Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Novel caged luciferin derivatives can prolong bioluminescence imaging in vitro and in vivo†
Chaochao Zhanga,
Mingliang Yuan a,
Guangxi Hana,
Yuqi Gaoa,
Chunchao Tanga,
Xiang Lia,
Lupei Dua and
Minyong Li*abc
a,
Guangxi Hana,
Yuqi Gaoa,
Chunchao Tanga,
Xiang Lia,
Lupei Dua and
Minyong Li*abc
aDepartment of Medicinal Chemistry, Key Laboratory of Chemical Biology (MOE), School of Pharmacy, Shandong University, Jinan, Shandong 250012, China. E-mail: mli@sdu.edu.cn; Fax: +86-531-8838-2076; Tel: +86-531-8838-2076
bState Key Laboratory of Microbial Technology, Shandong University, Jinan, Shandong 250100, China
cShenzhen Research Institute, Shandong University, Shenzhen, Guangdong 518057, China
First published on 30th May 2018
Abstract
Based on N-cyclobutylaminoluciferin (cybLuc), a set of high and efficient caged bioluminescent derivatives (Clucs) as firefly luciferase pro-substrates has been developed herein. After careful examination, these molecules exhibited low cytotoxicity and prolonged bioluminescence imaging up to 6 h in vitro and in vivo. Importantly, these caged luciferin derivatives have the potential to serve as long-term tracking tools to explore some biological process by using bioluminescent imaging.
Bioluminescent imaging (BLI) is a consistently sensitive, convenient, reliable and non-invasive imaging technique that has been comprehensively applied for monitoring a myriad of life processes,1 including cell proliferation and migration,2 tumor growth,3 protein–protein interactions,4 enzyme activities5 and other uses.6 The firefly luciferase-luciferin system is one of the most commonly used among the approximate 30 different bioluminescence systems.7 In the last two decades, to expand the application of firefly luciferase-luciferin bioluminescent imaging in vitro and in vivo, a lot of effort has been made.8 A mass of caged luciferin analogues has been well developed, which could serve as highly responsive bioluminescent probes for specific biomolecules.9 For instance, in order to test various enzymes activity, several bioluminescent probes have been well designed for aminopeptidase N,10 nitroreductase,11 β-galactosidase,12 as well as H2O2.13 Meanwhile, modified luciferins have also been developed, such as an alkyne-modified luciferin,14 6-substituted luciferin analogues,15 luciferin analogues containing a six-membered oxazine ring16 and a selenium analogue of firefly D-luciferin.17 However, the broad application of the luciferin is limited to strong attenuation of bioluminescent signals that are emitted below 600 nm, which results from the absorption and scattering of light by tissue.18 Moreover, the relatively short circulatory half-life of luciferin in vivo is another important factor to consider.
Recently, caged compounds, shielded by protecting groups have been utilized to distantly control the cellular functions19 or to investigate unknown biological phenomena.20 These the bio-inactive caged compounds can generate the biological activity when the protecting groups are removed.21 Specifically, a novel genetic technique for photo-mediated temporal and spatial control of gene activation in zebrafish embryos has been demonstrated as an alternative to the gene knockdown approach by using antisense, morpholino-modified oligonucleotides.22 In line with this direction, several caged luciferins have been well developed. For example, four bisdeoxycoelenterazine derivatives were synthesized which displayed the potential for improving the bioluminescence resonance energy transfer assays with reasonable light signal sustainability.23 Moreover, ten novel pro-substrates for Renilla luciferase were designed and synthesized by introducing ester protecting groups into the carbonyl group of the free luciferin, which could be efficiently activated by intracellular esterases or other hydrolases leading to a bioluminescence enhancement.24 As a result, caging strategy is very promising to prolong the time of bioluminescent imaging.
Recently, we introduced N-cyclobutylaminoluciferin (cybLuc) with robust red-shifted light emission and long-term emission which were higher than those of D-luciferin and aminoluciferin.25 In the current study, we designed and synthesized a series of caged compounds (Clucs, a–c, Scheme 1) based on cybLuc to prolong the bioluminescence duration. These caged compounds are proposed to release the free luciferins in an aqueous solution, which subsequently interact with firefly luciferase to generate the bioluminescent signals. It should be noted that among all three compounds, the caged luciferin 2 (Cluc-2) displayed prolonged bioluminescent imaging in vivo so that it may serve as a promising pro-substrate for BLI in diagnostic and therapeutic fields.
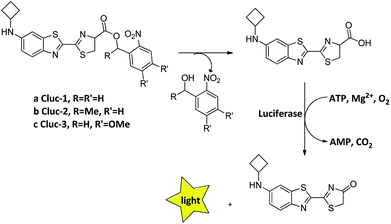 | ||
| Scheme 1 The structure of caged luciferin substrates that can release the substrates of firefly luciferase. | ||
Our caged luciferin derivatives can be conveniently prepared with a facile and efficient method26 as depicted in Scheme S1.† In brief, 1-(2-nitrophenyl)ethanones reacted with hydrazine hydrate in dry ethanol under reflux to provide 2-nitrobenzylidene-hydrazines, which were oxidized by activated manganese(IV)-oxide to obtain 1-(diazomethyl)-2-nitrobenzenes. A subsequent reaction of 1-(diazomethyl)-2-nitrobenzenes with cybLuc generated the caged derivatives (a–c). The details for the preparation of the compounds and their 1NMR, 13C NMR and ESI-HRMS spectra can be found in the ESI.†
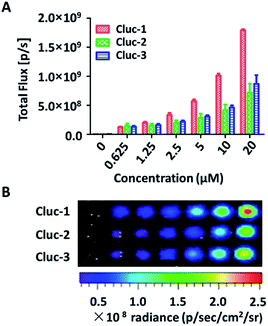
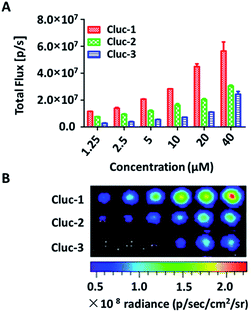
After obtaining the products, to test whether these compounds can release cybLuc in the aqueous solution, these caged luciferin derivatives at various concentrations (0–40 μM) were examined in Tris–HCl buffer (100 μL). The equal volume of Tris–HCl buffer containing 20 μg mL−1 luciferase and 2 mM ATP were added, and bioluminescence signals were detected with an exposure time of 1 s. As shown in Fig. 1, light emission intensities were enhanced with increasing caged luciferin derivatives concentration. These caged compounds displayed similar dose-dependent manner, and the Cluc-1 had the highest bioluminescence intensity. Overall, these interesting results indicated that these caged luciferin derivatives could release luciferins in Tris–HCl buffer so that they could serve as luciferin pro-substrates using in bioluminescence imaging.
The caged luciferin derivatives as imaging agents should be low cytotoxicity. Therefore, we used the way of Cell Counting Kit-8 to investigate the cell viability. The ES-2-Fluc cells (8 × 103 cells per well) were incubated in 96-well plates and the culture medium was removed after 24 h. Then a variety of dilutions of the caged luciferin derivatives in complete growth medium were added. After 12 h incubation, the solution of CCK-8 was added. Finally, the absorbance signals were recorded by a microplate reader at 450 nm, and no marked cytotoxicity was observed (Fig. S1†). The IC50 values of these caged luciferin derivatives were much greater than the concentration (40 μM) used in cell imaging, demonstrating good biocompatibility with live cells. Accordingly, we could further investigate the properties of caged luciferin derivatives.
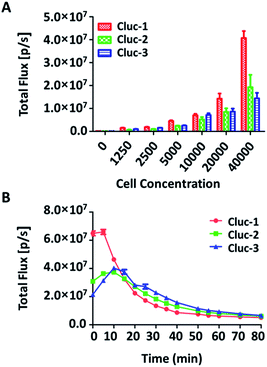
To evaluate the properties of caged luciferin derivatives in BLI at the cellular level, bioluminescence signals from ES-2-Fluc cancer cell lines with constitutive expression of Fluc were analysed. ES-2-Fluc cells were passed and plated (4 × 104 cells per well) in 96-well plates and cultured 24 h. Then the medium was removed and increasing concentrations of caged luciferin derivatives in Tris–HCl buffer (1.25 μM, 2.5 μM, 5 μM, 10 μM, 20 μM and 40 μM) were added. Meanwhile, ES-2-Fluc at various (0, 1250, 2500, 5000, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 20
000, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 40
000 and 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well) were passed and plated in 96-well plates and incubated for 24 h. Subsequently, the caged luciferin derivatives (40 μM) were added after removing the medium. The bioluminescence was measured at once with an exposure of 10 s and the bioluminescence intensity was collected. As shown in Fig. 2, with increasing concentrations of caged luciferin derivatives, the bioluminescent intensities increased in a dose-dependent manner. Similarly, the bioluminescent intensities of caged luciferin derivatives increased with increasing amounts of cells (Fig. 3A). Overall, these three different caged luciferin derivatives exhibited analogical properties that could release the substrates and produce similar bioluminescence intensity in cell. These results demonstrated that these caged luciferin derivatives could be potent luciferin bioluminescence substrates in the cellular level.
000 cells per well) were passed and plated in 96-well plates and incubated for 24 h. Subsequently, the caged luciferin derivatives (40 μM) were added after removing the medium. The bioluminescence was measured at once with an exposure of 10 s and the bioluminescence intensity was collected. As shown in Fig. 2, with increasing concentrations of caged luciferin derivatives, the bioluminescent intensities increased in a dose-dependent manner. Similarly, the bioluminescent intensities of caged luciferin derivatives increased with increasing amounts of cells (Fig. 3A). Overall, these three different caged luciferin derivatives exhibited analogical properties that could release the substrates and produce similar bioluminescence intensity in cell. These results demonstrated that these caged luciferin derivatives could be potent luciferin bioluminescence substrates in the cellular level.
 | ||
| Fig. 2 (A) Bioluminescence imaging of caged luciferin derivatives in ES-2-Fluc with different concentrations. (B) Bioluminescence imaging of caged luciferin derivatives in ES-2-Fluc. | ||
To further explore whether the caged luciferin derivatives can serve as a long-term tracking tool, a real-time imaging experiment was conducted at the cellular level. The bioluminescence signal was recorded every five minutes after ES-2-Fluc cells were treated with the caged luciferin derivatives. In brief, cybLuc displayed rapid bioluminescent signal decay and lost approximately 90% of its initial light intensity at 10 min (Fig. S2†). In contrast, other caged luciferin derivatives especially Cluc-2 and Cluc-3 exhibited a longer time to peak and slower kinetics characteristics (Fig. 3B). Moreover, the bioluminescent intensities of compounds Cluc-2 and Cluc-3 could stay about 1 h until they lost most of its light intensity even if they were lower than that of cybLuc. In general, these caged luciferin derivatives could release cybLuc slowly and prolong the time of bioluminescence imaging in cell. These promising results prompted us to employ the caged luciferin derivatives for bioluminescence imaging in living animals.
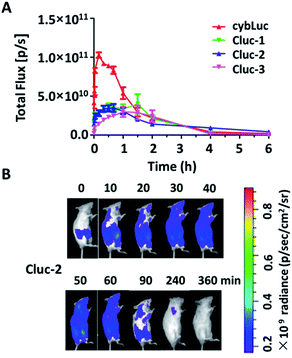
The potential of caged luciferin derivatives for the bio-imaging in vivo was further explored. Pathogen-free luciferase-expressing transgenic mice (about 24 g, female) were provided. CybLuc and caged luciferin derivatives were injected intraperitoneally (100 μL of 1 mM solutions in NS) into mice after they were anesthetized. Then bioluminescence imaging was immediately acquired with an exposure time of 1 s and the light signal was recorded every ten min until it disappeared. Every compound was evaluated using at least three mice. As depicted in Fig. 4, the bioluminescent intensity gradually increased to a maximum and then decayed over time. There existed differences between cybLuc and caged luciferin derivatives about the time of the maximum peak. In the case of cybLuc, the bioluminescence signal peaked at 20 min after injection then declined at a fast rate. However, the signals of Cluc-1 and Cluc-3 reached a plateau after 90 min and then dropped rapidly. And the bioluminescent intensity appeared at 40 min following Cluc-2 injection into the mice. Importantly, the light signal of Cluc-2 could be detected after 6 h. However, the bioluminescent intensities of these caged luciferin derivatives were also reduced as the same as the cellular level. These results indicated that the caged luciferin derivatives especially Cluc-2 were hydrolysed at a slower rate and they were more suitable for stable and long-time imaging study. Moreover, even if these three caged luciferin derivatives exhibited analogical bioluminescence intensity and dose–response analysis in vitro, the vivo imaging profile of Cluc-2 was different from Cluc-1 and Cluc-3. We propose that the presence of benzylic methyl group may affect the cell penetration or increase the steric hindrance and decrease its hydrolysis rate, and so that compound Cluc-2 could prolong the time of bioluminescence imaging in vivo. Therefore, these caged luciferin derivatives, particularly Cluc-2, could be used for exploring its application in biological fields.
Conclusions
In conclusion, herein we designed and synthesized a series of caged luciferin derivatives based on cybLuc and investigated their bioluminescence properties with firefly luciferase, and further displayed their applications both in vitro and in vivo. The experimental results clearly demonstrated that these caged luciferin derivatives can easily release the luciferin in Tris–HCl buffer and they displayed decent performance in the bioluminescence assay, so as to be practical pro-substrates of luciferase. Moreover, not only did they indicate a reasonable positive correlation between the concentration and bioluminescent intensities, but also, they implied a longer time and slower kinetics in cell. After careful evaluation in vivo, we found that they hydrolysed at a slower rate and got the luminous peak at prolong time. Surprisingly, the light signal of the caged luciferin 2 (Cluc-2) is suitable for stable and long-time imaging study since its signal can be detected after 6 h administration. Compared with our previously luciferin derivative cybLuc, our newly caged derivatives had longer duration at the same dose for in vivo bioluminescence imaging. As a whole, these caged luciferin derivatives have the potential to serve as novel luciferase pro-substrates to explore some biological process in relevant fields by using bioluminescent imaging.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present work was supported by grants from the National Natural Science Foundation of China (No. 81673393), the Science Technology and Innovation Committee of Shenzhen Municipality (No. JCYJ20160331183934512), the Taishan Scholar Program at Shandong Province, the Qilu/Tang Scholar Program at Shandong University, the Key Research and Development Project of Shandong Province (No. 2017CXGC1401) and the Major Project of Science and Technology of Shandong Province (No. 2015ZDJS04001).Notes and references
- M. A. Paley and J. A. Prescher, MedChemComm, 2014, 5, 255–267 RSC.
- J. E. Kim, S. Kalimuthu and B. C. Ahn, Nucl. Med. Mol. Imaging, 2015, 49, 3–10 CrossRef PubMed.
- Y. Inoue, F. Sheng, S. Kiryu, M. Watanabe, H. Ratanakanit, K. Izawa, A. Tojo and K. Ohtomo, Mol. Imaging, 2011, 10, 377–385 Search PubMed.
- A. Dragulescu-Andrasi, C. T. Chan, A. De, T. F. Massoud and S. S. Gambhir, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 12060–12065 CrossRef PubMed.
- W. Zhang., M. Chen., D. B. West and A. F. Purchio, Mol. Imaging, 2005, 4, 88–90 CrossRef.
- J. R. Pribaz, N. M. Bernthal, F. Billi, J. S. Cho, R. I. Ramos, Y. Guo, A. L. Cheung, K. P. Francis and L. S. Miller, J. Orthop. Res., 2012, 30, 335–340 CrossRef PubMed.
- Z. M. Kaskova, A. S. Tsarkova and I. V. Yampolsky, Chem. Soc. Rev., 2016, 45, 6048–6077 RSC.
- S. T. Adams Jr and S. C. Miller, Curr. Opin. Chem. Biol., 2014, 21, 112–120 CrossRef PubMed.
- N. Hananya and D. Shabat, Angew. Chem., 2017, 56, 16454–16463 CrossRef PubMed.
- J. Li, L. Chen, W. Wu, W. Zhang, Z. Ma, Y. Cheng, L. Du and M. Li, Anal. Chem., 2014, 86, 2747–2751 CrossRef PubMed.
- R. H. Wong, T. Kwong, K. H. Yau and H. Y. Au-Yeung, Chem. Commun., 2015, 51, 4440–4442 RSC.
- T. S. Wehrman, G. von Degenfeld, P. O. Krutzik, G. P. Nolan and H. M. Blau, Nat. Methods, 2006, 3, 295–301 CrossRef PubMed.
- W. Wu, J. Li, L. Chen, Z. Ma, W. Zhang, Z. Liu, Y. Cheng, L. Du and M. Li, Anal. Chem., 2014, 86, 9800–9806 CrossRef PubMed.
- R. C. Steinhardt, J. M. O'Neill, C. M. Rathbun, D. C. McCutcheon, M. A. Paley and J. A. Prescher, Chemistry, 2016, 22, 3671–3675 CrossRef PubMed.
- D. K. Sharma, S. T. Adams Jr, K. L. Liebmann and S. C. Miller, Org. Lett., 2017, 19, 5836–5839 CrossRef PubMed.
- D. M. Mofford, G. R. Reddy and S. C. Miller, J. Am. Chem. Soc., 2014, 136, 13277–13282 CrossRef PubMed.
- N. R. Conley, A. Dragulescu-Andrasi, J. Rao and W. E. Moerner, Angew. Chem., 2012, 51, 3350–3353 CrossRef PubMed.
- R. S. Negrin and C. H. Contag, In vivo imaging using bioluminescence: a tool for probing graft-versus-host disease, Nature Publishing Group, 2006, vol. 6, pp. 484–490 Search PubMed.
- G. C. Ellis-Davies, Nat. Methods, 2007, 4, 619–628 CrossRef PubMed.
- A. Reiner, J. Levitz and E. Y. Isacoff, Curr. Opin. Pharmacol., 2015, 20, 135–143 CrossRef PubMed.
- Y. Sasaki, R. Sugiura, Y. Nishiyama, H. Tanimoto, T. Morimoto and K. Kakiuchi, Tetrahedron, 2014, 70, 7973–7976 CrossRef.
- H. Ando, T. Furuta, R. Y. Tsien and H. Okamoto, Nat. Genet., 2001, 28, 317–325 CrossRef PubMed.
- J. Levi., A. De., Z. Chen and S. S. Gambhir, J. Am. Chem. Soc., 2007, 129, 11900–11901 CrossRef PubMed.
- M. Yuan, X. Ma, T. Jiang, Y. Gao, Y. Cui, C. Zhang, X. Yang, Y. Huang, L. Du, I. Yampolsky and M. Li, Org. Biomol. Chem., 2017, 15, 10238–10244 Search PubMed.
- W. Wu, J. Su, C. Tang, H. Bai, Z. Ma, T. Zhang, Z. Yuan, Z. Li, W. Zhou, H. Zhang, Z. Liu, Y. Wang, Y. Zhou, L. Du, L. Gu and M. Li, Anal. Chem., 2017, 89, 4808–4816 CrossRef PubMed.
- D. B. Thomason, BioTechniques, 1993, 15, 848–850 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Chemical synthesis and cytotoxicity test, bioluminescence imaging in vitro and in vivo, 1H NMR, 13C NMR and ESI-HRMS. See DOI: 10.1039/c8ra02312c |
| This journal is © The Royal Society of Chemistry 2018 |