nees;12 its derivatives,13 such as “Chuanhuning”,14,15 have been used in China to treat bacterial and viral infections for many years. Numerous andrographolide derivatives have been designed and synthesized in recent years.16 The antibacterial activities of andrographolide and its derivatives are not potent and show only minimal or marginal direct inhibition of bacterial growth; however, it was discovered that andrographolide inhibits the quorum sensing (QS) system17 of P. aeruginosa and that andrographolide derivatives block biofilm formation18 of P. aeruginosa, indicating that andrographolide and its derivatives can directly inhibit bacterial growth or/and infection by specific mechanisms and modes of action. On the other hand, some evidence has been found that the anti-inflammatory effects of andrographolide are related to regulation of the immune system19 and that andrographolide suppresses TLR4 expression and NF-κB signaling in multiple myeloma cells.20 Multi-targeting andrographolide and its analogs have potential use in the prevention and treatment of metabolic syndrome21 and stroke22via the NF-κB signaling pathway. The andrographolide derivative isoandrographolide significantly inhibited the release of NO and prostaglandin E2 and the production of interleukin-1β (IL-1β) and IL-6 in lipopolysaccharide (LPS)-stimulated J774A.1 macrophage cells in a dose-dependent manner.23 Andrographolide analog AL-1 improved insulin resistance by down-regulating the NF-κB signaling pathway.24 Our previous report25 revealed that some analogs of andrographolide play a role in the attenuation of innate immunity; these were identified as potential immunomodulatory inhibitors of TLR signaling with distinct regulation of NF-κB family members. Furthermore, one compound effectively reduced LPS-induced pulmonary injury in a mouse in vivo study; biochemical results showed that the nucleus translocation of phosphorylated p65 and serum pro-inflammatory cytokines decreased. Based upon these facts, we are interested in developing dual-function analogs of andrographolide as immunosuppressants to inhibit excessive immunity to “self” gastrointestinal microbiota and also as antibacterial agents to control infection by specific “commensal/mutualistic” gut flora; such compounds should be valuable in the treatment of IBD.
In our search for dual-functional 14-aryloxy andrographolide analogs inhibiting both bacterial growth and innate immunity, we launched a panel of screening platforms of these analogs to study their antibacterial activities against E. coli, S. aureus and E. faecalis and preliminary structure–activity relationship (SAR) studies as the first step. Selected active anti-bacterial compounds were explored as immunosuppressants to protect the host from abnormal immune response initiated by intestinal commensal/mutualistic bacteria. In this paper, we describe the synthesis and evaluation of a series of 14-aryloxy andrographolide compounds against E. coli, S. aureus and E. faecalis as well as against the IL-6/STAT3, TLR4/NF-κB and TNF-a/NF-κB signaling pathways of innate immune response and their preliminary SAR. It was discovered that analogs of andrographolide are more sensitive to E. faecalis and that active antibacterial compounds are greatly superior to andrographolide to attenuate innate immune response. Our results revealed that the andrographolide skeleton has immunosuppressant and antibacterial properties and that these analogs of andrographolide can potentially be used to treat IBD.
2. Results and discussion
2.1. Synthesis
The title derivatives were synthesized according to our previously reported synthesis;25,26 the synthesis is outlined in Scheme 1 (ArO groups are depicted in Table 1). Accordingly, 14α-3,19-acetonylidene-protected andrographolide (3a) was prepared by the reaction of andrographolide (1) with 2,2-dimethoxypropane catalyzed by PPTS, followed by a Mitsunobu reaction with acetic acid, which inverted 14α-OH of 3a into 14β-OAc of 4. Full hydrolysis of 4 by TsOH·H2O in MeOH/H2O (4/1) at 40 °C afforded 2 as the 14β-epimer of andrographolide; then, 3,19-protection of 2 afforded 14β-3,19-acetonylidene andrographolide (3b).| Cmpd | ArO | IC50 (μM)c (inhibition rate (%)d) | Cmpd | ArO | IC50 (μM) (inhibition rate (%)) | ||||
|---|---|---|---|---|---|---|---|---|---|
| EC | SA | EF | EC | SA | EF | ||||
| a EC: E. coli; SA: S. aureus; EF: E. faecalis. b “/”represents no inhibition or lower than 10% inhibition rate at 250 μM of tested compound. c IC50 value was generated when the inhibition rate at 250 μM of the tested compound was higher than 80%. d The inhibition rate at 250 μM of the tested compound is indicated in parentheses. | |||||||||
| 5a1 |
|
/ | (14) | 51.2 ± 11.6 | 5a8 |
|
(32) | (20) | (33) |
| 5b1 | (22) | (20) | (56) | 5b8 | / | / | (22) | ||
| 6a1 | / | (10) | 72.2 ± 9.2 | 6a8 | (21) | (17) | 83.3 ± 8.3 | ||
| 6b1 | (17) | (40) | 27.7 ± 3.5 | 6b8 | (21) | / | 89.7 ± 12.0 | ||
| 5a2 |
|
(20) | (20) | 56.7 ± 6.5 | 5a9 |
|
/ | (10) | (45) |
| 5b2 | (11) | (26) | (56) | 5b9 | / | (15) | (42) | ||
| 6a2 | (67) | (65) | 38.7 ± 4.5 | 6a9 | / | / | 38.3 ± 5.9 | ||
| 6b2 | (42) | (25) | 24.4 ± 3.2 | 6b9 | (17) | (40) | 53.3 ± 4.1 | ||
| 5a3 |
|
(21) | (18) | (60) | 5b10 |
|
(20) | (22) | (46) |
| 5b3 | / | (16) | (19) | 6b10 | (39) | (29) | 72.1 ± 9.4 | ||
| 6a3 | (31) | (17) | 112.9 ± 14.3 | 5b11 |
|
/ | / | (39) | |
| 6b3 | (9) | (44) | 140.3 ± 11.9 | 6b11 | / | / | (17) | ||
| 5a4 |
|
/ | / | (46) | 5a12 |
|
/ | / | (49) |
| 5b4 | (19) | (15) | (73) | 5b12 | / | (24) | (52) | ||
| 6a4 | / | / | (44) | 6a12 | / | (14) | 75.6 ± 17.8 | ||
| 6b4 | (60) | (28) | 19.6 ± 2.7 | 6b12 | (12) | (13) | 20.8 ± 4.5 | ||
| 5a5 |
|
(12) | (10) | (73) | 5b13 |
|
/ | / | (17) |
| 5b5 | (18) | (32) | (34) | 6b13 | 15 | 13 | 137.8 ± 5.4 | ||
| 6a5 | (11) | (10) | 50.4 ± 2.7 | 5b14 |
|
/ | / | (47) | |
| 6b5 | (23) | (37) | 21.8 ± 2.5 | 6b14 | (35) | (18) | (50) | ||
| 5b6 |
|
/ | / | 43 | 5a15 |
|
(13) | (11) | 25.5 ± 1.5 |
| 6b6 | (28) | (12) | 15.9 ± 9.0 | 5b15 | / | (33) | 38.4 ± 3.4 | ||
| 5b7 |
|
/ | / | / | 6a15 | (14) | (13) | 56.7 ± 5.5 | |
| 6b7 | / | 26 | 17.8 ± 4.2 | 6b15 | (14) | (41) | 29.3 ± 2.1 | ||
| 5a16 |
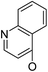
|
/ | (14) | 16.9 ± 5.9 | 6b17 |
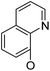
|
21.6 ± 1.7 | 37.5 ± 2.2 | 6.3 ± 1.5 |
| 5b16 | / | / | 15.5 ± 4.2 | 7b1 | (60) | (58) | 8.6 ± 0.7 | ||
| 6a16 | / | (22) | 65.8 ± 13.5 | 7b2 | (24) | (23) | 26.2 ± 2.8 | ||
| 6b16 | / | / | 58.5 ± 4.2 | 8b1 | (34) | (42) | 7.6 ± 0.8 | ||
| 5a17 |
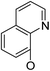
|
(44) | (35) | 10.4 ± 2.5 | 8b2 | (25) | (28) | (71) | |
| 5b17 | (47) | (27) | 19.3 ± 2.3 | 9b | 27.9 ± 1.5 | 27.6 ± 1.8 | 9.1 ± 2.1 | ||
| 6a17 | (70) | (66) | 16.3 ± 2.1 | Ciprofloxacin | 0.31 ± 0.06 | 0.4 ± 0.06 | 2.3 ± 0.6 | ||
Mitsunobu reactions of 3a or 3b with varied aromatic phenols were carried out in general from 0 °C to room temperature in anhydrous THF to afford 14β-aryloxy andrographolide 5b (5b1–5b17) or 14α-aryloxy andrographolide 5a (5a1–5a5, 5a8–5a9, 5a12, 5a15–5a17), respectively, in mild to moderate isolated yield depending on the distinct properties of the phenols. Deprotection of 3,19-acetonylidene from 5a or 5b afforded the corresponding alcohol 6a (6a1–6a5, 6a8–6a9, 6a12, 6a15–6a17) or 6b (6b1–6b17). As we noted before,26 14α-aryloxy derivatives are generally much less stable than their corresponding 14β-isomers, and some 14α-aryloxy compounds were not obtained or were obtained in very low yields. Furthermore, mono-acetylation or mono-silylation of 6b17 produced 7b1 (R = Ac) or 7b2 (R = TBS), which was oxidized into ketone 8b1 or 8b2 by Dess–Martin periodinane (DMP); finally, deprotection of 8b1 or 8b2 afforded 3-keto-19-alcohol product 9b.
2.2. Antibacterial activity and SAR analysis
Numerous bacteriostatic and bactericidal agents have been discovered and developed against bacterial growth and infection; however, bacterial resistance to currently used antibacterial drugs is becoming a major problem in clinical practice, especially hospital-acquired infections by these drug-resistant bacteria.27 To meet the demand of clinical medication for the treatment of digestive diseases caused by gastrointestinal bacterial infection, antibacterial drugs with diverse and novel structural origins are required.28On the basis of reported literature studies stating that andrographolide and some of its derivatives may function as antibiotics, we are pursuing the discovery of more potent and specific andrographolide derivatives for the development of chemotherapeutical agents against bacterial infection, especially for inhibiting gastrointestinal bacterial infection in the development of IBD. The strategy in this study was to submit these synthesized compounds to a preliminary screening test against E. coli, S. aureus and E. faecalis (ciprofloxacin was used as a reference compound; see Table 1). Because these andrographolide analogs did not obviously function as bactericidal agents (data not shown), bacteriostatic screening was employed with gradient concentrations and the percent growth was plotted versus test concentration to afford the IC50 values.29 The current antibacterial activity results are listed in Table 1.
In our study streamline, 5 series of 20 compounds bearing 14-(4′-nitro)phenoxy groups were first tested. It was found that all of these compounds are more selective against E. faecalis than against E. coli and S. aureus. 14β-3,19-Diol compounds (6b1–6b5) are more active against E. faecalis than the corresponding 14β-3,19-acetonylidene protected compounds (5b1–5b5). Furthermore, 14β-(3′-substituted 4′-nitro)phenoxy-3,19-diol compounds 6b4 and 6b5 are more sensitive to E. faecalis than 14β-(2′-substituted 4′-nitro)phenoxy-3,19-diol compounds 6b1, 6b2 and 6b3. It was observed that the activity patterns of the 14-(2′-fluro-4′-nitro)phenoxy and 14-(2′-carboxylate-4′-nitro)phenoxy derivatives are quite similar. The activity differences between 14α-(2′-substituted-4′-nitro)phenoxy groups and 14β-(2′-substituted-4′-nitro)phenoxy groups are variable, leading to an order of 5a1 > 5b1, 5a2 > 5b2, 6b1 > 6a1 and 6b2 > 6a2 when an electron-withdrawing group (EWG) is at the 2′-position but 6b3 < 6a3 when an electron-donating group (EDG) is at the 2′-position. Overall, an EWG at the 2′-position (5a1, 5a2, 6a1, 6a2, 6b1 and 6b2) was more favorable for antibacterial activity than an EDG at the 2′-position (5a3, 6a3 and 6b3). Compounds 6b4 and 6b5 with 14β-(3′-substituted 4′-nitro)phenoxy groups are more active to E. faecalis than the corresponding compounds 6a4 and 6a5 with 14α-(3′-substituted 4′-nitro)phenoxy groups, respectively.
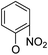
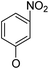
Then, 2′-nitro compounds and 3′-nitro compounds were explored; it was discovered that the 14β-3,19-diol isomers of 14-(2′-nitro)phenoxy analog 6b6 and 14-(3′-nitro)phenoxy analog 6b7 exhibited mild inhibitory activity to E. faecalis and were more active than the corresponding 3,19-acetonylidene protected compounds 5b6 and 5b7. 14α- and 14β-(2′-Carboxyl ester)phenoxy-3,19-diol analogs of 6a8 and 6b8 possessed similar but weak inhibitory activities to E. faecalis, indicating that 14-stereochemistry is not the key to their activity; meanwhile, their 3,19-acetonylidene protected analogs 5a8 and 5b8 were almost inactive. We compared the 14-(2′-carboxyl ester 4′-nitro)phenoxy analogs 6a1 and 6b1 with the 14-(2′-carboxyl ester)phenoxy analogs 6a8 and 6b8; the results suggested that the 4′-nitro group enhanced antibacterial activity against E. faecalis. A similar inhibition tendency was observed for 4′-carboxyl ester phenoxy analogs, where the 4′-carboxyl ester phenoxy-3,19-diol analogs of 6a9 and 6b9 exhibited weak activity against E. faecalis, whereas 14α-isomer 6a9 was slightly more active than 14β-isomer 6b9 against E. faecalis. However, the 4′-carboxyl ester phenoxy analogs 6a9 and 6b9 were more active against E. faecalis than the 2′-carboxyl ester phenoxy counterparts 6a8 and 6b8. Introduction of a cyano (CN) EWG at the 4′-position did not improve the antibacterial activity, and only 14β-isomer 6b10 showed very weak activity against E. faecalis. As above, all of these analogs were very weakly active or inactive against E. coli and S. aureus.
On the other hand, OMe as an EDG occupying the 2′-, 3′- or 4′-position afforded different results. 4′-OMe derivatives 5b11 and 6b11 showed very weak activity against E. faecalis and no antibacterial activity against E. coli and S. aureus; it is possible that 4′-EDG substitution was not as beneficial to antibacterial activity as 4′-EWG substitution, according to the comparison of 4′-OMe derivatives 5b11 and 6b11 with the 4′-CO2Et and 4′-CN derivatives 6a9, 6b9 and 6b10. 14β-(3′-OMe) derivative 6b13 was very weakly active, while its corresponding 14β-(3′-OMe-4′-nitro) analog 6b5 possessed mild inhibitory activity toward E. faecalis, indicating that the 4′-nitro group enhanced the inhibitory activity toward E. faecalis. 14α-2′-OMe-3,19-diol derivative 6a12 was weakly active against E. faecalis and 6b12 (14β) showed mild inhibitory activity toward E. faecalis, suggesting that the 14β-isomer is more stereochemically active for antibacterial activity. Meanwhile, the 4′-nitro group negatively influenced inhibitory activity toward E. faecalis; 6a12 and 6b12 were more active against E. faecalis than corresponding the 4′-nitro analogs 6a3 and 6b3, respectively.
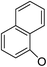
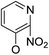
Based on the above data, further exploration to introduce more complex aryl groups at the 14-position were conducted. 14β-(1′-Naphthyloxy) compound 5b14 exhibited very weak inhibitory activity only to E. faecalis, and 14β-(1′-naphthyloxy)diol compound 6b14 expressed very weak activity against all three bacteria. Interestingly, the introduction of a 2′-nitro-pyridinyl-3-oxy group at the 14-position increased the activity of all four derivatives 5a15, 5b15, 6a15 and 6b15 against E. faecalis; 14α-3,19-acetonylidene analog 5a15 was the most active, while 14α-3,19-diol analog 6a15 was the least active. Moreover, 5b15 was more active than its corresponding 2′-nitro phenoxy derivative 5b6, but 6b15 was less active than 2′-nitro phenoxy analog 6b6. Corresponding to the replacement of phenoxy groups with pyridinyloxy groups, substitution of naphthyloxy by quinolinyloxy was subsequently studied. Unlike 14β-(1′-naphthyloxy) analogs 5b14 and 6b14, four analogs of 14-(4′-quinolinyloxy) derivatives 5a16 (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 6.8988), 5b16 (clog
P 6.8988), 5b16 (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 6.8988), 6a16 (clog
P 6.8988), 6a16 (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 4.8578) and 6b16 (clog
P 4.8578) and 6b16 (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 4.8578) expressed antibacterial activity against E. faecalis; however, none of these was active against E. coli, and two 14α-isomers, 5a16 and 6a16, were very weak inhibitors of S. aureus. The clog
P 4.8578) expressed antibacterial activity against E. faecalis; however, none of these was active against E. coli, and two 14α-isomers, 5a16 and 6a16, were very weak inhibitors of S. aureus. The clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of 3,19-protected compounds 5a16 and 5b16 were higher than 5.0 and higher than those of 6a16 and 6b16; 5a16 and 5b16 were more active against E. faecalis than the corresponding 3,19-diol compounds 6a16 and 6b16, respectively. Unlike the activity relationships between 5a15, 5b15, 6a15 and 6b15, the activity variation patterns against E. faecalis of 5a16 and 5b16 were parallel to those of 6a16 and 6b16 and 14β-isomers 5a16 and 6a16 were slightly more active than 14α-isomers 5b16 and 6b16, respectively. These data imply that a positive charge of nitrogen in the 14-aryloxy group under physiological conditions may play a role in antibacterial activity for the substitutions of phenoxy groups by pyridinyloxy groups and of naphthyloxy groups by quinolinyloxy groups.
P values of 3,19-protected compounds 5a16 and 5b16 were higher than 5.0 and higher than those of 6a16 and 6b16; 5a16 and 5b16 were more active against E. faecalis than the corresponding 3,19-diol compounds 6a16 and 6b16, respectively. Unlike the activity relationships between 5a15, 5b15, 6a15 and 6b15, the activity variation patterns against E. faecalis of 5a16 and 5b16 were parallel to those of 6a16 and 6b16 and 14β-isomers 5a16 and 6a16 were slightly more active than 14α-isomers 5b16 and 6b16, respectively. These data imply that a positive charge of nitrogen in the 14-aryloxy group under physiological conditions may play a role in antibacterial activity for the substitutions of phenoxy groups by pyridinyloxy groups and of naphthyloxy groups by quinolinyloxy groups.
Continued exploration in the quinolinyloxy line disclosed that 14-(8′-quinolinyloxy) derivatives exhibited novel antibacterial activity profiles. In contrast to the 14-(4′-quinolinyloxy) derivatives, the 14-(8′-quinolinyloxy) derivatives were active against all three bacteria; they also showed more selective inhibition of E. faecalis than of E. coli or S. aureus, similar to the other series of 14-aryloxy andrographolide derivatives in this work. Specifically, 14α-3,19-acetonylidene compound 5a17 (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 6.77355) was more active against E. faecalis than 14α-3,19-diol compound 6a17 (clog
P 6.77355) was more active against E. faecalis than 14α-3,19-diol compound 6a17 (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 4.73255), while 14β-3,19-acetonylidene compound 5b17 (clog
P 4.73255), while 14β-3,19-acetonylidene compound 5b17 (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 6.77355) was less active against E. faecalis than 14β-3,19-diol compound 6b17 (clog
P 6.77355) was less active against E. faecalis than 14β-3,19-diol compound 6b17 (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 4.73255); this activity pattern against E. faecalis is the same as that of 14-(2′-nitro-pyridinyl-3-oxy) derivatives 5a15, 5b15, 6a15 and 6b15. Importantly, 6b17 expressed good antibacterial activity against all three bacteria (Table 1) and was the most potent inhibitor of E. faecalis in this paper (Table 1). These data illustrate that the 14-(8′-quinolinyloxy) compounds showed superior results against all three bacteria compared to their corresponding 14-(4′-quinolinyloxy) compounds. 19-Acetylated 7b1 (clog
P 4.73255); this activity pattern against E. faecalis is the same as that of 14-(2′-nitro-pyridinyl-3-oxy) derivatives 5a15, 5b15, 6a15 and 6b15. Importantly, 6b17 expressed good antibacterial activity against all three bacteria (Table 1) and was the most potent inhibitor of E. faecalis in this paper (Table 1). These data illustrate that the 14-(8′-quinolinyloxy) compounds showed superior results against all three bacteria compared to their corresponding 14-(4′-quinolinyloxy) compounds. 19-Acetylated 7b1 (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 5.64055) and its 3-ketone 8b1 (clog
P 5.64055) and its 3-ketone 8b1 (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 5.37725) had almost no inhibitory activity toward E. coli and S. aureus but were still very active against E. faecalis. However, 19-silylated 7b2 (clog
P 5.37725) had almost no inhibitory activity toward E. coli and S. aureus but were still very active against E. faecalis. However, 19-silylated 7b2 (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 8.10955) had decreased inhibitory activity toward all three bacteria and possessed only mild activity against E. faecalis, while the antibacterial activity of 3-ketone 8b2 (clog
P 8.10955) had decreased inhibitory activity toward all three bacteria and possessed only mild activity against E. faecalis, while the antibacterial activity of 3-ketone 8b2 (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 7.85675) was very weak against all three bacteria. It is interesting that 3-keto-19-alcohol compound 9b (clog
P 7.85675) was very weak against all three bacteria. It is interesting that 3-keto-19-alcohol compound 9b (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 4.47495) regained the same antibacterial activity against all three bacteria (Table 1) as 6b17, although its inhibitory activity toward E. faecalis was slightly less potent than that of 6b17. By combining these data, it is suggested that in addition to the importance of the andrographolide skeleton, the 14-(8′-quinolinyloxy) group is essential to antibacterial activity; moreover, modifications at the 3- or/and 19-positions are not ignorable, and 19-acetylation greatly reduced inhibitory activity to E. coli and S. aureus while transformation of 3-alcohols into 3-ketones slightly influenced the antibacterial activity.
P 4.47495) regained the same antibacterial activity against all three bacteria (Table 1) as 6b17, although its inhibitory activity toward E. faecalis was slightly less potent than that of 6b17. By combining these data, it is suggested that in addition to the importance of the andrographolide skeleton, the 14-(8′-quinolinyloxy) group is essential to antibacterial activity; moreover, modifications at the 3- or/and 19-positions are not ignorable, and 19-acetylation greatly reduced inhibitory activity to E. coli and S. aureus while transformation of 3-alcohols into 3-ketones slightly influenced the antibacterial activity.
2.3. Inhibition of innate immune response and SAR analysis
Considering that initiation of a dysregulated immune response within the intestinal mucosa by “self” gut flora and/or food is due to immune defects in IBD, reduction of over-immunity is an effective treatment of IBD.3,30 TNF-α, the best studied NF-κB activator, is correlated with transformation of NF-κB by various stimuli from its inactive form to its active form. TLRs are involved in the regulation of innate and adaptive immunity,31 and LPS-activated TLR4 regulates the expression and nucleus translocation of NF-κB.32 IL-6 is a crucial pathogenic mediator in IBD that functions by triggering cellular effects and functions via STAT3 signaling. The proinflammatory IL-6/STAT3-dependent biological network is upregulated in active IBD patients and is considered to be an important pathogenic factor in IBD onset33 and progression.34 Based on these facts, immunosuppressants, corticosteroids and anti-TNF-α antibodies have become commonly used drugs in IBD treatment; they may affect IBD progression by interfering with cellular oxidative stress and cytokine production.35We utilized luciferase reporters bearing the promoter regions of TNF-α/NF-κB, TLR4/NF-κB, and IL-6/STAT3 (ref. 25) to examine the activities of the antibacterial active andrographolide analogs in the regulation of signaling pathways that govern inflammatory response (Table 2). It was noted that these derivatives did not show obvious cytotoxicity to AD-293 cells; DCB-3503 was used as a positive compound36 (Table 2). Our previous study revealed that compound 6b3 is active against the TNF-α/NF-κB and TLR4/NF-κB signaling pathways;25 however, 6b3 does not have antibacterial activity (Table 1). 14α-Compounds 5a17 and 6a17 are active against the IL-6/STAT3 signaling pathway but do not inhibit the NF-κB signaling pathways. 14β-Compounds 5b17 and 6b17 are active to suppress the three signaling pathways (Table 2), suggesting that 3,19-acetonylidene does not obviously affect the inhibition of these signaling pathways; although the feature of 3,19-acetonylidene may contribute to the distinct activities of 5b17 and 6b17 against E. faecalis, 6b17 is also 3 times more active than 5b17 against E. faecalis. Compounds 7b1 and 8b1 possess similar inhibitory activities to E. faecalis; however, 3-alcohol-19-acetylated compound 7b1 shows selective anti-TNF-α/NF-κB activity, while 3-keto-19-acetylated compound 8b1 is selectively active against the TLR4/NF-κB signaling pathway. Like their similarity in inhibitory activity against E. faecalis, E. coli and S. aureus (Table 1), 3-keto-19-alcohol compound 9b1 suppresses three signaling pathways in the same fashion as 6b17 (Table 2). These interesting data suggest that dual-functional analogs of andrographolide can behave as antibacterial and immunosuppressant agents and possibly have synergistic effects in IBD treatment.
| Entry | Cmpd | EC50 (μM) | CC50c (μM) | ||
|---|---|---|---|---|---|
| IL-6/STAT3a | TLR4/NF-κBa | TNF-α/NF-κBb | |||
| a Treated for 16 h. b Treated for 4 h. c Treated for 24 h. d Not applicable. | |||||
| 1 | 1 | >10 | >10 | >10 | >10 |
| 2 | 6b3 (ref. 25) | >10 | 3.49 ± 0.34 | 8.97 ± 1.03 | >10 |
| 3 | 5a17 | 4.71 ± 0.17 | >10 | >10 | >10 |
| 4 | 5b17 | 6.35 ± 0.77 | 2.85 ± 0.07 | 2.45 ± 0.49 | >10 |
| 5 | 6a17 | 5.62 ± 0.13 | >10 | >10 | >10 |
| 6 | 6b17 | 2.41 ± 0.31 | 2.65 ± 0.07 | 2.00 ± 0.28 | >10 |
| 7 | 7b1 | >10 | >10 | 4.91 ± 0.02 | >10 |
| 8 | 8b1 | >10 | 4.84 ± 0.31 | >10 | >10 |
| 9 | 9b | 6.13 ± 0.39 | 5.96 ± 0.03 | 2.03 ± 0.01 | >10 |
| 10 | DCB-3503 (ref. 36) | —d | — | — | 1.83 ± 0.39 |
3. Conclusion
Bacterial infections have long been regarded as a formidable enemy of mankind. Bacterial pathogens dramatically affect the normal function of host cells, including immune response, for their own benefit during infection; eventually, they destroy or damage host cells. As reconstitution of gut flora balance and correction of immune defects are two important strategies for IBD treatment, we attempted to discover dual-functional andrographolide analogs for use as antibacterial and immunosuppressant agents that could synergize ultimate efficacy.In this study, 14-aryloxy andrographolide derivatives were designed, synthesized and tested against the growth of E. coli, S. aureus and E. faecalis. Preliminary data suggest that the andrographolide skeleton is primarily more selective against E. faecalis; modifications at the 3- or/and 19-positions are not ignorable; the 14-aryloxy moiety strongly determines essential efficacy against bacteria growth; 14α- and 14β-isomers exhibit different antibacterial activities; and the substitution positions at the phenoxy groups and the structural features of the substitution groups may alter the inhibitory activities of the compounds. The current data imply that nitrogen-containing 14-aryloxy arenes may play an enhanced role in antibacterial activity upon the substitution of phenoxy groups by pyridinyloxy groups and of naphthyloxy groups by quinolinyloxy groups, suggesting the possibility that the nitrogen of the arene group is charged under physiological conditions. Moreover, it was revealed that the 14-(8′-quinolinyloxy) group is an important pharmacophore for antibacterial activity and that 14β-(8′-quinolinyloxy) andrographolide derivatives exhibit superb inhibitory activity toward E. faecalis. Importantly, andrographolide analogs with antibacterial activities also possess immunosuppressant activities against the IL-6/STAT3, TLR4/NF-κB and/or TNF-α/NF-κB signaling pathways, enabling us to conclude that dual functional andrographolide analogs can play synergistic roles in IBD treatment.
The results of this study suggest that the development of andrographloide analogs as antibacterial and immunosuppressant agents is possible. Further discovery of more potent structural cores, structural tuning of the 14-aryloxy substitutions and modifications at other positions are underway on the basis of these findings.
4. Materials and methods
4.1. General information for chemistry
1H and 13C NMR spectra were recorded on a Bruker AV-400 spectrometer at 400 and 100 MHz, respectively, in CD3Cl, CD3OD, (CD3)2SO and C6D6 as indicated. Coupling constants (J) are expressed in hertz (Hz). Chemical shifts (δ) of NMR are reported in parts per million (ppm) units relative to the solvent. High resolution ESI-MS spectra were recorded on an Applied Biosystems Q-STAR Elite ESI-LC-MS/MS mass spectrometer. Unless otherwise noted, materials were obtained from commercial suppliers and were used without further purification. Melting points were measured using a YRT-3 melting point apparatus (Shanghai, China) and were uncorrected.4.2. The preparation and characterization of 2, 3a, 3b, 4 and 5a1, 5b1, 6a1 and 6b1 were modified from our previous papers25,26
General method for synthesis of 5a and 5b. Under N2 atmosphere and at 0 °C, 0.5 g (1.28 mmol) 3a or 3b, assorted phenols (1.92 mmol) and 0.5 g PPh3 (1.92 mmol) were dissolved in 8.0 mL anhydrous THF. To the above mixture, a solution of 0.39 g (1.92 mmol) diisopropyl azodiformate (DIAD) in 2.0 mL anhydrous THF was added dropwise in 5 min, and the reaction progress was monitored by TLC. After the reaction was complete, the reaction mixture was treated with ethyl acetate and sol. sat. NaHCO3. The organic phase was washed with brine and dried over anhydrous Na2SO4. The residue was filtered, dried and subjected to silica gel chromatography using petroleum ether and ethyl acetate to yield product 5b or 5a.
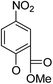
(14α)-(2′-Carboxy methyl ester-4′-nitro)phenoxy-3,19-acetonylidene andrographolide (5a1). White solid; mp 77.3 °C to 78.4 °C; 51% yield; 1H NMR (400 MHz, C6D6) δ 8.52 (d, J = 2.9 Hz, 1H), 7.75 (dd, J = 9.1, 2.9 Hz, 1H), 7.27–7.22 (m, 1H), 5.79 (d, J = 9.1 Hz, 1H), 4.87 (s, 1H), 4.81 (d, J = 5.7 Hz, 1H), 4.61 (s, 1H), 3.79 (d, J = 11.6 Hz, 1H), 3.73 (dd, J = 10.7, 6.1 Hz, 1H), 3.66 (dd, J = 10.7, 2.6 Hz, 1H), 3.45 (dd, J = 7.4, 3.3 Hz, 1H), 3.39 (s, 3H), 3.06 (d, J = 11.6 Hz, 1H), 2.51 (m, 1H), 2.27–2.16 (m, 2H), 1.89–1.79 (m, 1H), 1.79–1.69 (m, 1H), 1.62–1.47 (m, 3H), 1.39 (s, 3H), 1.31 (s, 3H), 1.09 (s, 3H), 1.05–0.96 (m, 2H), 0.94 (dd, J = 6.4, 5.0 Hz, 1H), 0.89 (s, 3H), 0.86–0.82 (m, 1H); 13C NMR (101 MHz, (CD3)2SO) δ 169.2, 164.5, 160.7, 151.6, 148.1, 141.3, 129.3, 127.2, 124.8, 122.1, 116.0, 109.0, 98.6, 76.2, 73.4, 70.9, 63.2, 55.3, 53.0, 51.8, 38.3, 37.6, 37.4, 34.4, 27.9, 26.2, 25.6, 25.2, 25.1, 23.0, 16.0; ESI-HRMS: m/z 592.2525 [M + Na]+, calcd for C31H39NNaO9, 592.2523.
(14β)-(2′-Carboxy methyl ester-4′-nitro)phenoxy-3,19-acetonylidene andrographolide (5b1). White solid; mp 104 °C to 106 °C; 82% yield; 1H NMR (400 MHz, C6D6) δ 8.57 (d, J = 2.9 Hz, 1H), 7.74 (dd, J = 9.1, 2.9 Hz, 1H), 7.25 (t, J = 6.2 Hz, 1H), 5.71 (d, J = 9.1 Hz, 1H), 4.86 (s, 1H), 4.80 (s, 1H), 4.42 (s, 1H), 3.78 (d, J = 11.5 Hz, 1H), 3.62 (m, 2H), 3.43 (dd, J = 7.1, 3.2 Hz, 1H), 3.35 (s, 3H), 3.06 (d, J = 11.5 Hz, 1H), 2.41 (dd, J = 16.5, 5.5 Hz, 1H), 2.26–2.14 (m, 2H), 1.78–1.67 (m, 2H), 1.59 (d, J = 10.8 Hz, 1H), 1.53–1.43 (m, 2H), 1.38 (s, 3H), 1.33 (s, 3H), 1.08 (s, 3H), 1.00 (m, 2H), 0.94 (d, J = 2.1 Hz, 1H), 0.91 (s, 3H), 0.87–0.83 (m, 1H); 13C NMR (101 MHz, C6D6) δ 167.90, 163.40, 160.05, 151.64, 148.46, 141.78, 128.31, 127.80, 124.20, 122.34, 114.01, 107.81, 99.48, 74.86, 73.23, 69.52, 64.21, 55.71, 51.99, 50.77, 38.37, 38.25, 37.61, 33.40, 26.17, 26.10, 25.89, 25.06, 24.41, 23.20, 16.70; ESI-HRMS: m/z 592.2523 [M + Na]+, calcd for C31H39NNaO9, 592.2515.
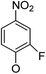
(14α)-(2′-Fluoro-4′-nitro)phenoxy-3,19-acetonylidene andrographolide (5a2). White solid; mp 147 °C to 149 °C; 47% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.26 (dd, J = 10.9, 2.7 Hz, 1H), 8.16 (m, 1H), 7.48 (t, J = 8.8 Hz, 1H), 6.97 (t, J = 6.3 Hz, 1H), 6.07 (d, J = 5.2 Hz, 1H), 4.83 (s, 1H), 4.74 (dd, J = 11.1, 5.5 Hz, 1H), 4.59 (s, 1H), 4.45 (dd, J = 11.1, 1.0 Hz, 1H), 3.82 (d, J = 11.6 Hz, 1H), 3.36 (dd, J = 9.2, 4.2 Hz, 1H), 3.07 (d, J = 11.6 Hz, 1H), 2.47–2.36 (m, 2H), 2.33 (dd, J = 10.3, 2.8 Hz, 1H), 2.03–1.91 (m, 2H), 1.89–1.77 (m, 1H), 1.69–1.55 (m, 2H), 1.54–1.45 (m, 1H), 1.28 (s, 3H), 1.25–1.20 (m, 2H), 1.23 (s, 3H), 1.10 (s, 3H), 0.86–0.80 (m, 1H), 0.76 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 168.64, 152.23, 151.34, 150.40, 150.30, 149.76, 147.60, 141.15, 141.07, 124.19, 121.28, 121.25, 115.75, 112.68, 112.45, 108.54, 98.15, 75.65, 73.01, 70.35, 62.74, 54.93, 51.35, 37.85, 37.14, 36.96, 33.83, 27.35, 25.73, 25.13, 24.96, 24.69, 22.59, 15.59; ESI-HRMS: m/z 552.2374 [M + Na]+, calcd for C29H36FNNaO7, 552.2368.
(14β)-(2′-Fluoro-4′-nitro)phenoxy-3,19-acetonylidene andrographolide (5b2). White solid; mp 114 °C to 117 °C; 72% yield; 1H NMR (400 MHz, C6D6) δ 7.57 (dd, J = 10.6, 2.6 Hz, 1H), 7.47 (m, 1H), 7.24–7.19 (m, 1H), 5.76 (m, 1H), 4.84 (d, J = 1.8 Hz, 1H), 4.71 (d, J = 5.1 Hz, 1H), 4.40 (d, J = 1.7 Hz, 1H), 3.78 (d, J = 11.6 Hz, 1H), 3.64–3.51 (m, 2H), 3.44 (dd, J = 7.0, 3.5 Hz, 1H), 3.06 (d, J = 11.5 Hz, 1H), 2.27–2.09 (m, 3H), 1.80–1.61 (m, 2H), 1.56–1.44 (m, 2H), 1.40 (s, 3H), 1.34 (s, 3H), 1.27–1.35 (m, 2H), 1.06 (s, 3H), 0.97 (td, J = 14.7, 13.8, 5.4 Hz, 2H), 0.89 (s, 3H), 0.87–0.90 (m, 1H); 13C NMR (101 MHz, C6D6) δ 167.99, 167.81, 153.15, 151.64, 151.49, 150.64, 149.80, 149.70, 148.26, 142.41, 142.34, 124.26, 120.68, 115.14, 113.08, 112.85, 107.85, 99.62, 74.78, 73.52, 69.61, 64.32, 55.55, 50.74, 38.40, 38.35, 37.62, 33.33, 26.12, 25.93, 25.12, 24.35, 23.24, 16.78; ESI-HRMS: m/z 552.2374 [M + Na]+, calcd for C29H36FNNaO7, 552.2418.
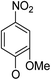
(14α)-(2′-Methoxy-4′-nitro)phenoxy-3,19-acetonylidene andrographolide (5a3)25,26. Pale yellow solid; mp 121 °C to 123 °C; 46% yield; 1HNMR (400 MHz, C6D6) δ 7.56 (dd, J = 8.8, 2.4 Hz, 1H), 7.45 (d, J = 2.5 Hz, 1H), 7.14–7.10 (m, 1H), 6.07–5.99 (m, 1H), 4.90 (s, 1H), 4.82 (s, 1H), 4.61 (s, 1H), 3.85–3.76 (m, 2H), 3.71 (dd, J = 10.8, 5.9 Hz, 1H), 3.46 (dd, J = 7.5, 3.7 Hz, 1H), 3.11–3.03 (m, 4H), 2.41–2.24 (m, 1H), 2.22–2.05 (m, 2H), 1.85 (m, 1H), 1.79–1.67 (m, 1H), 1.62–1.52 (m, 1H), 1.62–1.52 (m, 1H), 1.52–1.46 (m, 1H), 1.41 (s, 3H), 1.36 (s, 3H), 1.32 (m, 1H), 1.08 (s, 3H), 1.04–0.89 (m, 3H), 0.81 (s, 3H); 13C NMR (101 MHz, C6D6) δ 168.17, 151.11, 150.60, 147.17, 143.51, 124.80, 116.98, 115.61, 109.33, 107.40, 99.44, 75.17, 73.37, 69.90, 64.12, 55.82, 55.21, 51.25, 38.16, 38.04, 37.64, 34.06, 26.32, 26.01, 25.32, 25.20, 24.69, 23.16, 16.39; ESI-HRMS: m/z 564.2569 [M + Na]+, calcd for C30H39NNaO8, 564.2573.
(14β)-(2′-Methoxy-4′-nitro)phenoxy-3,19-acetonylidene andrographolide (5b3)25,26. Pale yellow solid; mp 129 °C to 131 °C; 51% yield; 1H NMR (400 MHz, C6D6) δ 7.56 (dd, J = 8.8, 2.6 Hz, 1H), 7.46 (d, J = 2.6 Hz, 1H), 7.18 (d, J = 1.5 Hz, 1H), 5.95 (d, J = 8.8 Hz, 1H), 4.90–4.86 (m, 1H), 4.85–4.80 (m, 1H), 4.39 (dd, J = 1.9, 1.0 Hz, 1H), 3.82–3.74 (m, 2H), 3.63–3.57 (m, 2H), 3.41 (dd, J = 7.3, 3.6 Hz, 1H), 3.10–3.02 (m, 1H), 3.06 (s, 3H), 2.19–2.10 (m, 3H), 1.77–1.60 (m, 2H), 1.52–1.44 (m, 1H), 1.44–1.37 (m, 1H), 1.40 (s, 3H), 1.36 (s, 3H), 1.34–1.25 (m, 2H), 1.06 (s, 3H), 0.99 (m, 1H), 0.94–0.80 (m, 3H), 0.85 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 168.12, 150.83, 150.47, 150.31, 148.06, 143.21, 128.02, 127.78, 127.54, 124.91, 116.92, 114.96, 107.68, 107.11, 99.35, 74.78, 72.86, 69.71, 64.04, 55.41, 55.12, 50.72, 38.16, 38.06, 37.43, 33.20, 26.08, 25.82, 25.55, 24.99, 24.32, 23.04, 16.44; ESI-HRMS: m/z 564.2584 [M + Na]+, calcd for C30H39NNaO8, 564.2573.
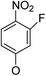
(14α)-(3′-Fluoro-4′-nitro)phenoxy-3,19-acetonylidene andrographolide (5a4). White solid; mp 137 °C to 139 °C; 51% yield; 1H NMR (400 MHz, C6D6) δ 7.46 (m, 1H), 6.34–6.26 (m, 1H), 6.05 (t, J = 7.9 Hz, 2H), 5.42 (s, 1H), 5.12 (m, 2H), 3.86 (d, J = 11.5 Hz, 1H), 3.69 (m, 2H), 3.50 (dd, J = 7.0, 3.2 Hz, 1H), 3.09 (d, J = 11.5 Hz, 1H), 2.32–2.22 (m, 2H), 2.03–1.91 (m, 2H), 1.86–1.64 (m, 4H), 1.43 (s, 3H), 1.41 (s, 3H), 1.50–1.32 (m, 2H), 1.11–1.06 (m, 2H), 1.04 (s, 3H), 1.01 (s, 3H); 13C NMR (101 MHz, C6D6) δ 171.37, 163.02, 162.91, 158.53, 155.89, 147.16, 145.92, 133.57, 131.56, 131.49, 128.03, 127.79, 110.42, 110.39, 109.14, 104.87, 104.63, 99.58, 74.86, 73.48, 69.98, 64.29, 52.30, 51.40, 38.39, 38.28, 38.16, 33.88, 30.73, 26.12, 25.86, 25.14, 24.56, 23.45, 16.99; ESI-HRMS: m/z 552.2365 [M + Na]+, calcd for C29H36FNNaO7, 552.2374.
(14β)-(3′-Fluoro-4′-nitro)phenoxy-3,19-acetonylidene andrographolide (5b4). White solid; mp 184 °C to 187 °C; 52% yield; 1H NMR (400 MHz, C6D6) δ 7.51 (t, J = 8.9 Hz, 1H), 7.20 (d, J = 1.6 Hz, 1H), 5.89–5.77 (m, 2H), 4.86 (d, J = 1.1 Hz, 1H), 4.54–4.48 (m, 1H), 4.41 (d, J = 0.7 Hz, 1H), 3.76 (d, J = 11.5 Hz, 1H), 3.50 (d, J = 3.8 Hz, 2H), 3.43 (dd, J = 6.8, 3.5 Hz, 1H), 3.06 (d, J = 11.5 Hz, 1H), 2.23–2.06 (m, 3H), 1.76–1.63 (m, 2H), 1.48–1.35 (m, 2H), 1.38 (s, 3H), 1.33 (s, 3H), 1.34–1.27 (m, 2H), 1.05 (d, J = 4.6 Hz, 3H), 1.04–0.95 (m, 1H), 0.95–0.87 (m, 2H), 0.89 (s, 3H); 13C NMR (101 MHz, C6D6) δ 167.85, 167.84, 161.50, 161.50, 161.40, 161.40, 158.59, 158.59, 155.95, 155.95, 151.01, 151.01, 148.11, 148.11, 132.01, 131.94, 131.94, 124.20, 124.20, 111.06, 111.06, 111.03, 111.03, 107.94, 107.94, 104.20, 104.20, 103.96, 103.96, 99.72, 99.72, 74.57, 74.57, 72.19, 72.19, 69.41, 69.41, 64.33, 64.33, 55.60, 55.60, 50.79, 50.79, 38.40, 38.40, 37.67, 37.67, 33.47, 33.47, 25.97, 25.97, 25.93, 25.93, 25.07, 25.07, 24.25, 24.25, 23.21, 23.21, 16.82, 16.82; ESI-HRMS: m/z 552.2415 [M + Na]+, calcd for C29H36FNNaO7, 552.2374.
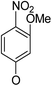
(14α)-(3′-Methoxy-4′-nitro)phenoxy-3,19-acetonylidene andrographolide (5a5). White solid; mp 127 °C to 129 °C; 41% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.00 (d, J = 9.1 Hz, 1H), 6.98 (dd, J = 10.0, 3.9 Hz, 1H), 6.86 (d, J = 2.4 Hz, 1H), 6.74 (dd, J = 9.1, 2.5 Hz, 1H), 6.03 (d, J = 5.2 Hz, 1H), 4.87 (s, 1H), 4.71 (dd, J = 11.0, 5.5 Hz, 1H), 4.61 (s, 1H), 4.36 (dd, J = 11.0, 0.9 Hz, 1H), 3.93 (s, 3H), 3.83 (d, J = 11.7 Hz, 1H), 3.39–3.35 (m, 1H), 3.08 (d, J = 11.6 Hz, 1H), 2.47–2.30 (m, 3H), 2.02–1.92 (m, 2H), 1.88–1.78 (m, 1H), 1.69–1.57 (m, 2H), 1.53–1.44 (m, 1H), 1.29 (s, 3H), 1.23 (s, 3H), 1.21–1.25 (m, 2H), 1.21–1.16 (m, 1H), 1.10 (s, 3H), 0.77 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 168.84, 161.72, 154.94, 150.46, 147.60, 133.08, 127.88, 124.70, 108.46, 106.86, 101.33, 98.16, 75.67, 71.68, 70.69, 62.73, 56.88, 54.95, 51.35, 37.92, 37.14, 36.96, 33.87, 27.38, 25.73, 25.14, 24.97, 24.72, 22.58, 15.60; ESI-HRMS: m/z 564.2569 [M + Na]+, calcd for C30H39NNaO8, 564.2573.
(14β)-(3′-Methoxy-4′-nitro)phenoxy-3,19-acetonylidene andrographolide (5b5). White solid; mp 169 °C to 171 °C; 75% yield; 1H NMR (400 MHz, C6D6) δ 7.60 (d, J = 9.0 Hz, 1H), 7.22 (m, 1H), 5.98 (d, J = 2.4 Hz, 1H), 5.54 (dd, J = 9.0, 2.5 Hz, 1H), 4.87 (d, J = 0.9 Hz, 1H), 4.76 (d, J = 5.4 Hz, 1H), 4.45 (s, 1H), 3.80–3.69 (m, 2H), 3.63 (m, 1H), 3.41 (m, 1H), 3.09 (s, 3H), 3.06 (m, 1H), 2.30–2.13 (m, 3H), 1.70 (m, 2H), 1.52 (t, J = 8.6 Hz, 1H), 1.45–1.36 (m, 1H), 1.38 (s, 3H), 1.35–1.28 (m, 2H), 1.32 (s, 3H), 1.06 (s, 3H), 1.04–0.96 (m, 1H), 0.95–0.90 (m, 1H), 0.98–0.82 (m, 1H), 0.87 (s, 3H); 13C NMR (101 MHz, C6D6) δ 168.23, 160.90, 155.43, 150.53, 148.07, 134.84, 127.80, 124.84, 107.95, 104.43, 101.58, 99.50, 74.86, 71.75, 69.87, 64.15, 55.72, 55.64, 51.01, 38.39, 38.21, 37.65, 33.57, 26.20, 25.92, 25.80, 25.08, 24.45, 23.16, 16.60; ESI-HRMS: m/z 564.2569 [M + Na]+, calcd for C30H39NNaO8, 564.2573.
(14β)-(2′-Nitro)phenoxy-3,19-acetonylidene andrographolide (5b6). White solid; mp 146 °C to 148 °C; 72% yield; 1H NMR (400 MHz, C6D6) δ 7.32–7.25 (m, 1H), 7.24–7.18 (m, 2H), 6.77–6.65 (m, 1H), 6.42–6.30 (m, 1H), 6.06–5.92 (m, 1H), 4.93–4.80 (m, 2H), 4.42–4.35 (m, 1H), 3.82 (d, J = 11.5 Hz, 1H), 3.71–3.55 (m, 2H), 3.45 (dd, J = 7.7, 3.3 Hz, 1H), 3.08 (d, J = 11.5 Hz, 1H), 2.40–2.29 (m, 1H), 2.26–2.11 (m, 2H), 1.86–1.67 (m, 2H), 1.62–1.47 (m, 3H), 1.42 (s, 3H), 1.36 (s, 3H), 1.39–1.31 (m, 1H), 1.12 (s, 3H), 1.05–0.91 (m, 3H), 0.87 (s, 3H); 13C NMR (101 MHz, C6D6) δ 168.02, 168.02, 161.21, 151.64, 149.29, 149.29, 148.41, 141.55, 141.55, 133.24, 133.24, 128.28, 128.22, 128.16, 128.04, 127.92, 127.80, 127.68, 125.77, 124.31, 121.71, 121.71, 115.78, 107.77, 99.27, 99.26, 99.26, 75.38, 73.27, 69.64, 64.11, 59.13, 55.72, 51.04, 38.39, 38.11, 37.63, 37.57, 33.61, 26.66, 25.93, 25.22, 24.72, 23.19, 16.49; ESI-HRMS: m/z 534.2467 [M + Na]+, calcd for C29H37NNaO7, 534.2468.
(14β)-(3′-Nitro)phenoxy-3,19-acetonylidene andrographolide (5b7). White solid; mp 163 °C to 165 °C; 32% yield; 1H NMR (400 MHz, C6D6) δ 7.51 (d, J = 7.1 Hz, 1H), 7.31 (d, J = 2.1 Hz, 1H), 7.20 (d, J = 6.8 Hz, 2H), 6.62 (t, J = 8.2 Hz, 1H), 6.52 (dd, J = 8.3, 1.8 Hz, 1H), 4.85 (s, 1H), 4.62 (d, J = 5.1 Hz, 1H), 4.43 (s, 1H), 3.77 (d, J = 11.6 Hz, 1H), 3.64 (dd, J = 10.8, 1.8 Hz, 1H), 3.58 (dd, J = 10.8, 5.5 Hz, 1H), 3.41 (dd, J = 7.1, 3.5 Hz, 1H), 3.06 (d, J = 11.5 Hz, 1H), 2.24–2.05 (m, 3H), 1.68 (m, 2H), 1.52–1.41 (m, 2H), 1.39 (s, 3H), 1.34 (s, 3H), 1.30 (m, 2H), 1.07 (s, 3H), 1.04–0.94 (m, 1H), 0.94–0.89 (m, 1H), 0.87 (d, J = 5.9 Hz, 1H), 0.84 (s, 3H); 13C NMR (101 MHz, C6D6) δ 168.13, 157.06, 150.18, 149.49, 147.85, 130.36, 128.16, 127.92, 127.80, 127.68, 124.91, 122.22, 116.85, 109.17, 107.97, 99.46, 74.86, 71.83, 69.76, 64.15, 55.58, 50.96, 38.36, 38.22, 37.61, 33.51, 26.20, 25.90, 25.79, 25.07, 24.38, 23.14, 16.58; ESI-HRMS: m/z 534.2463 [M + Na]+, calcd for C29H37NNaO7, 534.2468.
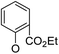
(14α)-(2′-Carboxy ethyl ester)phenoxy-3,19-acetonylidene andrographolide (5a8). White solid; mp 122 °C to 124 °C; 60% yield; 1H NMR (400 MHz, C6D6) δ 7.78 (dd, J = 7.7, 1.8 Hz, 1H), 7.21 (t, J = 6.0 Hz, 1H), 6.98–6.92 (m, 1H), 6.71 (t, J = 7.6 Hz, 1H), 6.38 (d, J = 8.2 Hz, 1H), 5.11 (s, 1H), 4.84 (s, 1H), 4.57 (s, 1H), 4.19–4.11 (m, 2H), 4.10–4.06 (m, 1H), 3.83–3.75 (m, 2H), 3.44 (dd, J = 7.9, 3.7 Hz, 1H), 3.07 (d, J = 11.5 Hz, 1H), 2.22–2.13 (m, 3H), 1.84 (m, 1H), 1.78–1.67 (m, 1H), 1.60–1.51 (m, 1H), 1.51–1.43 (m, 2H), 1.42 (s, 3H), 1.35 (s, 3H), 1.31 (d, J = 2.5 Hz, 1H), 1.09 (s, 3H), 1.06 (t, J = 7.1 Hz, 3H), 0.99–0.88 (m, 3H), 0.80 (s, 3H); 13C NMR (101 MHz, C6D6) δ 168.70, 165.60, 156.04, 149.51, 147.00, 132.85, 132.04, 125.51, 124.38, 122.50, 117.83, 109.13, 99.26, 75.48, 73.78, 70.37, 64.02, 60.96, 55.62, 51.54, 38.23, 38.07, 37.66, 34.21, 26.70, 26.05, 25.29, 25.24, 24.89, 23.15, 16.22, 14.10; ESI-HRMS: m/z 561.2824 [M + Na]+ calcd for C32H42NaO7, 561.2828.
(14β)-(2′-Carboxy ethyl ester)phenoxy-3,19-acetonylidene andrographolide (5b8). White solid; mp 168 °C to 170 °C; 78% yield; 1H NMR (400 MHz, C6D6) δ 7.80 (dd, J = 7.7, 1.8 Hz, 1H), 7.24–7.18 (m, 1H), 6.98–6.92 (m, 1H), 6.69 (m, 1H), 6.28 (d, J = 8.2 Hz, 1H), 5.04 (d, J = 5.4 Hz, 1H), 4.84 (d, J = 1.1 Hz, 1H), 4.44 (s, 1H), 4.19–4.04 (m, 2H), 4.01 (dd, J = 10.6, 1.4 Hz, 1H), 3.82 (d, J = 11.5 Hz, 1H), 3.68 (dd, J = 10.6, 5.5 Hz, 1H), 3.42 (dd, J = 7.6, 3.7 Hz, 1H), 3.08 (d, J = 11.5 Hz, 1H), 2.24–2.05 (m, 3H), 1.80–1.65 (m, 2H), 1.59 (d, J = 8.3 Hz, 1H), 1.53–1.43 (m, 1H), 1.41 (s, 3H), 1.40–1.31 (m, 2H), 1.37 (s, 3H), 1.11 (s, 3H), 1.04 (t, 3H), 1.00–0.88 (m, 3H), 0.85 (s, 3H); 13C NMR (101 MHz, C6D6) δ 168.70, 165.65, 155.99, 149.74, 148.21, 132.91, 132.15, 128.16, 127.92, 127.80, 127.68, 125.81, 123.92, 122.16, 116.61, 107.89, 99.29, 75.26, 73.23, 70.29, 64.14, 60.97, 55.83, 50.97, 38.39, 38.15, 37.64, 33.49, 26.55, 25.91, 25.87, 25.20, 24.64, 23.22, 16.51, 14.12; ESI-HRMS: m/z 561.2829 [M + Na]+, calcd for C32H42NaO7, 561.2828.
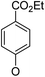
(14α)-(4′-Carboxy ethyl ester)phenoxy-3,19-acetonylidene andrographolide (5a9). White solid; mp 67.8 °C to 73.7 °C; 30% yield; 1H NMR (400 MHz, C6D6) δ 8.15–8.08 (m, 2H), 6.49 (d, J = 8.7 Hz, 2H), 4.85 (s, 2H), 4.65 (s, 1H), 4.16 (q, J = 7.1 Hz, 2H), 3.82–3.72 (m, 3H), 3.44 (dd, J = 7.8, 3.8 Hz, 1H), 3.06 (d, J = 11.6 Hz, 1H), 2.39–2.28 (m, 1H), 2.21–2.12 (m, 2H), 1.86–1.69 (m, 2H), 1.54 (t, J = 10.1 Hz, 2H), 1.43–1.38 (m, 1H), 1.42 (s, 3H), 1.34 (s, 3H), 1.33–1.29 (m, 1H), 1.09 (s, 3H), 1.05 (t, J = 7.1 Hz, 3H), 1.00–0.93 (m, 2H), 0.85 (t, J = 6.1 Hz, 2H), 0.75 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 169.4, 165.7, 161.0, 150.8, 148.1, 131.9, 125.3, 123.7, 115.8, 109.0, 98.6, 76.1, 71.8, 71.3, 63.2, 60.9, 55.4, 51.8, 38.3, 37.6, 37.4, 34.3, 27.8, 26.2, 25.6, 25.4, 25.2, 23.1, 16.1, 14.7; ESI-HRMS: m/z 561.2822 [M + Na]+, calcd for C32H42NaO7, 561.2828.
(14β)-(4′-Carboxy ethyl ester)phenoxy-3,19-acetonylidene andrographolide (5b9). White solid; mp 122 °C to 125 °C; 45% yield; 1H NMR (400 MHz, C6D6) δ 8.14–8.09 (m, 2H), 7.23–7.18 (m, 1H), 6.45 (d, J = 8.8 Hz, 2H), 4.86–4.78 (m, 2H), 4.44 (s, 1H), 4.16 (q, J = 7.1 Hz, 2H), 3.80–3.71 (m, 2H), 3.68 (t, J = 7.2 Hz, 1H), 3.38 (dd, J = 7.4, 3.6 Hz, 1H), 3.05 (d, J = 11.5 Hz, 1H), 2.16 (m, 3H), 1.75–1.63 (m, 2H), 1.52 (d, J = 10.2 Hz, 1H), 1.46–1.36 (m, 1H), 1.37 (s, 3H), 1.35–1.26 (m, 2H), 1.33 (s, 3H), 1.06 (s, 3H), 1.07–1.01 (m, 3H), 1.01–0.83 (m, 3H), 0.81 (s, 3H); 13C NMR (101 MHz, C6D6) δ 168.29, 165.52, 160.29, 149.91, 147.98, 132.07 (2C), 125.26, 124.92, 115.12 (2C), 107.85, 75.00, 71.48, 70.01, 64.14, 60.70, 55.66, 51.07, 38.37, 38.18, 37.64, 33.49, 26.32, 25.91, 25.68, 25.11, 24.47, 23.17, 16.49, 14.21; ESI-HRMS: m/z 561.2825 [M + Na]+, calcd for C32H42NaO7, 561.2828.
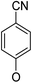
(14β)-(4′-Cyano)phenoxy-3,19-acetonylidene andrographolide (5b10). White solid; mp 177 °C to 179 °C; 42% yield; 1H NMR (400 MHz, C6D6) δ 7.22–7.18 (m, 1H), 6.97–6.91 (m, 2H), 6.12–6.07 (m, 2H), 4.85 (d, J = 1.1 Hz, 1H), 4.66 (s, 1H), 4.42 (s, 1H), 3.77 (d, J = 11.5 Hz, 1H), 3.63–3.56 (m, 2H), 3.43 (dd, J = 7.1, 3.6 Hz, 1H), 3.06 (d, J = 11.5 Hz, 1H), 2.23–2.04 (m, 3H), 1.74–1.64 (m, 2H), 1.51–1.38 (m, 2H), 1.41 (s, 3H), 1.35 (s, 3H), 1.34–1.24 (m, 2H), 1.07 (s, 1H), 0.98 (m, 1H), 0.97 (m, 1H), 0.92–0.84 (m, 2H), 0.83 (s, 3H); 13C NMR (101 MHz, C6D6) δ 168.06, 159.36, 150.35, 148.00, 134.09, 124.74, 118.44, 115.68, 107.84, 106.04, 99.53, 74.78, 71.45, 69.64, 64.18, 55.54, 50.88, 38.31, 38.25, 37.60, 33.44, 26.12, 25.91, 25.72, 25.08, 24.33, 23.13, 16.59; ESI-HRMS: m/z 514.2566 [M + Na]+ calcd for C30H37NNaO5, 514.2569.
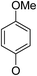
(14β)-(4′-Methoxy)phenoxy-3,19-acetonylidene andrographolide (5b11). White solid; mp 149 °C to 151 °C; 48% yield; 1H NMR (400 MHz, C6D6) δ 7.20–7.17 (m, 1H), 6.69–6.62 (m, 2H), 6.57–6.51 (m, 2H), 4.86–4.78 (m, 2H), 4.43 (q, J = 1.4 Hz, 1H), 4.01 (dd, J = 10.5, 1.8 Hz, 1H), 3.82 (d, J = 11.5 Hz, 1H), 3.75–3.68 (m, 1H), 3.41 (m, 1H), 3.30 (s, 3H), 3.08 (d, J = 11.5 Hz, 1H), 2.21–2.05 (m, 3H), 1.82–1.64 (m, 2H), 1.55–1.43 (m, 2H), 1.41 (s, 3H), 1.38–1.29 (m, 2H), 1.37 (s, 3H), 1.08 (s, 3H), 1.02–0.90 (m, 2H), 0.89–0.79 (m, 1H), 0.83 (s, 3H); 13C NMR (101 MHz, C6D6) δ 168.82, 155.47, 150.74, 149.23, 148.08, 126.05, 117.95, 117.95, 115.12, 115.12, 107.93, 99.28, 75.34, 72.90, 70.43, 64.10, 55.63, 55.06, 51.21, 38.34, 38.11, 37.64, 33.66, 26.57, 25.98, 25.63, 25.24, 24.72, 23.18, 16.43; ESI-HRMS: m/z 519.2725 [M + Na]+, calcd for C30H40NaO6, 519.2723.
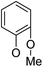
(14α)-(2′-Methoxy)phenoxy-3,19-acetonylidene andrographolide (5a12). White solid; mp 48.7 °C to 52.6 °C; 57% yield; 1H NMR (400 MHz, C6D6) δ 6.81 (ddd, J = 8.1, 6.9, 2.3 Hz, 1H), 6.69–6.61 (m, 2H), 6.48 (d, J = 8.2 Hz, 1H), 5.15 (d, J = 5.2 Hz, 1H), 4.82 (s, 1H), 4.62 (s, 1H), 4.21 (dd, J = 10.6, 1.6 Hz, 1H), 3.82 (d, J = 11.5 Hz, 1H), 3.75 (dd, J = 10.6, 5.6 Hz, 1H), 3.46 (dd, J = 7.7, 3.7 Hz, 1H), 3.28 (s, 3H), 3.08 (d, J = 11.5 Hz, 1H), 2.25–2.12 (m, 3H), 1.87 (m, 1H), 1.77–1.66 (m, 1H), 1.61–1.52 (m, 1H), 1.50–1.41 (m, 2H), 1.43 (s, 3H), 1.39 (s, 3H), 1.36–1.27 (m, 2H), 1.09 (s, 3H), 1.02–0.87 (m, 4H), 0.82 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 169.8, 151.3, 149.8, 147.9, 145.9, 126.1, 124.1, 121.2, 119.2, 113.1, 109.1, 98.6, 76.2, 73.3, 71.7, 63.2, 56.0, 55.3, 51.8, 38.4, 37.6, 37.5, 34.4, 27.9, 26.2, 25.7, 25.2, 25.0, 23.1, 16.0; ESI-HRMS: m/z 519.2719 [M + Na]+, calcd for C30H40NaO6, 519.2723.
(14β)-(2′-Methoxy)phenoxy-3,19-acetonylidene andrographolide (5b12). White solid; mp 130 °C to 131 °C; 60% yield; 1H NMR (400 MHz, C6D6) δ 7.13–7.10 (m, 1H), 6.80 (m, 1H), 6.65 (m, 1H), 6.58 (dd, J = 7.9, 1.6 Hz, 1H), 6.47 (dd, J = 8.1, 1.3 Hz, 1H), 5.18 (d, J = 5.4 Hz, 1H), 4.82 (d, J = 1.2 Hz, 1H), 4.42 (s, 1H), 4.15 (dd, J = 10.6, 1.5 Hz, 1H), 3.83 (d, J = 11.5 Hz, 1H), 3.65 (dd, J = 10.6, 5.5 Hz, 1H), 3.41 (dd, J = 7.7, 3.7 Hz, 1H), 3.29 (s, 3H), 3.09 (d, J = 11.5 Hz, 1H), 2.26–2.04 (m, 3H), 1.83–1.62 (m, 2H), 1.55–1.44 (m, 2H), 1.42 (s, 3H), 1.39 (s, 3H), 1.38–1.28 (m, 2H), 1.08 (s, 3H), 1.05–0.94 (m, 1H), 0.94–0.88 (m, 1H), 0.88–0.83 (m, 1H), 0.85 (s, 3H); 13C NMR (101 MHz, C6D6) δ 169.08, 151.78, 149.42, 148.27, 145.92, 128.15, 128.03, 127.91, 127.79, 127.67, 126.39, 124.00, 121.11, 120.16, 112.54, 107.85, 99.30, 75.39, 73.44, 70.56, 64.14, 55.72, 55.11, 51.10, 38.34, 38.12, 37.66, 33.56, 26.57, 26.00, 25.64, 25.29, 24.74, 23.24, 16.46; ESI-HRMS: m/z 519.2715 [M + Na]+, calcd for C30H40NaO6, 519.2723.
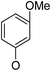
(14β)-(3′-Methoxy)phenoxy-3,19-acetonylidene andrographolide (2b13). White solid; mp 168 °C to 170 °C; 58% yield; 1H NMR (400 MHz, C6D6) δ 7.25–7.19 (m, 1H), 6.97 (t, J = 8.2 Hz, 1H), 6.43 (dd, J = 8.2, 2.0 Hz, 1H), 6.38 (t, J = 2.2 Hz, 1H), 6.19 (d, J = 8.2 Hz, 1H), 4.90 (s, 1H), 4.84 (d, J = 0.9 Hz, 1H), 4.46 (s, 1H), 3.93 (d, J = 10.6 Hz, 1H), 3.81 (d, J = 11.5 Hz, 1H), 3.76–3.68 (m, 1H), 3.40 (dd, J = 7.7, 3.7 Hz, 1H), 3.30 (s, 3H), 3.07 (d, J = 11.5 Hz, 1H), 2.34–2.25 (m, 1H), 2.15 (m, 2H), 1.79–1.65 (m, 2H), 1.58 (d, J = 10.4 Hz, 1H), 1.51–1.42 (m, 1H), 1.41 (s, 3H), 1.40–1.37 (m, 1H), 1.37–1.30 (m, 2H), 1.36 (s, 3H), 1.09 (s, 3H), 1.02–0.92 (m, 2H), 0.88 (dd, J = 12.9, 2.1 Hz, 1H), 0.81 (s, 3H); 13C NMR (101 MHz, C6D6) δ 168.61, 161.60, 158.18, 149.42, 147.97, 130.45, 125.84, 107.90, 107.58, 107.42, 102.64, 99.25, 75.36, 71.37, 70.39, 64.06, 55.70, 54.79, 51.24, 38.40, 38.10, 37.66, 33.61, 26.66, 25.95, 25.59, 25.20, 24.72, 23.16, 16.40; ESI-HRMS: m/z 519.2717 [M + Na]+ calcd for C30H40NaO6, 519.2723.
(14β)-(Naphthyl-1′-oxy)-3,19-acetonylidene andrographolide (5b14). White solid; mp 158 °C to 161 °C; 46% yield; 1H NMR (400 MHz, C6D6) δ 8.16 (dd, J = 6.2, 3.5 Hz, 1H), 7.59–7.54 (m, 1H), 7.39 (dd, J = 11.0, 4.2 Hz, 1H), 7.30 (d, J = 8.3 Hz, 1H), 7.26–7.21 (m, 2H), 7.11–7.06 (m, 1H), 6.02 (d, J = 7.6 Hz, 1H), 4.96 (d, J = 5.6 Hz, 1H), 4.85 (s, 1H), 4.51 (s, 1H), 3.88 (d, J = 7.2 Hz, 1H), 3.78 (dd, J = 10.7, 5.6 Hz, 1H), 3.70 (d, J = 11.5 Hz, 1H), 3.06 (dd, J = 7.6, 3.7 Hz, 1H), 2.98 (d, J = 11.5 Hz, 1H), 2.24–2.03 (m, 3H), 1.74 (dd, J = 20.3, 8.7 Hz, 2H), 1.40–1.33 (m, 1H), 1.32 (s, 3H), 1.31–1.24 (m, 1H), 1.28 (s, 3H), 1.12 (m, 1H), 0.98–0.85 (m, 1H), 0.92 (s, 3H), 0.84–0.73 (m, 1H), 0.71 (s, 3H), 0.70–0.60 (m, 2H); 13C NMR (101 MHz, C6D6) δ 168.55, 152.54, 149.60, 147.88, 135.21, 127.02, 126.27, 126.02, 125.83, 125.54, 122.47, 121.65, 107.86, 105.47, 99.17, 74.98, 71.22, 70.42, 64.02, 56.55, 50.80, 38.39, 37.95, 37.74, 33.27, 26.47, 25.68, 25.07, 24.08, 23.17, 16.35, 14.06; ESI-HRMS: m/z 539.2768 [M + Na]+, calcd for C33H40NaO5, 539.2773.
(14α)-(2′-Nitro-pyridinyl-3′-oxy)-3,19-acetonylidene andrographolide (5a15). White solid; mp 174 °C to 176 °C; 53% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.21 (dd, J = 4.5, 1.0 Hz, 1H), 8.05–8.00 (m, 1H), 7.84 (m, 4.6 Hz, 1H), 6.94 (t, J = 6.1 Hz, 1H), 6.07 (d, J = 5.2 Hz, 1H), 4.81 (s, 1H), 4.73 (m, 1H), 4.50–4.43 (m, 2H), 3.83 (d, J = 11.7 Hz, 1H), 3.38–3.34 (m, 1H), 3.08 (d, J = 11.6 Hz, 1H), 2.48–2.41 (m, 1H), 2.40–2.29 (m, 2H), 1.96 (m, 2H), 1.81 (m, 1H), 1.68–1.55 (m, 2H), 1.52–1.43 (m, 1H), 1.28 (m, 3H), 1.23 (s, 3H), 1.21–1.25 (m, 1H), 1.21–1.13 (m, 2H), 1.11 (s, 3H), 0.77 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 168.57, 151.44, 149.03, 147.50, 144.23, 140.41, 129.46, 126.43, 124.08, 108.52, 98.14, 75.70, 73.14, 70.22, 62.73, 54.90, 51.35, 37.86, 37.13, 36.90, 33.88, 27.41, 25.75, 25.15, 24.74, 24.72, 22.57, 15.57; ESI-HRMS: m/z 535.2415 [M + Na]+, calcd for C28H36N2NaO7, 535.2420.
(14β)-(2′-Nitro-pyridinyl-3′-oxy)-3,19-acetonylidene andrographolide (5b15). White solid; mp 104 °C to 107 °C; 42% yield; 1H NMR (400 MHz, C6D6) δ 7.53 (d, J = 4.6 Hz, 1H), 6.32 (m, 1H), 6.10 (m, 1H), 4.82 (s, 1H), 4.76 (s, 1H), 4.33 (s, 1H), 3.82 (d, J = 11.5 Hz, 1H), 3.51 (m, 2H), 3.44 (m, 1H), 3.07 (d, J = 11.5 Hz, 1H), 2.30–2.12 (m, 3H), 1.87–1.66 (m, 2H), 1.63–1.47 (m, 3H), 1.42 (s, 3H), 1.39–1.31 (m, 1H), 1.36 (s, 3H), 1.10 (s, 3H), 1.04–0.88 (m, 3H), 0.90 (s, 3H); 13C NMR (101 MHz, C6D6) δ 167.65, 152.42, 151.04, 148.50, 143.65, 140.77, 127.47, 124.75, 123.67, 107.70, 99.31, 75.29, 73.91, 69.17, 64.12, 55.77, 51.06, 38.40, 38.13, 37.58, 33.67, 26.56, 26.05, 25.93, 25.21, 24.63, 23.19, 16.51; ESI-HRMS: m/z 535.2415 [M + Na]+, calcd for C28H36N2NaO7, 535.2420.
(14α)-(Quinolyl-4′-oxy)-3,19-acetonylidene andrographolide (5a16). White solid; mp 97.8 °C to 102.9 °C; 40% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.79 (d, J = 5.2 Hz, 1H), 8.07 (d, J = 8.3 Hz, 1H), 7.99 (d, J = 8.4 Hz, 1H), 7.79–7.74 (m, 1H), 7.57 (t, J = 7.6 Hz, 1H), 7.11–7.04 (m, 2H), 6.12 (d, J = 6.9 Hz, 1H), 4.88–4.81 (m, 2H), 4.63 (s, 1H), 4.47–4.42 (m, 1H), 3.76 (d, J = 11.7 Hz, 1H), 3.27 (dd, J = 9.2, 3.9 Hz, 1H), 3.02 (d, J = 11.5 Hz, 1H), 2.31 (d, J = 11.7 Hz, 1H), 2.02 (s, 1H), 1.92 (s, 1H), 1.76–1.57 (m, 3H), 1.53–1.43 (m, 1H), 1.42–1.30 (m, 2H), 1.23 (s, 3H), 1.20 (s, 3H), 1.15–1.12 (m, 2H), 1.10 (s, 1H), 1.06–1.04 (m, 3H), 0.66 (d, J = 9.5 Hz, 3H), 0.65 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 169.4, 159.3, 151.9, 151.5, 149.5, 148.2, 132.1, 130.5, 129.2, 126.5, 124.9, 121.8, 109.0, 103.0, 98.6, 76.1, 72.1, 71.1, 63.1, 55.2, 51.8, 38.3, 37.5, 37.3, 34.3, 27.9, 26.2, 25.6, 25.5, 25.1, 23.0, 16.0; ESI-HRMS: m/z 518.2902 [M + H]+, calcd for C32H40NO5, 518.2906.
(14β)-(Quinolyl-4′-oxy)-3,19-acetonylidene andrographolide (5b16). White solid; mp 122 °C to 124 °C; 53% yield; 1H NMR (400 MHz, C6D6) δ 8.63 (d, J = 5.1 Hz, 1H), 8.31–8.25 (m, 1H), 7.98 (dd, J = 8.4, 1.4 Hz, 1H), 7.42 (m, 1H), 7.37–7.30 (m, 1H), 5.66–5.62 (m, 1H), 4.89–4.82 (m, 2H), 4.51 (d, J = 1.7 Hz, 1H), 3.76–3.65 (m, 3H), 3.10 (dd, J = 7.1, 3.5 Hz, 1H), 2.98 (d, J = 11.5 Hz, 1H), 2.23–2.05 (m, 3H), 1.80–1.63 (m, 2H), 1.34–1.24 (m, 2H), 1.30 (s, 3H), 1.28 (s, 3H), 1.14 (m, 1H), 1.00–0.91 (m, 1H), 0.90 (s, 3H), 0.76 (s, 3H), 0.74–0.59 (m, 3H); 13C NMR (101 MHz, C6D6) δ 168.22, 158.91, 151.10, 150.54, 150.43, 147.82, 130.19, 129.97, 128.15, 127.91, 127.79, 127.67, 126.07, 125.40, 121.97, 121.27, 107.91, 100.92, 99.36, 74.50, 71.28, 69.95, 64.12, 56.67, 50.62, 38.42, 38.08, 37.75, 33.15, 26.10, 25.73, 25.63, 24.94, 23.67, 23.18, 16.53; ESI-HRMS: m/z 518.2900 [M + H]+, calcd for C32H40NaO5, 518.2906.
(14α)-(Quinolyl-8′-oxy)-3,19-acetonylidene andrographolide (5a17). White solid; mp 143 °C to 146 °C; 42% yield; 1H NMR (400 MHz, C6D6) δ 8.64 (dd, J = 4.1, 1.7 Hz, 1H), 7.46 (dd, J = 8.4, 1.7 Hz, 1H), 7.27–7.22 (m, 1H), 7.08 (dd, J = 8.2, 1.3 Hz, 1H), 6.99 (t, J = 7.8 Hz, 1H), 6.88 (m, 1H), 6.74 (dd, J = 8.3, 4.1 Hz, 1H), 6.08 (d, J = 5.6 Hz, 1H), 4.82 (s, 1H), 4.69 (s, 1H), 4.39 (dd, J = 10.8, 1.6 Hz, 1H), 3.88 (dd, J = 10.8, 5.6 Hz, 1H), 3.79 (d, J = 11.5 Hz, 1H), 3.41 (dd, J = 8.1, 3.9 Hz, 1H), 3.06 (d, J = 11.5 Hz, 1H), 2.35 (m, 1H), 2.25–2.11 (m, 2H), 1.81 (m, 1H), 1.74–1.64 (m, 1H), 1.55–1.48 (m, 1H), 1.48–1.40 (m, 1H), 1.42 (s, 3H), 1.36 (s, 3H), 1.32–1.23 (m, 2H), 1.08 (s, 3H), 0.99–0.78 (m, 3H), 0.71 (s, 3H); 13C NMR (101 MHz, C6D6) δ 169.33, 153.10, 149.55, 149.07, 147.00, 142.46, 135.98, 130.11, 126.78, 126.52, 123.22, 121.41, 119.68, 109.42, 99.18, 75.65, 75.33, 70.92, 64.00, 55.65, 51.54, 38.19, 38.00, 37.68, 34.09, 26.83, 26.09, 25.63, 25.39, 25.04, 23.18, 16.12; ESI-HRMS: m/z 518.2901 [M + H]+, calcd for C32H40NO5, 518.2906.
(14β)-(Quinolyl-8′-oxy)-3,19-acetonylidene andrographolide (5b17). White solid; mp 146 °C to 148 °C; 87% yield; 1H NMR (400 MHz, C6D6) δ 8.67 (dd, J = 4.1, 1.7 Hz, 1H), 7.49 (dd, J = 8.3, 1.7 Hz, 1H), 7.30–7.24 (m, 1H), 7.08 (m, 1H), 7.01 (m, 1H), 6.75 (m, 2H), 5.97 (d, J = 5.5 Hz, 1H), 4.84 (d, J = 1.2 Hz, 1H), 4.54 (s, 1H), 4.25 (dd, J = 10.7, 1.6 Hz, 1H), 3.84–3.75 (m, 2H), 3.28 (m, 1H), 3.06 (d, J = 11.5 Hz, 1H), 2.37 (m, 1H), 2.30–2.21 (m, 1H), 2.21–2.13 (m, 1H), 1.74 (m, 1H), 1.69–1.58 (m, 2H), 1.39 (s, 3H), 1.36–1.30 (m, 1H), 1.34 (s, 3H), 1.29–1.19 (m, 2H), 1.04 (m, 3H), 1.01–0.92 (m, 1H), 0.84 (m, 2H), 0.75 (s, 3H); 13C NMR (101 MHz, C6D6) δ 169.13, 153.03, 149.84, 149.10, 148.26, 142.17, 135.83, 130.10, 126.66, 126.65, 122.68, 121.51, 117.63, 107.87, 99.13, 75.55, 74.63, 70.62, 64.03, 55.77, 51.17, 38.37, 37.99, 37.69, 33.57, 26.74, 25.99, 25.99, 25.31, 24.74, 23.30, 16.25; ESI-HRMS: m/z 518.2880 [M + H]+, calcd for C32H40NO5, 518.2906.
General method for synthesis of 6a and 6b. At room temperature, 1.0 mmol 5a or 5b were dissolved in 15 mL methanol, and 0.1 mmol TsOH·H2O was added. The reaction was stirred at rt and was generally complete in 5 min. After the reaction was complete, the reaction mixture was treated with sol. sat. NaHCO3 and extracted with ethyl acetate. The organic phase was washed with brine and dried over anhydrous Na2SO4. The solvent was removed in vacuo, and the residue was further purified using flash column chromatography eluting with petroleum ether and ethyl acetate to afford 6a or 6b.
(14α)-(2′-Carboxy ethyl ester-4′-nitro)phenoxy-andrographolide (6a1). White solid; mp 82.6 °C to 92.7 °C; 97% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.51 (d, J = 2.9 Hz, 1H), 8.44 (dd, J = 9.2, 3.0 Hz, 1H), 7.40 (d, J = 9.3 Hz, 1H), 6.99–6.93 (m, 1H), 6.05 (d, J = 5.4 Hz, 1H), 5.00 (d, J = 4.9 Hz, 1H), 4.81–4.74 (m, 2H), 4.47 (s, 1H), 4.39 (dd, J = 11.0, 1.5 Hz, 1H), 4.09 (dd, J = 7.5, 2.9 Hz, 1H), 3.79 (s, 3H), 3.79–3.74 (m, 1H), 3.24–3.11 (m, 2H), 2.48–2.44 (m, 1H), 2.38–2.27 (m, 2H), 1.96–1.86 (m, 2H), 1.70 (d, J = 13.2 Hz, 1H), 1.57–1.43 (m, 3H), 1.31 (m, 1H), 1.17–1.10 (m, 1H), 1.08–1.01 (m, 1H), 1.04 (s, 3H), 0.52 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 169.2, 164.5, 160.7, 151.7, 148.2, 141.3, 129.2, 127.2, 124.6, 122.1, 116.0, 108.5, 78.8, 73.4, 70.9, 63.0, 55.5, 54.8, 53.1, 42.7, 38.9, 37.8, 36.9, 28.2, 24.9, 24.3, 23.5, 14.9; ESI-HRMS: m/z 552.2203 [M + Na]+, calcd for C28H35NNaO9, 552.2210.
(14β)-(2′-Carboxy ethyl ester-4′-nitro)phenoxy-andrographolide (6b1). White solid; mp 190 °C to 192 °C; 78% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.58 (d, J = 2.9 Hz, 1H), 8.49 (dd, J = 9.2, 3.0 Hz, 1H), 7.44 (d, J = 9.3 Hz, 1H), 7.02 (t, J = 6.9 Hz, 1H), 6.15 (d, J = 5.2 Hz, 1H), 4.99 (d, J = 4.8 Hz, 1H), 4.83 (s, 1H), 4.77 (dd, J = 11.1, 5.5 Hz, 1H), 4.55 (s, 1H), 4.41 (d, J = 11.0 Hz, 1H), 4.07 (dd, J = 7.5, 2.8 Hz, 1H), 3.82 (s, 3H), 3.75 (dd, J = 11.0, 2.8 Hz, 1H), 3.20 (m, 1H), 3.02–2.95 (m, 1H), 2.44 (m, 1H), 2.46–2.30 (m, 2H), 1.90 (m, 2H), 1.71 (m, 1H), 1.55–1.38 (m, 2H), 1.38–1.24 (m, 2H), 1.10–1.00 (m, 1H), 1.04 (s, 3H), 0.86 (m, 1H), 0.54 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 168.69, 163.67, 160.48, 151.11, 147.59, 140.66, 129.03, 126.96, 124.68, 120.98, 115.50, 107.80, 78.25, 72.78, 70.46, 62.47, 55.62, 54.18, 52.51, 42.12, 38.67, 37.35, 36.04, 27.71, 25.03, 23.86, 22.95, 14.54; ESI-HRMS: m/z 552.2204 [M + Na]+, calcd for C28H35NNaO9, 552.2210.
(14α)-(2′-Fluoro-4′-nitro)phenoxy-andrographolide (6a2). White solid; mp 172 °C to 175 °C; 87% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.25 (dd, J = 10.9, 2.7 Hz, 1H), 8.18–8.13 (m, 1H), 7.48 (t, J = 8.7 Hz, 1H), 6.96 (t, J = 6.3 Hz, 1H), 6.05 (d, J = 5.3 Hz, 1H), 5.03 (d, J = 4.9 Hz, 1H), 4.79 (s, 1H), 4.74 (m, 1H), 4.54 (s, 1H), 4.44 (d, J = 12.2 Hz, 1H), 4.11 (m, 1H), 3.78 (dd, J = 11.0, 2.9 Hz, 1H), 3.19 (m, 2H), 2.45 (s, 1H), 2.42–2.26 (m, 2H), 1.98–1.87 (m, 2H), 1.71 (m, 1H), 1.54 (m, 3H), 1.38–1.25 (m, 1H), 1.18–1.10 (m, 2H), 1.06 (s, 3H), 0.54 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 168.64, 152.22, 151.45, 150.42, 150.32, 149.74, 147.67, 141.12, 141.04, 124.11, 121.27, 121.23, 115.72, 112.68, 112.45, 108.05, 78.36, 73.02, 70.34, 62.53, 55.12, 54.30, 42.18, 38.43, 37.36, 36.35, 27.77, 24.80, 23.84, 23.01, 14.52; ESI-HRMS: m/z 512.2054 [M + Na]+, calcd for C26H32FNNaO7, 512.2061.
(14β)-(2′-Fluoro-4′-nitro)phenoxy-andrographolide (6b2). White solid; mp 162 °C to 165 °C; 84% yield; 1H NMR (400 MHz, CD3OD) δ 8.19–8.11 (m, 2H), 7.34 (t, J = 8.4 Hz, 1H), 7.13–7.04 (m, 1H), 6.03–5.97 (m, 1H), 4.72 (dd, J = 11.2, 5.5 Hz, 1H), 4.56 (s, 1H), 4.45 (dd, J = 11.2, 1.4 Hz, 1H), 4.02 (d, J = 11.1 Hz, 1H), 3.32 (m, 1H), 3.21 (dd, J = 11.8, 4.1 Hz, 1H), 2.52 (m, 1H), 2.45–2.34 (m, 2H), 2.07–1.92 (m, 2H), 1.87–1.78 (m, 1H), 1.73–1.46 (m, 3H), 1.41–1.28 (m, 1H), 1.22 (m, 1H), 1.17 (s, 3H), 1.10–0.98 (m, 1H), 0.64 (s, 3H); 13C NMR (101 MHz, CD3OD) δ 171.05, 154.37, 152.98, 151.89, 151.83, 151.73, 148.94, 143.42, 143.34, 126.12, 122.24, 122.20, 116.71, 113.79, 113.56, 108.54, 80.83, 74.64, 72.22, 64.88, 57.72, 56.33, 43.63, 40.24, 38.93, 37.90, 28.89, 26.76, 25.19, 23.39, 15.42; ESI-HRMS: m/z 512.2099 [M + Na]+, calcd for C26H32FNNaO7, 512.2061.
(14α)-(2′-Methoxy-4′-nitro)phenoxy-andrographolide (6a3)25,26. Yellow solid; mp 155 °C to 159 °C; 87% yield; 1H NMR (400 MHz, CD3OD) δ 7.95 (dd, J = 8.8, 2.6 Hz, 1H), 7.92 (d, J = 2.6 Hz, 1H), 7.16 (d, J = 8.8 Hz, 1H), 7.12–7.03 (m, 1H), 5.87 (d, J = 5.7 Hz, 1H), 4.91 (s, 1H), 4.73 (dd, J = 10.9, 5.8 Hz, 1H), 4.67 (s, 1H), 4.46 (dd, J = 10.9, 1.8 Hz, 1H), 4.10 (d, J = 11.1 Hz, 1H), 3.98 (s, 3H), 3.38 (t, J = 5.4 Hz, 1H), 2.58–2.40 (m, 3H), 2.09–1.95 (m, 2H), 1.90–1.83 (m, 1H), 1.80–1.60 (m, 3H), 1.44–1.32 (m, 1H), 1.32–1.17 (m, 2H), 1.22 (s, 3H), 0.66 (s, 3H); 13C NMR (101 MHz, CD3OD) δ 171.44, 153.11, 152.54, 151.94, 148.72, 144.43, 126.17, 118.27, 116.57, 109.47, 108.44, 80.88, 74.75, 72.58, 64.91, 57.17, 56.86, 56.29, 43.65, 39.84, 38.87, 38.15, 28.96, 26.36, 25.17, 23.35, 15.43; ESI-HRMS: m/z 524.2253 [M + Na]+, calcd for C27H35NNaO8, 524.2260.
(14β)-(2′-Methoxy-4′-nitro)phenoxy-andrographolide (6b3)25,26. Yellow solid; mp 151 °C to 153 °C; 90% yield; 1H NMR (400 MHz, CDCl3) δ 7.89 (m, 1H), 7.84–7.79 (m, 1H), 7.13–7.05 (m, 1H), 6.88 (dd, J = 9.2, 4.2 Hz, 1H), 5.71–5.65 (m, 1H), 4.83 (d, J = 1.9 Hz, 1H), 4.58 (dd, J = 11.0, 5.8 Hz, 1H), 4.42 (dd, J = 11.0, 1.8 Hz, 1H), 4.34 (s, 1H), 4.16–4.08 (m, 1H), 3.96 (s, 3H), 3.38 (dd, J = 11.6, 3.5 Hz, 1H), 3.28 (d, J = 11.1 Hz, 1H), 2.58 (d, J = 52.7 Hz, 2H), 2.44–2.36 (m, 2H), 2.32–2.21 (m, 1H), 1.97 (m, 1H), 1.89 (dd, J = 9.3, 2.7 Hz, 1H), 1.85–1.78 (m, 1H), 1.78–1.60 (m, 2H), 1.52 (m, 1H), 1.26–1.16 (m, 2H), 1.22 (s, 3H), 1.14–1.03 (m, 1H), 0.61 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 168.99, 151.84, 151.03, 150.56, 147.09, 143.34, 124.42, 117.39, 116.00, 108.22, 107.50, 80.35, 77.37, 77.26, 77.05, 76.73, 73.32, 70.60, 64.04, 56.36, 55.75, 55.06, 42.82, 38.93, 37.65, 36.64, 28.16, 25.71, 23.70, 22.77, 15.12; ESI-HRMS: m/z 524.2253 [M + Na]+, calcd for C27H35NNaO8, 524.2260.
(14α)-(3′-Fluoro-4′-nitro)phenoxy-andrographolide (6a4). White solid; mp 191 °C to 194 °C; 86% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.10 (t, J = 9.1 Hz, 1H), 7.65 (s, 1H), 7.13 (dd, J = 13.4, 2.6 Hz, 1H), 6.90 (dd, J = 9.3, 2.3 Hz, 1H), 5.11 (s, 1H), 5.04 (d, J = 4.9 Hz, 1H), 4.93–4.86 (m, 1H), 4.89 (s, 3H), 4.11 (m, 1H), 3.82 (m, 1H), 3.24 (m, 2H), 2.31 (m, 1H), 2.03–1.91 (m, 2H), 1.89–1.59 (m, 6H), 1.40–1.27 (m, 1H), 1.26–1.11 (m, 2H), 1.05 (m, 3H), 0.62 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 172.00, 163.60, 163.49, 157.78, 155.17, 149.66, 146.99, 131.27, 130.46, 130.40, 128.16, 111.88, 111.86, 107.72, 105.08, 104.84, 78.29, 72.99, 71.10, 62.58, 54.48, 51.23, 42.27, 38.45, 37.77, 36.50, 29.11, 27.80, 23.99, 22.95, 14.77; ESI-HRMS: m/z 512.2055 [M + Na]+, calcd for C26H32FNNaO7, 512.2061.
(14β)-(3′-Fluoro-4′-nitro)phenoxy-andrographolide (6b4). Yellow solid; mp 163 °C to 166 °C; 90% yield; 1H NMR (400 MHz, CD3OD) δ 8.20 (t, J = 9.0 Hz, 1H), 7.18–7.06 (m, 2H), 6.99 (m, 1H), 5.97 (d, J = 5.3 Hz, 1H), 4.70 (dd, J = 11.1, 5.5 Hz, 1H), 4.57 (s, 1H), 4.40 (dd, J = 11.1, 1.3 Hz, 1H), 4.04 (d, J = 11.1 Hz, 1H), 3.61 (q, J = 7.0 Hz, 3H), 3.33 (s, 1H), 3.24 (m, 1H), 2.56 (m, 1H), 2.47–2.36 (m, 2H), 2.02 (m, 1H), 1.98–1.91 (m, 1H), 1.89–1.79 (m, 1H), 1.63 (m, 3H), 1.42–1.20 (m, 2H), 1.18 (s, 3H), 1.13–1.01 (m, 1H), 0.66 (s, 3H); 13C NMR (101 MHz, CD3OD) δ 171.07, 163.94, 163.83, 159.93, 157.31, 152.70, 148.90, 132.84, 132.77, 129.41, 126.15, 113.08, 113.05, 108.63, 106.14, 105.90, 80.82, 73.80, 72.22, 64.90, 57.52, 56.34, 43.65, 40.25, 38.95, 38.09, 28.91, 26.76, 25.20, 23.39, 15.44; ESI-HRMS: m/z 512.2093 [M + Na]+, calcd for C26H32FNNaO7, 512.2061.
(14α)-(3′-methoxy-4′-nitro)phenoxy-andrographolide (6a5). White solid; mp 131 °C to 133 °C; 81% yield; 1H NMR (400 MHz, (CD3)2SO) δ 7.99 (d, J = 9.1 Hz, 1H), 6.96 (t, J = 6.8 Hz, 1H), 6.85 (d, J = 2.4 Hz, 1H), 6.73 (dd, J = 9.1, 2.4 Hz, 1H), 6.01 (d, J = 5.0 Hz, 1H), 5.04 (d, J = 4.9 Hz, 1H), 4.83 (s, 1H), 4.71 (dd, J = 11.0, 5.4 Hz, 1H), 4.56 (s, 1H), 4.39–4.33 (m, 1H), 4.12 (dd, J = 7.4, 2.9 Hz, 1H), 3.78 (dd, J = 11.0, 2.8 Hz, 1H), 3.26–3.14 (m, 2H), 2.44 (dd, J = 5.8, 3.0 Hz, 1H), 2.36–2.28 (m, 2H), 1.97–1.87 (m, 2H), 1.76–1.68 (m, 1H), 1.60–1.48 (m, 3H), 1.36–1.22 (m, 2H), 1.18–1.13 (m, 3H), 1.06 (s, 3H), 0.90–0.76 (m, 1H), 0.55 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 168.7, 161.5, 155.8, 151.9, 146.6, 134.2, 128.7, 123.8, 109.1, 104.6, 101.7, 80.4, 71.8, 70.4, 64.1, 56.7, 55.8, 55.2, 42.8, 38.8, 37.7, 37.0, 28.1, 25.4, 23.6, 22.7, 15.2; ESI-HRMS: m/z 524.2252 [M + Na]+, calcd for C27H35NNaO8, 524.2260.
(14β)-(3′-Methoxy-4′-nitro)phenoxy-andrographolide (6b5). Yellow solid; mp 126 °C to 129 °C; 85% yield; 1H NMR (400 MHz, CD3OD) δ 8.02 (d, J = 9.1 Hz, 1H), 7.15 (t, J = 6.6 Hz, 1H), 6.85 (d, J = 2.5 Hz, 1H), 6.71 (dd, J = 9.1, 2.5 Hz, 1H), 5.98 (d, J = 5.4 Hz, 1H), 4.72 (dd, J = 11.0, 5.5 Hz, 1H), 4.60 (s, 1H), 4.42 (dd, J = 11.0, 1.3 Hz, 1H), 4.06 (d, J = 11.1 Hz, 1H), 3.99 (s, 3H), 3.35 (d, J = 4.2 Hz, 1H), 3.25 (dd, J = 11.8, 4.1 Hz, 1H), 2.60–2.52 (m, 1H), 2.42 (m, 2H), 2.10–2.00 (m, 1H), 1.97 (m, 1H), 1.90–1.81 (m, 1H), 1.76–1.64 (m, 1H), 1.64–1.54 (m, 2H), 1.42–1.30 (m, 1H), 1.24 (m, 1H), 1.20 (s, 3H), 1.15–1.05 (m, 1H), 0.68 (s, 3H); 13C NMR (101 MHz, CD3OD) δ 171.33, 163.25, 156.92, 152.16, 148.93, 135.26, 129.27, 126.63, 108.56, 107.53, 102.64, 80.83, 73.31, 72.52, 64.88, 57.55, 57.34, 56.33, 43.65, 40.24, 38.95, 38.03, 28.91, 26.71, 25.20, 23.38, 15.41; ESI-HRMS: m/z 524.2254 [M + Na]+, calcd for C27H35NNaO8, 524.2260.
(14β)-(2′-Nitro)phenoxy-andrographolide (6b6). Yellow solid; mp 167 °C to 168 °C; 89% yield; 1H NMR (400 MHz, CDCl3) δ 7.89 (dd, J = 8.1, 1.6 Hz, 1H), 7.62–7.55 (m, 1H), 7.18 (t, J = 7.7 Hz, 1H), 7.13 (dd, J = 10.1, 3.9 Hz, 1H), 6.92 (d, J = 8.3 Hz, 1H), 5.64 (d, J = 5.6 Hz, 1H), 4.84 (d, J = 15.2 Hz, 1H), 4.66 (dd, J = 10.9, 6.0 Hz, 1H), 4.44 (dd, J = 10.9, 2.0 Hz, 1H), 4.31 (s, 1H), 4.16–4.09 (m, 1H), 3.43 (dd, J = 11.5, 4.6 Hz, 1H), 3.28 (d, J = 11.1 Hz, 1H), 2.56–2.45 (m, 1H), 2.38 (dd, J = 13.2, 3.5 Hz, 1H), 2.26 (m, 1H), 2.09 (s, 3H), 1.98 (t, J = 12.3 Hz, 1H), 1.89 (d, J = 10.4 Hz, 1H), 1.85–1.78 (m, 1H), 1.78–1.63 (m, 2H), 1.59 (m, 1H), 1.31–1.18 (m, 2H), 1.24 (s, 3H), 1.10 (m, 1H), 0.58 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 168.74, 152.64, 149.38, 147.27, 141.14, 134.14, 126.28, 123.69, 122.33, 115.90, 108.07, 80.31, 77.37, 77.25, 77.05, 76.73, 73.14, 70.30, 64.10, 55.80, 54.86, 42.80, 38.89, 37.63, 36.54, 28.12, 25.81, 23.69, 22.63, 15.12; ESI-HRMS: m/z 494.2158 [M + Na]+, calcd for C26H33NNaO7, 494.2155.
(14β)-(3′-Nitro)phenoxy-andrographolide (6b7). Yellow solid; mp 144 °C to 146 °C; 93% yield; 1H NMR (400 MHz, CDCl3) δ 7.94 (m, 1H), 7.69 (t, J = 2.3 Hz, 1H), 7.58–7.49 (m, 1H), 7.20 (m, 1H), 7.17–7.11 (m, 1H), 5.62 (m, 1H), 4.87 (dd, J = 2.5, 1.3 Hz, 1H), 4.72–4.63 (m, 1H), 4.45–4.35 (m, 2H), 4.16–4.08 (m, 1H), 3.41 (m, 1H), 3.33–3.25 (m, 1H), 2.51 (m, 1H), 2.46–2.38 (m, 1H), 2.37–2.23 (m, 1H), 2.29 (s, 3H), 2.01–1.93 (m, 1H), 1.93–1.87 (m, 1H), 1.83 (m, 1H), 1.74 (m, 1H), 1.62 (m, 2H), 1.34–1.18 (m, 2H), 1.24 (s, 3H), 1.18–1.06 (m, 1H), 0.60 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 168.90, 156.99, 151.67, 149.36, 146.91, 130.85, 124.19, 122.41, 117.26, 109.70, 108.28, 80.29, 77.38, 77.06, 76.74, 71.95, 70.39, 64.05, 55.77, 55.15, 42.80, 39.00, 37.68, 36.91, 28.05, 25.74, 23.68, 22.72, 15.10; ESI-HRMS: m/z 494.2156 [M + Na]+, calcd for C26H33NNaO7, 494.2155.
(14α)-(2′-Carboxy ethyl ester)phenoxy-andrographolide (6a8). White solid; mp 157 °C to 159 °C; 96% yield; 1H NMR (400 MHz, C6D6) δ 7.78 (dd, J = 7.7, 1.8 Hz, 1H), 7.21 (t, J = 6.0 Hz, 1H), 6.98–6.92 (m, 1H), 6.71 (t, J = 7.6 Hz, 1H), 6.38 (d, J = 8.2 Hz, 1H), 5.11 (s, 1H), 4.84 (s, 1H), 4.57 (s, 1H), 4.19–4.11 (m, 2H), 4.09 (dd, J = 8.8, 4.0 Hz, 1H), 3.78 (dd, J = 17.5, 8.6 Hz, 2H), 3.44 (dd, J = 7.9, 3.7 Hz, 1H), 3.07 (d, J = 11.5 Hz, 1H), 2.22–2.13 (m, 3H), 1.89–1.80 (m, 1H), 1.78–1.67 (m, 1H), 1.60–1.51 (m, 1H), 1.51–1.43 (m, 2H), 1.42 (s, 3H), 1.35 (s, 3H), 1.31 (d, J = 2.5 Hz, 1H), 1.09 (s, 3H), 1.06 (t, J = 7.1 Hz, 3H), 0.99–0.88 (m, 3H), 0.80 (s, 3H); 13C NMR (101 MHz, C6D6) δ 168.70, 165.60, 156.04, 149.51, 147.00, 132.85, 132.04, 125.51, 124.38, 122.50, 117.83, 109.13, 99.26, 75.48, 73.78, 70.37, 64.02, 60.96, 55.62, 51.54, 38.23, 38.07, 37.66, 34.21, 26.70, 26.05, 25.29, 25.24, 24.89, 23.15, 16.22, 14.10; ESI-HRMS: m/z: 521.2509 [M + Na]+, calcd for C29H38NaO7, 521.2515.
(14β)-(2′-Carboxy ethyl ester)phenoxy-andrographolide (6b8). White solid; mp 181 °C to 182 °C; 88% yield; 1H NMR (400 MHz, CDCl3) δ 7.88 (dd, J = 7.8, 1.7 Hz, 1H), 7.54–7.48 (m, 1H), 7.15 (t, J = 7.6 Hz, 1H), 7.04 (t, J = 7.0 Hz, 1H), 6.91 (d, J = 8.3 Hz, 1H), 5.61 (d, J = 5.3 Hz, 1H), 4.83 (d, J = 1.9 Hz, 1H), 4.58 (dd, J = 10.7, 5.4 Hz, 1H), 4.52 (dd, J = 10.7, 1.7 Hz, 1H), 4.34 (dd, J = 14.7, 7.6 Hz, 3H), 4.14 (d, J = 11.1 Hz, 1H), 3.46–3.36 (m, 1H), 3.33–3.25 (m, 1H), 2.47–2.25 (m, 4H), 2.22–2.21 (m, 1H), 2.02–1.90 (m, 1H), 1.88–1.78 (m, 2H), 1.78–1.61 (m, 2H), 1.53–1.46 (m, 1H), 1.36 (t, 3H), 1.32–1.16 (m, 2H), 1.24 (s, 3H), 1.10–0.99 (m, 1H), 0.56 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 169.45, 165.73, 155.78, 150.94, 147.11, 133.38, 132.26, 124.95, 123.22, 122.60, 117.01, 108.19, 80.33, 77.36, 77.25, 77.04, 76.73, 73.39, 70.98, 64.09, 61.16, 55.76, 54.90, 42.81, 38.81, 37.63, 36.55, 28.12, 25.64, 23.68, 22.64, 15.07, 14.29 ESI-HRMS: m/z 521.2517 [M + Na]+, calcd for C29H39NaO7, 521.2515.
(14α)-(4′-Carboxy ethyl ester)phenoxy-andrographolide (6a9). White solid; mp 183 °C to 185 °C; 84% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.00–7.91 (m, 2H), 7.09 (d, J = 8.9 Hz, 2H), 6.93 (t, J = 6.2 Hz, 1H), 5.88 (d, J = 5.5 Hz, 1H), 5.01 (d, J = 4.7 Hz, 1H), 4.82 (s, 1H), 4.73 (dd, J = 10.9, 5.6 Hz, 1H), 4.58 (s, 1H), 4.33–4.26 (m, 3H), 4.10 (d, J = 5.1 Hz, 1H), 3.77 (d, J = 10.8 Hz, 1H), 3.24–3.13 (m, 2H), 2.43 (s, 1H), 2.37–2.27 (m, 2H), 1.96–1.87 (m, 2H), 1.71 (d, J = 13.1 Hz, 1H), 1.58–1.45 (m, 3H), 1.38–1.24 (m, 1H), 1.31 (t, J = 7.1 Hz, 3H), 1.12 (dd, J = 15.9, 12.0 Hz, 2H), 1.05 (s, 3H), 0.52 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 169.4, 165.7, 161.0, 150.9, 148.2, 131.9, 125.3, 123.7, 115.8, 108.5, 78.8, 71.8, 71.2, 63.0, 60.9, 55.6, 54.8, 42.7, 38.9, 37.9, 36.9, 28.3, 25.2, 24.3, 23.5, 21.2, 15.0, 14.7, 14.5; ESI-HRMS: m/z 521.2507 [M + Na]+, calcd for C29H38NaO7, 521.2515.
(14β)-(4′-Carboxy ethyl ester)phenoxy-andrographolide (6b9). White solid; mp 136 °C to 139 °C; 95% yield; 1H NMR (400 MHz, CD3OD) δ 8.07–8.01 (m, 2H), 7.12 (t, J = 7.3 Hz, 1H), 7.09–7.04 (m, 2H), 5.88 (d, J = 5.4 Hz, 1H), 4.70 (dd, J = 10.9, 5.5 Hz, 1H), 4.56 (s, 1H), 4.40–4.31 (m, 3H), 4.03 (d, J = 11.1 Hz, 1H), 3.29 (s, 1H), 3.20 (dd, J = 11.9, 4.1 Hz, 1H), 2.55–2.47 (m, 1H), 2.45–2.30 (m, 2H), 2.08–1.91 (m, 2H), 1.86–1.79 (m, 1H), 1.71–1.58 (m, 1H), 1.58–1.48 (m, 2H), 1.42–1.27 (m, 4H), 1.24–1.14 (m, 1H), 1.17 (s, 3H), 1.09–0.96 (m, 1H), 0.63 (s, 3H); 13C NMR (101 MHz, CD3OD) δ 171.50, 167.59, 162.09, 151.93, 148.95, 132.93, 126.89, 125.30, 116.59, 108.53, 80.79, 72.88, 72.66, 64.90, 62.07, 57.61, 56.29, 43.62, 40.21, 38.95, 37.99, 28.87, 26.65, 25.18, 23.36, 15.43, 14.68; ESI-HRMS: m/z 521.2509 [M + Na]+, calcd for C29H38NaO7, 521.2515.
(14β)-(4′-Cyano)phenoxy-andrographolide (6b10). White solid; mp 182 °C to 184 °C; 76% yield; 1H NMR (400 MHz, CD3OD) δ 7.73–7.68 (m, 2H), 7.13–7.06 (m, 3H), 5.86 (d, J = 5.5 Hz, 1H), 4.85 (s, 1H), 4.65 (dd, J = 11.0, 5.5 Hz, 1H), 4.52 (s, 1H), 4.31 (dd, J = 10.9, 1.4 Hz, 1H), 3.99 (d, J = 11.1 Hz, 1H), 3.30–3.35 (m, 1H), 3.18 (dd, J = 11.8, 4.2 Hz, 1H), 2.53–2.45 (m, 1H), 2.41–2.28 (m, 2H), 2.02–1.89 (m, 2H), 1.82–1.76 (m, 1H), 1.63 (m, 1H), 1.51 (m, 2H), 1.36–1.24 (m, 1H), 1.20–1.15 (m, 1H), 1.14 (s, 3H), 1.06–0.97 (m, 1H), 0.60 (s, 3H); 13C NMR (101 MHz, CD3OD) δ 171.30, 161.67, 152.20, 148.92, 135.62, 126.56, 119.68, 117.60, 108.51, 106.25, 80.79, 72.91, 72.40, 64.87, 57.60, 56.33, 43.62, 40.21, 38.93, 38.04, 28.86, 26.67, 25.17, 23.35, 15.40; ESI-HRMS: m/z 474.2252 [M + Na]+ calcd for C27H33NNaO5, 474.2256.
(14β)-(4′-Methoxy)phenoxy-andrographolide (6b11). White solid; mp 178 °C to 180 °C; 75% yield; 1H NMR (400 MHz, (CD3)2SO) δ 6.97–6.88 (m, 4H), 6.87–6.82 (m, 1H), 5.66 (d, J = 5.4 Hz, 1H), 4.83–4.77 (m, 1H), 4.60 (dd, J = 10.7, 5.4 Hz, 1H), 4.46–4.40 (m, 1H), 4.33 (dd, J = 10.7, 1.4 Hz, 1H), 3.78 (d, J = 10.9 Hz, 1H), 3.72 (s, 3H), 3.21 (d, J = 10.8 Hz, 1H), 3.09 (dd, J = 11.2, 4.5 Hz, 1H), 2.30 (m, 1H), 2.21–2.12 (m, 2H), 1.97–1.83 (m, 2H), 1.71 (m, 1H), 1.61–1.22 (m, 4H), 1.14–0.91 (m, 2H), 1.05 (s, 3H), 0.55 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 169.24, 154.49, 150.27, 149.39, 147.73, 125.79, 117.95, 117.95, 114.82, 114.82, 107.75, 78.30, 72.48, 71.16, 62.55, 55.38, 55.36, 54.22, 42.16, 38.59, 37.38, 36.24, 27.74, 24.95, 23.87, 22.96, 14.58; ESI-HRMS: m/z 479.2417 [M + Na]+, calcd for C27H36NaO6, 479.2410.
(14α)-(2′-Methoxy)phenoxy-andrographolide (6a12). White solid; mp 155.2 °C to 161.6 °C; 92% yield; 1H NMR (400 MHz, (CD3)2SO) δ 7.07–7.03 (m, 2H), 6.97 (d, J = 7.5 Hz, 1H), 6.93–6.87 (m, 1H), 6.82 (dd, J = 6.8, 5.7 Hz, 1H), 5.58 (d, J = 5.3 Hz, 1H), 5.04 (d, J = 4.8 Hz, 1H), 4.79 (s, 1H), 4.57 (dd, J = 10.7, 5.4 Hz, 1H), 4.52 (s, 1H), 4.36 (dd, J = 10.7, 1.2 Hz, 1H), 4.12 (dd, J = 7.4, 2.7 Hz, 1H), 3.81–3.74 (m, 1H), 3.76 (s, 3H), 3.22 (dd, J = 10.9, 7.7 Hz, 1H), 3.16 (dd, J = 10.4, 5.0 Hz, 1H), 2.36–2.26 (m, 2H), 2.21–2.11 (m, 1H), 1.94–1.81 (m, 2H), 1.70 (d, J = 12.9 Hz, 1H), 1.62–1.51 (m, 2H), 1.50–1.43 (m, 1H), 1.30 (m, 1H), 1.15–1.07 (m, 2H), 1.05 (s, 3H), 0.53 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 169.8, 151.2, 149.9, 148.0, 145.9, 126.0, 124.0, 121.2, 118.9, 113.1, 108.6, 78.8, 73.3, 71.7, 63.0, 56.0, 55.5, 54.8, 42.7, 38.9, 37.9, 36.9, 28.3, 24.9, 24.4, 23.5, 15.0; ESI-HRMS: m/z 479.2406 [M + Na]+, calcd for C27H36NaO6, 479.2410.
(14β)-(2′-Methoxy)phenoxy-andrographolide (6b12). White solid; mp 175 °C to 178 °C; 88% yield; 1H NMR (400 MHz, CDCl3) δ 7.13–7.02 (m, 1H), 7.01–6.83 (m, 4H), 5.56 (d, J = 5.4 Hz, 1H), 4.81 (d, J = 1.8 Hz, 1H), 4.55–4.40 (m, 2H), 4.32 (s, 1H), 4.13 (t, J = 8.9 Hz, 1H), 3.92–3.81 (m, 3H), 3.47–3.35 (m, 1H), 3.30 (d, J = 11.1 Hz, 1H), 2.42–2.33 (m, 1H), 2.32–2.21 (m, 2H), 2.02–1.90 (m, 1H), 1.88–1.73 (m, 3H), 1.73–1.65 (m, 1H), 1.60–1.52 (m, 1H), 1.32–1.24 (m, 1H), 1.23 (s, 3H), 1.22–1.16 (m, 1H), 1.10 (td, J = 13.2, 4.2 Hz, 1H), 0.59 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 169.77, 151.35, 150.68, 147.23, 145.29, 125.46, 124.33, 121.09, 119.97, 112.35, 108.12, 80.45, 73.42, 71.20, 64.14, 55.80, 55.69, 55.03, 42.87, 38.88, 37.69, 36.64, 28.22, 25.55, 23.73, 22.70, 15.10; ESI-HRMS: m/z 479.2400 [M + Na]+, calcd for C27H36NaO6, 479.2410.
(14β)-(3′-Methoxy)phenoxy-andrographolide (6b13). White solid; mp 156 °C to 158 °C; 92% yield; 1H NMR (400 MHz, (CD3)2SO) δ 7.29–7.16 (m, 1H), 6.94 (dd, J = 7.2, 6.0 Hz, 1H), 6.64–6.49 (m, 3H), 5.79 (d, J = 5.3 Hz, 1H), 4.99 (d, J = 4.9 Hz, 1H), 4.81 (s, 1H), 4.66 (dd, J = 10.7, 5.4 Hz, 1H), 4.52 (s, 1H), 4.28 (dd, J = 10.7, 1.1 Hz, 1H), 4.10 (dd, J = 7.5, 2.8 Hz, 1H), 3.79–3.37 (m, 1H), 3.74 (s, 3H), 3.19 (dd, J = 10.8, 7.7 Hz, 1H), 3.05–2.98 (m, 1H), 2.43–2.35 (m, 1H), 2.33–2.22 (m, 2H), 1.92 (t, J = 10.2 Hz, 2H), 1.70 (d, J = 13.2 Hz, 1H), 1.47 (m, 3H), 1.36–1.21 (m, 1H), 1.11–1.05 (m, 1H), 1.02 (s, 3H), 0.94 (m, 1H), 0.55 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 169.09, 160.65, 157.69, 149.66, 147.70, 130.25, 125.73, 107.71, 107.55, 107.35, 102.01, 78.24, 71.14, 71.04, 62.52, 55.66, 55.16, 54.21, 42.13, 38.68, 37.41, 36.17, 27.75, 24.91, 23.88, 22.93, 14.56; ESI-HRMS: m/z 479.2400 [M + Na]+, calcd for C27H36NaO6, 479.2410.
(14β)-(Naphthyl-1′-oxy)andrographolide (6b14). White solid; mp 172 °C to 175 °C; 88% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.07 (d, J = 8.1 Hz, 1H), 7.92 (d, J = 7.7 Hz, 1H), 7.62–7.41 (m, 4H), 7.14 (t, J = 7.2 Hz, 1H), 7.01 (d, J = 7.5 Hz, 1H), 5.99 (d, J = 5.2 Hz, 1H), 4.84 (s, 1H), 4.83–4.79 (m, 1H), 4.76 (d, J = 4.9 Hz, 1H), 4.62 (s, 1H), 4.44 (dd, J = 10.8, 1.0 Hz, 1H), 4.05–4.00 (m, 1H), 3.63 (dd, J = 11.0, 2.8 Hz, 1H), 3.07 (dd, J = 10.8, 7.6 Hz, 1H), 2.45–2.33 (m, 2H), 2.33–2.22 (m, 2H), 1.96–1.86 (m, 2H), 1.63–1.54 (m, 1H), 1.24 (m, 3H), 1.06 (m, 1H), 0.80 (s, 3H), 0.69 (dd, J = 12.6, 2.2 Hz, 1H), 0.58–0.47 (m, 1H), 0.43 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 169.13, 168.88, 151.95, 150.00, 147.65, 134.31, 127.60, 126.69, 126.08, 125.97, 125.58, 124.93, 121.86, 121.66, 121.00, 107.65, 106.45, 99.49, 77.83, 71.21, 62.40, 56.28, 53.62, 48.13, 41.83, 38.72, 38.61, 37.39, 35.72, 27.56, 24.92, 23.78, 22.59, 20.75, 20.75, 14.45; ESI-HRMS: m/z 499.2462 [M + Na]+, calcd for C30H36NaO5, 499.2460.
(14α)-(2′-Nitro-pyridinyl-3′-oxy)andrographolide (6a15). White solid; mp 140 °C to 142 °C; 83% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.21 (dd, J = 4.5, 1.1 Hz, 1H), 8.02 (dd, J = 8.5, 1.0 Hz, 1H), 7.83 (dd, J = 8.5, 4.6 Hz, 1H), 6.93 (t, J = 6.0 Hz, 1H), 6.06 (d, J = 5.4 Hz, 1H), 5.02 (d, J = 4.9 Hz, 1H), 4.77 (s, 1H), 4.72 (dd, J = 11.2, 5.6 Hz, 1H), 4.46 (dd, J = 11.1, 1.3 Hz, 1H), 4.40 (s, 1H), 4.12 (dd, J = 7.4, 2.9 Hz, 1H), 3.79 (dd, J = 11.0, 2.8 Hz, 1H), 3.26–3.13 (m, 2H), 2.47–2.41 (m, 1H), 2.36–2.25 (m, 2H), 1.91 (m, 2H), 1.71 (m, 1H), 1.63–1.44 (m, 3H), 1.32 (m, 1H), 1.19–1.11 (m, 1H), 1.10–1.02 (m, 1H), 1.06 (s, 3H), 0.55 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 168.58, 151.60, 149.00, 147.58, 144.24, 140.35, 129.44, 126.35, 123.96, 108.04, 78.36, 73.08, 70.19, 62.54, 55.07, 54.28, 42.19, 38.44, 37.30, 36.38, 27.77, 24.62, 23.82, 23.02, 14.51; ESI-HRMS: m/z 495.2102 [M + Na]+, calcd for C25H32N2NaO7, 495.2107.
(14β)-(2′-Nitro-pyridinyl-3′-oxy)andrographolide (6b15). White solid; mp 146 °C to 149 °C; 90% yield; 1H NMR (400 MHz, CD3OD) δ 8.18 (dd, J = 4.6, 1.2 Hz, 1H), 7.90 (dd, J = 8.5, 1.1 Hz, 1H), 7.75 (dd, J = 8.5, 4.6 Hz, 1H), 7.16 (td, J = 7.3, 1.5 Hz, 1H), 6.05 (d, J = 5.5 Hz, 1H), 4.90 (s, 1H), 4.73 (dd, J = 11.2, 5.6 Hz, 1H), 4.54 (s, 1H), 4.47 (dd, J = 11.2, 1.4 Hz, 1H), 4.07 (d, J = 11.1 Hz, 1H), 3.34 (d, J = 2.9 Hz, 1H), 3.28 (dd, J = 11.9, 4.1 Hz, 1H), 2.54 (m, 1H), 2.47–2.33 (m, 2H), 2.04 (m, 1H), 1.95 (m, 1H), 1.90–1.82 (m, 1H), 1.77–1.53 (m, 3H), 1.41–1.26 (m, 2H), 1.21 (s, 3H), 1.09–0.99 (m, 1H), 0.66 (s, 3H); 13C NMR (101 MHz, CD3OD) δ 170.88, 153.35, 151.17, 149.05, 145.93, 141.67, 130.25, 126.69, 125.80, 108.52, 80.74, 74.60, 72.00, 64.95, 57.59, 56.03, 43.64, 40.20, 38.89, 37.88, 28.93, 26.91, 25.20, 23.29, 15.47; ESI-HRMS: m/z 495.2101 [M + Na]+, calcd for C25H32N2NaO7, 495.2107.
(14α)-(Quinolyl-4′-oxy)andrographolide (6a16). White solid; mp 102.6 °C to 104.8 °C; 83% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.75 (d, J = 5.2 Hz, 1H), 8.03 (d, J = 7.5 Hz, 1H), 7.95 (d, J = 8.3 Hz, 1H), 7.77–7.71 (m, 1H), 7.53 (t, J = 7.6 Hz, 1H), 7.05 (d, J = 5.3 Hz, 1H), 7.01 (t, J = 6.8 Hz, 1H), 6.08 (d, J = 5.3 Hz, 1H), 4.96 (d, J = 4.9 Hz, 1H), 4.84–4.76 (m, 2H), 4.55 (s, 1H), 4.41 (d, J = 11.0 Hz, 1H), 4.05 (dd, J = 7.4, 2.8 Hz, 1H), 3.68 (dd, J = 11.0, 2.8 Hz, 1H), 3.13 (dd, J = 11.1, 7.6 Hz, 1H), 3.10–3.02 (m, 1H), 2.43–2.34 (m, 1H), 2.25 (d, J = 11.9 Hz, 1H), 1.94 (d, J = 11.4 Hz, 1H), 1.89–1.83 (m, 1H), 1.63 (d, J = 13.6 Hz, 1H), 1.49–1.41 (m, 1H), 1.40–1.35 (m, 2H), 0.98 (s, 3H), 0.85–0.67 (m, 3H), 0.64–0.56 (m, 1H), 0.40 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 169.4, 159.3, 151.8, 151.6, 149.4, 148.3, 130.5, 129.2, 126.5, 124.9, 121.8, 121.1, 108.5, 103.0, 78.8, 72.1, 71.1, 63.0, 55.5, 54.7, 42.6, 38.9, 37.7, 36.8, 28.2, 25.3, 24.2, 23.5, 14.9; ESI-HRMS: m/z 478.2587 [M + H]+, calcd for C29H36NO5, 478.2593.
(14β)-(Quinolyl-4′-oxy)andrographolide (6b16). White solid; mp 175 °C to 177 °C; 88% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.78 (d, J = 5.2 Hz, 1H), 8.07 (d, J = 7.6 Hz, 1H), 7.99 (d, J = 8.1 Hz, 1H), 7.81–7.74 (m, 1H), 7.21 (t, J = 7.2 Hz, 1H), 7.07 (d, J = 5.3 Hz, 1H), 6.13 (d, J = 5.2 Hz, 1H), 4.84 (s, 1H), 4.78 (d, J = 4.8 Hz, 1H), 4.66 (s, 1H), 4.48 (d, J = 11.1 Hz, 1H), 4.04–3.97 (m, 1H), 3.61 (dd, J = 11.0, 2.9 Hz, 1H), 3.06 (dd, J = 10.9, 7.6 Hz, 1H), 2.44 (d, J = 8.6 Hz, 1H), 2.31–2.24 (m, 3H), 1.89 (d, J = 9.3 Hz, 2H), 1.65–1.52 (m, 2H), 1.31–1.24 (m, 2H), 1.24–1.13 (m, 2H), 1.05–0.94 (m, 1H), 0.78 (s, 3H), 0.71–0.59 (m, 2H), 0.44 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 169.0, 166.5, 153.0, 147.9, 147.7, 145.9, 135.4, 129.6, 124.5, 123.8, 121.2, 120.7, 108.4, 104.2, 78.6, 74.8, 70.6, 62.8, 56.7, 54.6, 42.5, 39.3, 37.9, 36.6, 28.0, 25.7, 24.3, 23.3, 15.0; ESI-HRMS: m/z 478.2588 [M + H]+, calcd for C29H36NO5, 478.2593.
(14α)-(Quinolyl-8′-oxy)andrographolide (6a17). White solid; mp 151 °C to 153 °C; 79% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.89 (dd, J = 4.1, 1.7 Hz, 1H), 8.39 (dd, J = 8.4, 1.7 Hz, 1H), 7.69 (dd, J = 8.2, 0.9 Hz, 1H), 7.62–7.51 (m, 2H), 7.28 (dd, J = 7.6, 1.0 Hz, 1H), 6.90 (t, J = 6.3 Hz, 1H), 6.06 (d, J = 5.3 Hz, 1H), 5.00 (d, J = 4.9 Hz, 1H), 4.76–4.68 (m, 2H), 4.56–4.49 (m, 2H), 4.09 (dd, J = 7.5, 2.8 Hz, 1H), 3.71 (dd, J = 11.0, 2.7 Hz, 1H), 3.19–3.00 (m, 2H), 2.27–2.18 (m, 2H), 2.17–2.07 (m, 1H), 1.81 (m, 2H), 1.64 (m, 1H), 1.40 (m, 2H), 1.26–1.20 (m, 2H), 1.15 (m, 1H), 1.03 (m, 1H), 1.00 (s, 3H), 0.36 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 169.9, 152.7, 150.1, 149.9, 147.8, 141.3, 136.7, 130.0, 127.2, 126.1, 122.9, 122.5, 116.3, 108.6, 78.7, 74.0, 71.8, 63.0, 55.4, 54.6, 42.6, 38.8, 37.8, 36.6, 28.2, 25.1, 24.3, 23.4, 14.8; ESI-HRMS: m/z 478.2588 [M + H]+, calcd for C29H36NO5, 478.2593.
(14β)-(Quinolyl-8′-oxy)andrographolide (6b17). White solid; mp 134 °C to 136 °C; 76% yield; 1H NMR (400 MHz, CD3OD) δ 8.88 (dd, J = 4.3, 1.7 Hz, 1H), 8.40 (dd, J = 8.3, 1.7 Hz, 1H), 7.67–7.58 (m, 3H), 7.31–7.22 (m, 2H), 6.09 (d, J = 5.5 Hz, 1H), 4.89 (d, J = 1.0 Hz, 1H), 4.83–4.79 (m, 1H), 4.62–4.55 (m, 2H), 3.91 (d, J = 11.1 Hz, 1H), 3.63 (q, J = 7.0 Hz, 1H), 3.21 (d, J = 10.3 Hz, 1H), 2.52 (dd, J = 11.9, 4.2 Hz, 1H), 2.48–2.34 (m, 2H), 2.32–2.21 (m, 1H), 2.06 (m, 2H), 1.77–1.68 (m, 1H), 1.48–1.34 (m, 1H), 1.30–1.24 (m, 1H), 1.20 (m, 2H), 1.09 (m, 1H), 0.99 (s, 3H), 0.84 (m, 1H), 0.58 (m, 1H), 0.49 (s, 3H); 13C NMR (101 MHz, CD3OD) δ 171.84, 153.59, 151.90, 150.48, 149.14, 141.29, 138.32, 131.55, 128.29, 127.54, 123.50, 122.60, 112.92, 108.19, 80.50, 73.65, 73.10, 64.79, 57.59, 55.73, 43.35, 40.01, 38.80, 37.39, 28.65, 26.58, 25.10, 23.18, 15.28; ESI-HRMS: m/z 500.2407 [M + Na]+, calcd for C29H35NNaO5, 500.2413.
8b1 . White solid; mp 56 °C to 59 °C; 90% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.87 (d, J = 4.2 Hz, 1H), 8.37 (d, J = 8.3 Hz, 1H), 7.71–7.47 (m, 3H), 7.25 (d, J = 7.6 Hz, 1H), 7.02 (t, J = 7.2 Hz, 1H), 6.11 (d, J = 5.3 Hz, 1H), 4.88 (s, 1H), 4.78 (dd, J = 10.9, 5.5 Hz, 1H), 4.60 (s, 1H), 4.50 (d, J = 10.9 Hz, 1H), 4.35 (d, J = 11.3 Hz, 1H), 3.78 (d, J = 11.3 Hz, 1H), 2.31 (q, J = 9.3, 7.7 Hz, 3H), 2.17–2.08 (m, 1H), 2.04–1.91 (m, 2H), 1.87 (s, 3H), 1.75–1.53 (m, 3H), 1.35 (s, 1H), 1.27–1.11 (m, 3H), 1.08–0.94 (m, 2H), 0.86 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 211.7, 170.5, 169.7, 152.6, 150.0, 149.9, 147.4, 140.8, 136.6, 129.9, 127.2, 126.7, 122.5, 122.2, 113.9, 109.1, 73.1, 71.7, 65.8, 55.5, 54.9, 51.6, 38.9, 37.2, 37.0, 35.0, 25.7, 24.7, 20.9, 20.7, 14.3; ESI-HRMS: m/z 518.2536 [M + H]+, calcd for C31H36NO6, 518.25426.
8b2 . White solid; mp 125 °C to 127 °C; 86% yield; 1H NMR (400 MHz, (CD3)2SO) δ 8.85 (dd, J = 4.1, 1.7 Hz, 1H), 8.36 (dd, J = 8.4, 1.7 Hz, 1H), 7.64 (d, J = 7.4 Hz, 1H), 7.59–7.50 (m, 2H), 7.24 (d, J = 6.8 Hz, 1H), 7.02 (t, J = 6.8 Hz, 1H), 6.10 (d, J = 5.3 Hz, 1H), 4.86 (s, 1H), 4.77 (dd, J = 10.8, 5.5 Hz, 1H), 4.57 (s, 1H), 4.52–4.45 (m, 1H), 3.67 (d, J = 10.1 Hz, 1H), 3.36 (d, J = 10.3 Hz, 1H), 2.28 (m, 4H), 2.09 (m, 1H), 1.97 (m, 1H), 1.76–1.68 (m, 1H), 1.60 (m, 1H), 1.57–1.49 (m, 1H), 1.40–1.33 (m, 1H), 1.30–1.27 (m, 1H), 0.97–0.90 (m, 1H), 0.79 (s, 3H), 0.75 (s, 9H), 0.63 (s, 3H), −0.09 (d, J = 1.7 Hz, 6H); 13C NMR (101 MHz, (CD3)2SO) δ 212.4, 169.7, 152.6, 150.1, 149.8, 147.9, 140.8, 136.6, 129.9, 127.2, 126.6, 122.5, 122.2, 113.9, 108.9, 73.1, 71.7, 65.9, 55.4, 55.0, 53.5, 38.9, 37.4, 36.9, 35.6, 26.8, 26.1, 26.0, 25.8, 24.9, 21.3, 18.2, 14.4, −5.3, −5.4; ESI-HRMS: m/z 590.3297 [M + H]+, calcd for C35H48NO5Si, 590.3302.
A solution of 0.20 g (0.34 mmol) 8b2 in 4.0 mL dry DCM was cooled to −20 °C and treated with 4.0 mL trifluoroacetic acid (TFA) for 20 min. The reaction mixture was diluted with ethyl acetate and carefully treated with sol. sat. NaHCO3. The organic phase was washed with sol. sat. NaHCO3 and brine, then dried over anhydrous Na2SO4. The residue was filtered, dried and purified by silica gel column chromatography (petroleum ether/ethyl acetate) to afford (14β)-(quinolyl-8′-oxy)-3-keto andrographolide (9b) in 81% yield. 9b: white solid; mp 141 °C to 143 °C; 1H NMR (400 MHz, (CD3)2SO) δ 8.85 (dd, J = 4.1, 1.7 Hz, 1H), 8.36 (dd, J = 8.3, 1.7 Hz, 1H), 7.64 (d, J = 7.5 Hz, 1H), 7.60–7.50 (m, 2H), 7.24 (d, J = 6.8 Hz, 1H), 7.01 (dd, J = 7.3, 6.1 Hz, 1H), 6.10 (d, J = 5.3 Hz, 1H), 4.85 (s, 1H), 4.77 (dd, J = 10.8, 5.5 Hz, 1H), 4.56 (s, 1H), 4.49 (dd, J = 10.8, 1.1 Hz, 1H), 4.43 (t, J = 5.4 Hz, 1H), 3.66 (dd, J = 10.9, 5.7 Hz, 1H), 3.16 (dd, J = 10.9, 5.2 Hz, 1H), 2.42 (m, 1H), 2.28 (m, 3H), 2.06 (m, 1H), 2.00–1.90 (m, 1H), 1.67 (m, 1H), 1.62–1.47 (m, 2H), 1.34 (m, 1H), 1.25–1.18 (m, 1H), 0.99–0.87 (m, 1H), 0.80 (s, 3H), 0.65 (s, 3H); 13C NMR (101 MHz, (CD3)2SO) δ 212.64, 169.30, 152.13, 149.76, 149.45, 147.42, 140.31, 136.18, 129.50, 126.72, 126.19, 122.12, 121.79, 113.51, 108.43, 72.64, 71.29, 63.84, 55.38, 54.75, 53.68, 38.55, 37.11, 36.98, 35.21, 25.32, 24.24, 20.10, 14.15; ESI-HRMS: m/z 476.2431 [M + H]+, calcd for C29H34NO5, 476.2437.
Bacteriostatic screening was performed by applying a modified protocol of the National Committee on Clinical Laboratory Standards (NCCLS).29 Briefly, E. coli, S. aureus and E. faecalis cells (American Type Culture Collection, ATCC) were grown in LB plate medium for one passage, then seeded in LB liquid medium and cultured at 37 °C for 12 h. After being diluted 1000 times, 190 μL diluted cells were aliquoted to 96-well plates; then, 10 μL stock solution of carrier or a gradient amount of the tested compound or the positive compound, ciprofloxacin, were added; the final concentration of DMSO was 1% in all wells. The blank controls contained 1% DMSO and LB medium but no bacterial cells. The cultures were incubated at 37 °C for 24 h, and the growth data were expressed as OD630; the IC50 values were calculated based on growth data versus test concentration.
Cytotoxicity of testing compounds. AD-293 cells were plated in a 96-well plate at a concentration of 1.0 × 105 cells per well overnight to allow cell attachment. Working solutions of the tested compounds, the positive drug, DCB-3503
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 of a cryptopleurine analog, or the DMSO vehicle as a control were dispensed appropriately into the partitioned 96-well plates, which were then incubated for another 24 h. Then, the medium was discarded and the cells were incubated for 4 h at 37 °C in MTT solution (final concentration 0.5 mg mL−1). The solution was then replaced with 100 μL DMSO to dissolve the violet formazan crystals in the intact cells. Cell growth was assessed by MTT according to the manufacturer's protocol. The absorbance was measured at 570 nm as the reference wavelength. The cytotoxicity as the CC50 value (concentration of 50% cell growth/viability inhibition) was calculated based on the percentage of cell viability data compared to the control group. Each concentration was repeated 3 times independently.
36 of a cryptopleurine analog, or the DMSO vehicle as a control were dispensed appropriately into the partitioned 96-well plates, which were then incubated for another 24 h. Then, the medium was discarded and the cells were incubated for 4 h at 37 °C in MTT solution (final concentration 0.5 mg mL−1). The solution was then replaced with 100 μL DMSO to dissolve the violet formazan crystals in the intact cells. Cell growth was assessed by MTT according to the manufacturer's protocol. The absorbance was measured at 570 nm as the reference wavelength. The cytotoxicity as the CC50 value (concentration of 50% cell growth/viability inhibition) was calculated based on the percentage of cell viability data compared to the control group. Each concentration was repeated 3 times independently.
Signaling pathway reporter assay. Reporter cells were treated with 50 ng mL−1 TNF-α to stimulate the NF-κB signaling pathway, 1 μg mL−1 LPS to stimulate the TLR4/NF-κB signaling pathway, and 2.5 μg mL−1 IL-6 to stimulate the IL-6/STAT3 signaling pathway. The medium was removed at the end of the treatment, cell extracts were prepared, and the luciferase activity was measured using a Luciferase assay kit (Promega) according to the manufacturer's instructions. EC50 was defined as the concentration of drug that inhibited stimulator-triggered luciferase reporter activation by 50% after continuous drug exposure for 4 (TNF-α/NF-κB) or 16 (IL-6/STAT3 and TLR4/NF-κB) hours. Each concentration was repeated 3 times independently.
Author contributions
GCZ, DW and YW conceived and supervised the project, analyzed the data and wrote the paper. GCZ, FL, DS, XN, ZL and DW designed the andrographolide derivatives; FL, DS, XN, ZL and DW conducted the syntheses of andrographolide derivatives. YW, XML, SRC, QZ, and YTW designed the biological experiments. XML, SRC and QZ conducted the biology experiments. All authors have read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported in part by the Natural Science Foundation of China (30973621) and Six Major Talents of Jiangsu Province of China (2014). This work was partially supported by the Macao Science and Technology Development Fund 041/2014/A1, Research Fund of the University of Macao MYRG 2016-00105-ICMS-QRCM, and MYRG 2017-00116-ICMS-QRCM to YW, and MYRG2015-00098-ICMS-QRCM to YTW.References
- (a) C. Abraham and J. H. Cho, N. Engl. J. Med., 2009, 361, 2066–2078 CrossRef CAS; (b) A. N. Ananthakrishnan, Nat. Rev. Gastroenterol. Hepatol., 2015, 12, 205–217 CrossRef; (c) Inflammatory bowel disease, Nature, 2016, 540, S97–S140 CrossRef.
- (a) L. Jostins, S. Ripke and R. K. Weersma, et al. , Nature, 2012, 491, 119–124 CrossRef CAS; (b) T. T. Macdonald and G. Monteleone, Science, 2005, 307, 1920–1925 CrossRef CAS.
- K. O. Arseneau, H. Tamagawa, T. T. Pizarro and F. Cominelli, Curr. Gastroenterol., 2007, 9, 508–512 CrossRef.
- C. G. Knutson, A. Mangerich, Y. Zeng, A. R. Raczynski, R. G. Liberman, P. Kang, W. Ye, E. G. Prestwich, K. Lu, J. S. Wishnok, J. R. Korzenik, G. N. Wogan, J. G. Fox, P. C. Dedon and S. R. Tannenbaum, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, E2332–E2341 CrossRef CAS.
- (a) M. F. Neurath, Nat. Rev. Immunol., 2014, 14, 329–342 CrossRef CAS; (b) C. Luo and H. Zhang, Mediators Inflammation, 2017, 11, 5126048 Search PubMed.
- (a) P. P. Tak and G. S. Firestein, J. Clin. Invest., 2017, 107, 7–11 CrossRef; (b) K. Mitsuyama, S. Matsumoto, J. Masuda, H. Yamasakii, K. Kuwaki, H. Takedatsu and M. Sata, Anticancer Res., 2007, 27, 3749–3756 CAS.
- L. Sherwood, W. Joanne and W. Christopher, Prescott's Microbiology, 2013, New York, McGraw Hill, 9th edn, pp. 713–721 Search PubMed.
- R. Saxena and V. K. Sharma, A Metagenomic Insight Into the Human Microbiome: Its Implications in Health and Disease, in Medical and Health Genomics, ed. D. Kumar and S. Antonarakis, Elsevier Science, 2016 Search PubMed.
- (a) A. Shawki and D. F. McCole, Nat. Rev. Gastroenterol. Hepatol., 2017, 3, 41–50 Search PubMed; (b) E. Balish and T. Warner, Am. J. Pathol., 2002, 160, 2253–2257 CrossRef CAS; (c) Y. Zhou, H. Chen, H. He, Y. Du, J. Hu, Y. Li, Y. Li, Y. Zhou, H. Wang, Y. Chen and Y. Nie, Medicine), 2016, 95, e5019 CrossRef CAS.
- (a) C. S. Pedamallu, A. S. Bhatt, S. Bullman, S. Fowler, S. S. Freeman, J. Durand, J. Jung, F. Duke, V. Manzo, D. Cai, A. Ananthakrishnan, A. I. Ojesina, A. Ramachandran, D. Gevers, R. J. Xavier, A. K. Bhan, M. Meyerson and V. Yajnik, Nat. Rev. Gastroenterol. Hepatol., 2016, 2, 563–566 Search PubMed; (b) D. Bettenworth, T. M. Nowacki, A. Friedrich, K. Becker, J. Wessling and J. Heidemann, World J. Gastroenterol., 2013, 19, 4418–4421 CrossRef; (c) M. Chiba, S. Hoshina, M. Kono, M. Tobita, T. Fukushima, M. Iizuka and S. Watanabe, Scand. J. Gastroenterol., 2001, 36, 615–620 CrossRef CAS.
- F. Guarner and J. Malagelada, Lancet, 2003, 361, 512–519 CrossRef.
- (a) M. K. Gorter, Recl. Trav. Chim. Pays-Bas, 1911, 30, 151–160 CrossRef; (b) R. J. C. Kleipool, Nature, 1952, 169, 33–34 CrossRef CAS; (c) M. P. Cava, W. R. Chan, L. J. Haynes, L. F. Johnson and B. Weinstein, Tetrahedron, 1962, 18, 397–403 CrossRef CAS.
- (a) R. N. Chakravarti and D. Chakravarti, Indian Med. Gaz., 1951, 86, 96–97 CAS; (b) T. Zhang, Zhongyaocai, 2000, 23, 366–368 CAS; (c) X. Liu, Y. Wang and G. Li, Zhongyaocai, 2003, 26, 135–138 Search PubMed; (d) P. K. Singha, S. Roy and S. Dey, Fitoterapia, 2003, 74, 692–694 CrossRef; (e) S. Gupta, K. P. Mishra and L. Ganju, Arch. Virol., 2017, 162, 611–623 CrossRef CAS.
- Chinese Pharmacopoeia Commission, Chinese Pharmacopoeia Part 2, 2015, pp. 619–620 Search PubMed.
- (a) R. S. Chan, L. Ding, G. Q. Chen, Q. C. Pan, Z. L. Zhao and K. M. Smith, Proc. Soc. Exp. Biol. Med., 1991, 197, 59–66 CrossRef; (b) A. Basak, S. Cooper, A. G. Roberg, U. K. Banik, M. Chretien and N. G. Seidah, Biochem. J., 1999, 338, 107–113 CrossRef CAS.
- (a) C. Aromdee, Expert Opin. Ther. Pat., 2014, 24, 1129–1138 CrossRef CAS; (b) B. Zhou, D. Zhang and X. Wu, Mini-Rev. Med. Chem., 2013, 13, 298–309 CAS; (c) C. Aromdee, Expert Opin. Ther. Pat., 2012, 22, 169–180 CrossRef CAS; (d) T. Kasemsuk, P. Piyachaturawat, R. Bunthawong, U. Sirion, K. Suksen, A. Suksamrarn and R. Saeeng, Eur. J. Med. Chem., 2017, 138, 952–963 CrossRef CAS.
- H. T. Li, H. R. Qin, W. H. Wang, C. J. Li, C. M. Wu and J. X. Song, China J. Chin. Mater. Med., 2006, 1, 1016–1017 Search PubMed.
- X. Jiang, P. Yu, J. Jiang, Z. Zhang, Z. Wang, Z. Yang, Z. Tian, S. C. Wright, J. W. Larrick and Y. Wang, Eur. J. Med. Chem., 2009, 4, 2936–2943 CrossRef.
- (a) C. Calabrese, S. H. Berman, J. G. Babish, X. Ma, L. Shinto, M. Dorr, K. Wells, A. A. Wenner and L. J. Standish, Phytother. Res., 2000, 14, 333–338 CrossRef CAS; (b) Y. C. Shen, C. F. Chen and W. F. Chiou, Br. J. Pharmacol., 2002, 35, 399–406 CrossRef; (c) Y. F. Xia, B. Q. Ye, Y. D. Li, J. G. Wang, X. J. He, X. Lin, X. Yao, D. Ma, A. Slungaard, R. P. Hebbel, N. S. Key and J. G. Geng, J. Immunol., 2004, 173, 4207–4217 CrossRef CAS PubMed; (d) M. A. Hidalgo, A. Romero, J. Figueroa, P. Cortes, I. I. Concha, J. L. Hancke and R. A. Burgos, Br. J. Pharmacol., 2005, 144, 680–686 CrossRef CAS.
- H. Gao and J. Wang, Andrographolide inhibits multiple myeloma cells by inhibiting the TLR4/NF-kappaB signaling pathway, Mol. Med. Rep., 2016, 13, 1827–1832 CrossRef CAS.
- M. T. Islam, Front. Pharmacol., 2017, 8, 571 CrossRef.
- C. H. Yang, T. L. Yen, C. Y. Hsu, P. A. Thomas, J. R. Sheu and T. Jayakumar, Int. J. Mol. Sci., 2017, 18, E1638 CrossRef PubMed.
- C. V. Chandrasekaran, P. Thiyagarajan, H. B. Deepak and A. Agarwal, Int. Immunopharmacol., 2011, 11, 79–84 CrossRef CAS PubMed.
- Y. Li, H. Yan, Z. Zhang, G. Zhang, Y. Sun, P. Yu, Y. Wang and L. Xu, Br. J. Pharmacol., 2015, 172, 3151–3158 CrossRef CAS.
- X. Nie, S.-R. Chen, K. Wang, Y. Peng, Y.-T. Wang, D. Wang, Y. Wang and G.-C. Zhou, Sci. Rep., 2017, 7, 4738 CrossRef.
- (a) Z. Liu, W.-K. Law, D. Wang, X. Nie, D. Sheng, G. Song, K. Guo, P. Wei, P. Ouyang, C.-W. Wong and G.-C. Zhou, RSC Adv., 2014, 4, 13533–13545 RSC; (b) Y. Peng, J. Li, Y. Sun, J. Y.-W. Chan, D. Sheng, K. Wang, P. Wei, P. Ouyang, D. Wang, S. M. Y. Lee and G.-C. Zhou, RSC Adv., 2015, 5, 22510–22526 RSC.
- W. Leung, G. Malhi, B. M. Willey, A. J. McGeer, B. Borgundvaag, R. Thanabalan, P. Gnanasuntharam, B. Le, A. V. Weizman, K. Croitoru, M. S. Silverberg, A. H. Steinhart and G. C. Nguyen, Journal of Crohn's & Colitis, 2012, 6, 743–749 Search PubMed.
- WHO, Antimicrobial resistance: global report on surveillance, 2014 Search PubMed.
- (a) NCCLS, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically M7-A5, National Committee on Clinical Laboratory Standards, 2000, vol. 20, p. 2 Search PubMed; (b) NCCLS, Susceptibility Testing of Mycobacteria, Nocardia, and Other Aerobic Actinomycetes; Tentative Standard, Second Edition, M24-T2, National Committee on Clinical Laboratory Standards, 2000, vol. 20, p. 26 Search PubMed; (c) V. Samoylenko, M. R. Jacob, S. I. Khan, J. Zhao, B. L. Tekwani, J. O. Midiwo, L. A. Walker and I. Muhammad, Nat. Prod. Commun., 2009, 4, 791–796 CAS.
- (a) G. Bamias, M. R. Nyce, S. A. De La Rue and F. Cominelli, Ann. Intern. Med., 2005, 143, 895–904 CrossRef CAS PubMed; (b) D. K. Podolsky, N. Engl. J. Med., 2002, 347, 417–429 CrossRef CAS PubMed.
- K. A. Shirey, W. Lai, A. J. Scott, M. Lipsky, P. Mistry, L. M. Pletneva, C. L. Karp, J. McAlees, T. L. Gioannini, J. Weiss, W. H. Chen, R. K. Ernst, D. P. Rossiqnol, F. Gusovsky, J. C. Blanco and S. N. Vogel, Nature, 2013, 497, 498–502 CrossRef CAS.
- J. Xing, R. Li, N. Li, J. Zhang, Y. Li, P. Gong, D. Gao, H. Liu and Y. Zhang, Mol. Cell. Biochem., 2015, 407, 89–95 CrossRef CAS.
- (a) R. Carey, I. Jurickova, E. Ballard, E. Bonkowski, X. Han, H. Xu and L. A. Denson, Inflammatory Bowel Dis., 2008, 14, 446–457 CrossRef; (b) S. Zundler and M. F. Neurath, Vaccines, 2016, 4, 5 CrossRef.
- (a) S. R. Vavricka, J. A. Galván, H. Dawson, A. Soltermann, L. Biedermann, M. Scharl, A. M. Schoepfer, G. Rogler, M. B. PrinzVavricka, L. Terracciano, A. Navarini, I. Zlobec, A. Lugli and T. Greuter, J. Crohn's Colitis, 2018, 12, 347–354 CrossRef; (b) C. Luo and H. Zhang, Mediators Inflammation, 2017, 5126048 Search PubMed.
- A. Piechota-Polanczyk and J. Fichna, Naunyn-Schmiedeberg's Arch. Pharmacol., 2014, 387, 605–620 CrossRef CAS PubMed.
- Y. Wang, H. C. Wong, E. A. Gullen, W. Lam, X. Yang, Q. Shi, K. H. Lee and Y. C. Cheng, PLoS One, 2012, 7, e51138 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra01063c |
| ‡ These authors contributed equally to this work. |
| § Current address: Department of Pharmaceutical and Chemical Engineering, Taizhou Polytechnic College, Taizhou 225300, Jiangsu, PR China. |
| This journal is © The Royal Society of Chemistry 2018 |