Open Access Article
Open Access ArticleReal-time simultaneous detection of microbial contamination and determination of an ultra low-content active pharmaceutical ingredient in tazarotene gel by near-infrared spectroscopy
Qian Xie†
,
Ruanqi Wu†,
Xiaoxiao Zhong†,
Yanhong Dong and
Qi Fan *
*
School of Pharmacy, Chongqing Medical University, Chongqing 400016, China. E-mail: fanqi787@cqmu.edu.cn; Tel: +86 23 6848 5161
First published on 30th July 2018
Abstract
This paper proposes and proves a real-time and non-destructive strategy for sensitive and simultaneous detection of microbial contamination and determination of an ultra low-content active pharmaceutical ingredient in tazarotene gel by near-infrared (NIR) spectroscopy. In this experiment, 88 samples of tazarotene gel (0.41–0.65 mg g−1 of tazarotene) were prepared using the standard addition method. Among them, 47 samples were inoculated with 50 μl of different concentrations of Escherichia coli (E. coli) DH5a in Luria–Bertani (LB) broth to give 1–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1 of E. coli DH5a in the gel, 6 samples with 50 μl of LB broth, and 35 samples with nothing. Based on the gel NIR transflectance spectra, E. coli DH5a in the gel was detected by the counter propagation artificial neural network (CP-ANN) model with a classification accuracy of 100.0%, while tazarotene in the gel was simultaneously determined by the partial least squares regression (PLS) model with a root mean square error of cross-validation of 0.0232 mg g−1. Furthermore, 9 samples of real tazarotene gel were used to verify the practicality of the established NIR spectroscopy. The developed NIR strategy can be used to correctly and quickly release the pharmaceutical gels, required for sensitive and simultaneous control of microbial contamination and the active pharmaceutical ingredient (API) content, to the next stage.
CFU g−1 of E. coli DH5a in the gel, 6 samples with 50 μl of LB broth, and 35 samples with nothing. Based on the gel NIR transflectance spectra, E. coli DH5a in the gel was detected by the counter propagation artificial neural network (CP-ANN) model with a classification accuracy of 100.0%, while tazarotene in the gel was simultaneously determined by the partial least squares regression (PLS) model with a root mean square error of cross-validation of 0.0232 mg g−1. Furthermore, 9 samples of real tazarotene gel were used to verify the practicality of the established NIR spectroscopy. The developed NIR strategy can be used to correctly and quickly release the pharmaceutical gels, required for sensitive and simultaneous control of microbial contamination and the active pharmaceutical ingredient (API) content, to the next stage.
Introduction
Pharmaceutical products (PPs) are at risk of microbial contamination until they are used. Therefore, the detection of microbial contamination in PPs is of fundamental interest for pharmaceutical analysts. Up to now, the pharmacopoeia methods for the detection of microbial contamination in PPs require an incubation period of at least three days.1–5 Because of the rapid proliferation of microorganisms, the pharmacopoeia methods cannot be used to monitor in real time the microbial contamination in PPs, especially in the manufacturing process. In addition, the pharmacopeia methods also have other drawbacks, such as tedious sample preparation and testing, sample destruction, microbial pollution, and being one-parameter.Near-infrared (NIR) spectroscopy, which can characterize a variety of properties of an analyte containing the X–H groups (X = C, N, O, S), is becoming a popular analytical method for drug quality control because it is sample-nondestructive, reagent-free, real-time, available for process control, and multi-parameter.6–8 The possibility to detect and quantify bacterial contaminations in liquid PPs with fiber optic NIR spectroscopy has been explored by Quintelas et al. (E. coli lowest content 9.6 CFU ml−1).9
However, we could not find, so far, the real-time sensitive NIR spectroscopy for the detection of microbial contamination in pharmaceutical gels, although the active pharmaceutical ingredient (API) in pharmaceutical gels has been determined using NIR spectroscopy by Blanco et al.10 (API content 1.25–3.75% w/w), Rosas et al.11 (API content 1.0–1.5% w/w), and Donoso et al.12 (API content about 0.5–2% w/w). And the tazarotene content in gels has been determined using high performance liquid chromatography (HPLC) in Chinese Pharmacopoeia5 (API content 0.05–0.1% w/w), using high performance thin layer chromatography (HPTLC) by Patel et al.13 (API content 0.05% w/w), using ultraviolet (UV) spectrophotometry by Jogarami et al.14 (API content 0.1% w/w), but not using NIR spectroscopy. Pharmaceutical gels are one of the important semi-solid PPs for topical application to the skin or the body cavity.5 The unique advantages of pharmaceutical gels include reducing greatly the systemic adverse effects, escaping from the first-pass metabolism, and noninvasive and convenient use.15 For example, tazarotene gel (tazarotene chemical structure in Fig. 1; labeled content 0.05% w/w, that is, 0.5 mg g−1) is well accepted for treatment with psoriasis.16
Accordingly, in this paper, the feasibility of real-time, non-destructive, sensitive and simultaneous analysis of E. coli DH5a contamination and ultra low-content tazarotene in tazarotene gel was evaluated by using Fourier transform NIR spectroscopy with chemometric techniques, mainly on the basis of the characteristic absorptions of the X–H groups of E. coli DH5a and tazarotene. Using this real-time simultaneous NIR approach, pharmaceutical gels can be rapidly analyzed and released to the next stage. This work can extend the NIR applications in the simultaneous analysis of both microbial contamination and ultra low-content API in a pharmaceutical gel.
Experimental
Samples and reference values
A total of 88 sterile samples of tazarotene gel, including nine API contents 0.41, 0.43, 0.46, 0.48, 0.51, 0.54, 0.58, 0.61, and 0.65 mg g−1 (82–130% of 0.5 mg g−1), were prepared with tazarotene (assay 99.7%) and jojoba oil by using standard addition method, filled into the lacquer-coated aluminum tubes, and sealed by an authorized manufacturer, Chongqing Huapont Pharmaceutical Co., Ltd. (Chongqing, China).Before the NIR spectra acquirement, about 7.5 g of each sterile sample of translucent tazarotene gel in a lacquer-coated aluminum tube was squeezed into an empty disposable sterile ziplock transparent plastic bag (8 cm × 12 cm) and inoculated with 50 μl of different concentrations of E. coli DH5a in Luria–Bertani (LB) broth, 50 μl of LB broth, or nothing. Both E. coli DH5a and LB broth were supplied by the Key Laboratory of Molecular Biology on Infectious Diseases, Chinese Ministry of Education, Chongqing Medical University (Chongqing, China). After in time and gently pressing to remove the most of trapped air, the bag was zipped immediately and kneaded rapidly and gently to mix the contents. The above-mentioned operations were conducted in a class A clean bench in a class B clean room. Finally, 47 positive samples with 50 μl of different concentrations of E. coli DH5a in LB broth (1–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1 of E. coli DH5a in the gel) and 41 negative samples without E. coli DH5a (including 6 samples with 50 μl of LB broth and 35 samples with nothing) were obtained.
CFU g−1 of E. coli DH5a in the gel) and 41 negative samples without E. coli DH5a (including 6 samples with 50 μl of LB broth and 35 samples with nothing) were obtained.
Furthermore, 9 samples of real tazarotene gel produced by Chongqing Huapont Pharmaceutical Co., Ltd. (Chongqing, China) were used to verify the practicality of the established NIR spectroscopy. And 9 real samples were proven uncontaminated using the Chinese pharmacopoeia method.5 The tazarotene contents were determined as 0.49–0.52 mg g−1 (98–104% of 0.5 mg g−1) using the HPLC.5
Near-infrared instrument and spectral measurements
The near-infrared instrument, used in this experiment, was Antaris II FT-NIR analyzer (Thermo Fisher Scientific, USA), which was equipped with an indium gallium arsenide detector, an integrating sphere, and a transflectance accessory. The instrument was controlled with the software RESULT 3.0. The chemometric analysis software was TQ Analyst 8.0 (Thermo Fisher Scientific, USA) and Matlab 8.0 (The Math Works, USA).After the sample in bag was laid flat and squeezed to fill up the space between the window of integrating sphere and the transflectance accessory, the sample NIR spectrum was immediately measured within the range of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–4000 cm−1 using the selected resolution, co-averaged scans, and specimen holder (transflectance accessory) thickness. The resolution was selected from 4, 8 and 16 cm−1 for more sample information in less time; the co-averaged scans from 16, 32, 48, 64, and 128 for less noise in less time; and the specimen holder thickness from 0.5, 1, and 2 mm for moderate absorption. The spectrum of ambient background, prior to sample, was measured under the selected conditions to eliminate the interferences from H2O and CO2 in the air on the sample spectrum.
000–4000 cm−1 using the selected resolution, co-averaged scans, and specimen holder (transflectance accessory) thickness. The resolution was selected from 4, 8 and 16 cm−1 for more sample information in less time; the co-averaged scans from 16, 32, 48, 64, and 128 for less noise in less time; and the specimen holder thickness from 0.5, 1, and 2 mm for moderate absorption. The spectrum of ambient background, prior to sample, was measured under the selected conditions to eliminate the interferences from H2O and CO2 in the air on the sample spectrum.
In addition, the NIR spectra of E. coli DH5a, LB broth, tazarotene, jojoba oil, and the blank bag were also measured in the same way as the tazarotene gel.
Detection of E. coli DH5a contamination in tazarotene gel
In the detection, both linear and nonlinear models, the discriminant analysis (DA) and counter propagation artificial neural network (CP-ANN) models, were used to validate each other. In the above-mentioned two models, 48 calibration samples were consisted of 24 positive samples with 50 μl of different concentrations of E. coli DH5a in LB broth (1–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1 of E. coli DH5a in the gel) and 24 negative samples, which include 3 negative samples with 50 μl of LB broth and 21 negative samples with nothing. And 40 validation samples were consisted of 23 positive samples with 50 μl of different concentrations of E. coli DH5a in LB broth (1–4
CFU g−1 of E. coli DH5a in the gel) and 24 negative samples, which include 3 negative samples with 50 μl of LB broth and 21 negative samples with nothing. And 40 validation samples were consisted of 23 positive samples with 50 μl of different concentrations of E. coli DH5a in LB broth (1–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1 of E. coli DH5a in the gel), 3 negative samples with 50 μl of LB broth, and 14 negative samples with nothing.
CFU g−1 of E. coli DH5a in the gel), 3 negative samples with 50 μl of LB broth, and 14 negative samples with nothing.
The sample spectra were preprocessed separately for the DA and CP-ANN models. The preprocessing techniques were selected from no preprocessing (NP), multiplicative scatter correction (MSC) or standard normal variate (SNV) for eliminating the interference of particle size and compactness, derivative for deducting the background and separating the overlapping signals, and smoothing for denoising. The spectral sub-ranges, respectively for the DA and CP-ANN models, were selected mainly on the basis of the characteristic absorptions of the X–H groups of E. coli DH5a in order to reduce irrelevant variables and data redundancy. The DA and CP-ANN models were established separately by using the scores of selected principal components (PCs) of spectral data for calibration samples and the reference values. The cumulative contribution rate of the selected PCs was over 85%.17
The DA and CP-ANN models were validated respectively by using the scores of selected PCs of spectral data for validation samples and the reference values. The DA model was evaluated with the classification accuracy of calibration (CAC) and validation (CAV), and the CP-ANN model with the classification accuracy of calibration (CAC), validation (CAV), and cross-validation (CACV).
Determination of ultra low-content tazarotene in tazarotene gel
In the determination, the partial least squares regression (PLS) model was used. 44 calibration samples, including 20 positive samples with 50 μl of different concentrations of E. coli DH5a in LB broth, 3 negative samples with 50 μl of LB broth, and 21 negative samples with nothing, were distributed in the range of 0.41–0.65 mg g−1 of tazarotene in the gel. And 44 validation samples, including 27 positive samples with 50 μl of different concentrations of E. coli DH5a in LB broth, 3 negative samples with 50 μl of LB broth, and 14 negative samples with nothing, were also distributed in the range of 0.41–0.65 mg g−1 of tazarotene contents.The sample spectra were preprocessed by the chemometric techniques selected from NP, MSC or SNV, derivative, smoothing, mean centering (MC), and variance scaling (VS). The spectral sub-ranges were selected mainly on the basis of the characteristic absorptions of C–H groups in tazarotene. The PLS model was built by using the scores of selected factors of spectral data for calibration samples and the reference values. The used factors were confirmed by the smallest root mean square error of cross-validation (RMSECV).
The PLS model was validated by using the factor scores of spectral data for validation samples and the reference values. And the PLS model was evaluated with the correlation coefficient of calibration (Rc), validation (Rv), and cross-validation (Rcv) between reference values and predicted values; the root mean square error of calibration (RMSEC), validation (RMSEV), and RMSECV; and the bias.
Results and discussion
Spectral measurements
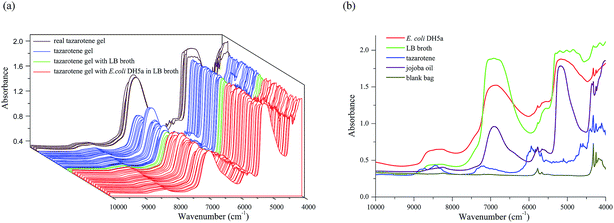
The NIR transflectance spectra of 97 translucent samples of tazarotene gel, including 88 simulated samples and 9 real samples, were measured within the range of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–4000 cm−1 using the resolution of 8 cm−1, 64 co-averaged scans, the specimen holder thickness of 1 mm, and the data collection time of about 31.65 seconds, as shown in Fig. 2a. In Fig. 2a, the brown lines represent 9 real samples, the blue lines 35 samples with nothing, the green lines 6 samples with 50 μl of LB broth, and the red lines 47 samples with 50 μl of different concentrations of E. coli DH5a in LB broth.
000–4000 cm−1 using the resolution of 8 cm−1, 64 co-averaged scans, the specimen holder thickness of 1 mm, and the data collection time of about 31.65 seconds, as shown in Fig. 2a. In Fig. 2a, the brown lines represent 9 real samples, the blue lines 35 samples with nothing, the green lines 6 samples with 50 μl of LB broth, and the red lines 47 samples with 50 μl of different concentrations of E. coli DH5a in LB broth.
And the NIR transflectance spectra of E. coli DH5a, LB broth, tazarotene, jojoba oil, and the blank bag are given in Fig. 2b respectively in red, green, blue, purple, and dark yellow. It has been demonstrated that the above-mentioned five materials are NIR responsive and can be characterized with the NIR transflectance spectroscopy.
Detection of E. coli DH5a contamination in tazarotene gel
For the detection, the vital preprocessing techniques are listed in Table 1. It can be found from Table 1 that the performances of both DA and CP-ANN models are improved with MSC or SNV. That is, the influence of scattering on the transflectance spectra cannot be ignored in the detection for the nature of E. coli DH5a and the translucent gel. Moreover, the performances of both DA and CP-ANN models are not improved with smoothing. We speculate that the application of 64 co-averaged scans has greatly reduced noise in the NIR spectra.| Item | Model | Preprocessing | Spectral sub-ranges (cm−1) | Number of PCs/cumulative contribution rate (%) | CAC (%) | CAV (%) | CACV (%) |
|---|---|---|---|---|---|---|---|
| a DA for discriminant analysis; CP-ANN counter propagation artificial neural network; PC principal component; CAC classification accuracy of calibration; CAV classification accuracy of validation; CACV classification accuracy of cross-validation; MSC multiplicative scatter correction; SNV standard normal variate; NP no preprocessing; FD first derivative; SD second derivative; SGS Savitzky–Golay smoothing; NDS Norris derivative smoothing. | |||||||
| DA | 1 | NP | 9000–8000 | 3/100.0 | 95.8 | 95.0 | |
| 2 | MSC | 6400–5400 | 3/99.9 | 100.0 | 100.0 | ||
| 3 | SNV | 3/99.9 | 100.0 | 100.0 | |||
| 4 | FD | 3/97.7 | 58.3 | 62.5 | |||
| 5 | SD | 3/70.3 | 72.9 | 82.5 | |||
| 6 | SGS | 3/100.0 | 95.8 | 95.0 | |||
| 7 | NDS | 3/98.7 | 58.3 | 65.0 | |||
| 8 | MSC + FD + SGS | 3/96.1 | 83.3 | 82.5 | |||
| 9 | MSC | 9000–8000 | 3/99.5 | 97.9 | 95.0 | ||
| 6400–5400 | |||||||
| 4450–4100 | |||||||
| 10 | MSC | 9000–8000 | 3/99.8 | 91.7 | 87.5 | ||
| 7300–4800 | |||||||
| 4450–4100 | |||||||
| CP-ANN | 11 | NP | 9000–8000 | 2/99.9 | 100.0 | 92.5 | 96.0 |
| 12 | MSC | 6400–5400 | 2/99.7 | 100.0 | 100.0 | 100.0 | |
| 13 | SNV | 2/99.6 | 100.0 | 100.0 | 100.0 | ||
| 14 | SGS | 2/99.9 | 100.0 | 90.0 | 96.0 | ||
| 15 | NDS | 2/98.0 | 98.0 | 67.5 | 73.0 | ||
| 16 | MSC + FD + SGS | 2/94.7 | 100.0 | 92.5 | 83.0 | ||
The red line in Fig. 2b indicates that the informative spectral sub-ranges for E. coli DH5a are 9000–8000 cm−1, 7300–4800 cm−1, and 4450–4100 cm−1. Since two strong overtones of O–H in H2O are respectively around 6900 cm−1 and 5150 cm−1, two portions 7300–6400 cm−1 and 5400–4800 cm−1 are removed from 7300–4800 cm−1. On the other hand, the sub-range of 4450–4100 cm−1 is also not used for modeling because it may include the strong combination bands of alkyl, aromatic and the polymer of the plastic bag. Finally, the spectral sub-ranges for modelling were 9000–8000 cm−1 and 6400–5400 cm−1. The above-mentioned sub-ranges can be mainly attributed to the overtones and combination bands of C–H in E. coli DH5a, such as the second overtones of alkyl near 8696–8264 cm−1 and aromatic near 8834 cm−1, and the first overtones of alkyl near 5882–5555 cm−1 and aromatic near 6000 cm−1.18 It is noted that the selected spectral sub-ranges overlap partially with 7000–4000 cm−1 used by Rodriguez-Saona et al.19 to analyze bacterial contaminations in liquids, and 6000–5400 cm−1 used by Quintelas et al.9 to analyze E. coli contaminations in liquid PPs. Moreover, both the DA model 9 (using 4450–4100 cm−1) and 10 (using 7300–6400 cm−1, 5400–4800 cm−1, and 4450–4100 cm−1) have lower classification accuracies than the model 2 although all of them use MSC. This result proves that it is correct to remove 7300–6400 cm−1, 5400–4800 cm−1, and 4450–4100 cm−1 from the spectral sub-ranges for modelling.
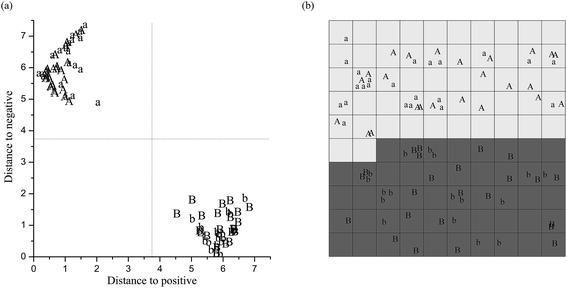
The performances of the DA and CP-ANN models are listed in Table 1. The optimized DA model is the model 2 or 3. It reaches the CAC 100.0% and the CAV 100.0% by using MSC or SNV, small spectral sub-ranges 9000–8000 cm−1 and 6400–5400 cm−1, and fewer PCs the first three (cumulative contribution rate 99.9%). Similarly, the optimized CP-ANN model is the model 12. It reaches the CAC 100.0%, the CAV 100.0%, and the CACV 100.0% by using MSC, 9000–8000 cm−1 and 6400–5400 cm−1, and the first two PCs (cumulative contribution rate 99.7%). Fig. 3 indicates that the model 2 and 12 distribute respectively the positive samples and the negative samples (with or without LB broth) of tazarotene gel logically and distinctly in two separate two-dimensional zones in both calibration and validation.
Therefore, the E. coli DH5a contamination in tazarotene gel (1–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1) can be in time, nondestructively, and sensitively detected with the linear DA model or the nonlinear CP-ANN model (using less PCs; with a better representational ability) based on the NIR spectra. And the specificity of the models is confirmed by three experimental results as below. First, the positive samples (with 50 μl of E. coli DH5a in LB broth) and the negative samples (with 50 μl of LB broth), with or without E. coli DH5a, are classified into two subgroups. Second, all negative samples (with 50 μl of LB broth or nothing), without E. coli DH5a, are classified in one subgroup. Third, two stronger overtones of LB broth than E. coli DH5a, respectively near 7300–6400 cm−1 and 5400–4800 cm−1, are not used to build the DA and CP-ANN models. That is, the recognition of E. coli DH5a in tazarotene gel is mainly based on the characteristic NIR absorptions of E. coli DH5a rather than LB broth and/or tazarotene gel. Moreover, in the validation and prediction, the false positive or false negative results were reduced because the number of positive and negative samples in the calibration samples is equal.
CFU g−1) can be in time, nondestructively, and sensitively detected with the linear DA model or the nonlinear CP-ANN model (using less PCs; with a better representational ability) based on the NIR spectra. And the specificity of the models is confirmed by three experimental results as below. First, the positive samples (with 50 μl of E. coli DH5a in LB broth) and the negative samples (with 50 μl of LB broth), with or without E. coli DH5a, are classified into two subgroups. Second, all negative samples (with 50 μl of LB broth or nothing), without E. coli DH5a, are classified in one subgroup. Third, two stronger overtones of LB broth than E. coli DH5a, respectively near 7300–6400 cm−1 and 5400–4800 cm−1, are not used to build the DA and CP-ANN models. That is, the recognition of E. coli DH5a in tazarotene gel is mainly based on the characteristic NIR absorptions of E. coli DH5a rather than LB broth and/or tazarotene gel. Moreover, in the validation and prediction, the false positive or false negative results were reduced because the number of positive and negative samples in the calibration samples is equal.
Comparing with the NIR method reported previously by Quintelas et al.9 for analyzing E. coli contaminations in liquid PPs (the spectral sub-range for modelling 6000–5400 cm−1), both the DA and CP-ANN models use the spectral information at first overtones 6400–5400 cm−1 with the second overtones 9000–8000 cm−1. Obviously, high-frequency regions are less affected by the overlapping bands than low-frequency regions. We speculate that it is beneficial to detect the E. coli DH5a contamination as low as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1 using the second overtones of alkyl and aromatic 9000–8000 cm−1 but not to use 5400–4800 cm−1 and 4450–4100 cm−1. The established DA and CP-ANN models are very important in practice because the pharmaceutical gel is inhomogeneous and its sample preparation requires much tedious operations than the liquid PPs.
CFU g−1 using the second overtones of alkyl and aromatic 9000–8000 cm−1 but not to use 5400–4800 cm−1 and 4450–4100 cm−1. The established DA and CP-ANN models are very important in practice because the pharmaceutical gel is inhomogeneous and its sample preparation requires much tedious operations than the liquid PPs.
Determination of ultra low-content tazarotene in tazarotene gel
For the determination, the important preprocessing techniques are listed in Table 2. As can be seen from Table 2, the PLS model performances are significantly improved with the combination of SNV and MC, but not improved by smoothing. If not considering MC, the preprocessing technique for quantitative analysis, this situation is similar to that in the detection of E. coli DH5a contamination in tazarotene gel.| Model | Preprocessing | Spectral sub-ranges (cm−1) | Factor number | Rc | Rv | Rcv | RMSEC (mg g−1) | RMSEV (mg g−1) | RMSECV (mg g−1) | Bias (mg g−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| a PLS for partial least squares regression; Rc correlation coefficient of calibration; Rv correlation coefficient of validation; Rcv correlation coefficient of cross-validation; RMSEC root mean square error of calibration; RMSEV root mean square error of validation; RMSECV root mean square error of cross-validation; NP no preprocessing; SNV standard normal variate; MSC multiplicative scatter correction; SGS Savitzky–Golay smoothing; FD first derivative; NDS Norris derivative smoothing; SD second derivative; MC mean centering; VS variance scaling. | ||||||||||
| 1 | NP | 9100–7900 | 8 | 0.9688 | 0.9373 | 0.9345 | 0.0187 | 0.0257 | 0.0273 | 0 |
| 2 | MSC | 7600–6500 | 8 | 0.9761 | 0.9554 | 0.9506 | 0.0162 | 0.0226 | 0.0240 | 0 |
| 3 | SNV | 6400–5600 | 8 | 0.9761 | 0.9549 | 0.9477 | 0.0162 | 0.0231 | 0.0244 | 0 |
| 4 | SGS | 4700–4500 | 8 | 0.9688 | 0.9373 | 0.9345 | 0.0187 | 0.0257 | 0.0273 | 0 |
| 5 | MC | 8 | 0.9743 | 0.9589 | 0.9343 | 0.0173 | 0.0212 | 0.0277 | 0 | |
| 6 | FD + NDS | 8 | 0.9566 | 0.9491 | 0.8653 | 0.0231 | 0.0327 | 0.0393 | 0 | |
| 7 | SD + NDS | 8 | 0.9182 | 0.7478 | 0.5669 | 0.0308 | 0.0572 | 0.0684 | 0 | |
| 8 | MC + VS | 7 | 0.9725 | 0.9358 | 0.9478 | 0.0178 | 0.0262 | 0.0247 | 0 | |
| 9 | MSC + MC | 7 | 0.9757 | 0.9542 | 0.9525 | 0.0160 | 0.0222 | 0.0236 | 0 | |
| 10 | SNV + MC | 7 | 0.9780 | 0.9667 | 0.9491 | 0.0154 | 0.0207 | 0.0232 | 0 | |
| 11 | MSC + MC + VS | 7 | 0.9739 | 0.9521 | 0.9464 | 0.0171 | 0.0233 | 0.0246 | 0 | |
| 12 | SNV + MC + VS | 7 | 0.9790 | 0.9571 | 0.9507 | 0.0162 | 0.0215 | 0.0235 | 0 | |
| 13 | SNV + MC | 9100–7900 | 8 | 0.9745 | 0.9363 | 0.9336 | 0.0163 | 0.0267 | 0.0279 | 0 |
| 7600–7200 | ||||||||||
| 6150–5600 | ||||||||||
| 4700–4500 | ||||||||||
The blue line in Fig. 2b indicates that the informative spectral sub-ranges for tazarotene are 9100–7900 cm−1, 7600–6500 cm−1, 6400–5600 cm−1, and 4700–4000 cm−1. Because the sub-range of 4500–4000 cm−1 may include the strong combination bands of alkyl, aromatic, and the polymer of the plastic bag, it is removed from 4700–4000 cm−1. Finally, the spectral sub-ranges for modelling were 9100–7900 cm−1, 7600–6500 cm−1, 6400–5600 cm−1, and 4700–4500 cm−1. The above-mentioned sub-ranges can be mainly attributed to the overtones and combination bands of C–H in tazarotene. For example, the second overtones of CH3 and CH2 are about 8696–8264 cm−1; and phenyl about 8834 cm−1. The first overtones of CH3 appear near 5905, 5876, and 5872 cm−1; CH2 in linear near 5680 cm−1; CH2 in cyclic near 6060, 5791, and 5697 cm−1; and phenyl near 6000 cm−1. The combination bands of CH3 appear near 7355, 7263, and 4545–4500 cm−1; CH2 near 7186 and 7080 cm−1; and phenyl and pyridyl near 4700–4500 cm−1.18 It is noted that the spectral sub-range of 7600–6500 cm−1 might include the overtone of O–H in H2O around 6900 cm−1. However, we can find from Table 2 that the portion around 6900 cm−1 is useful to determine tazarotene in the gel, although it might overlap with the overtone of O–H in H2O around 6900 cm−1, because the model 10 (use of the portion around 6900 cm−1) has higher Rc, Rv, and Rcv, and smaller RMSEC, RMSEV, and RMSECV than the model 13 (no use of the portion around 6900 cm−1).
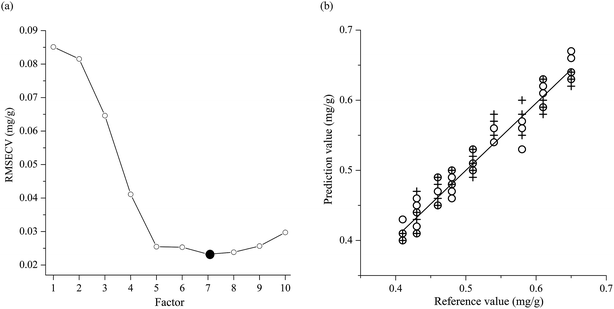
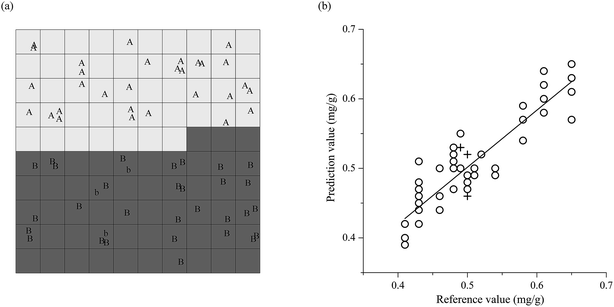
The performances of the PLS model are listed in Table 2. It can be seen from Table 2 that the PLS model reaches the RMSECV 0.0232 mg g−1 by optimizing into the model 10, as in Fig. 4. It is built by using the combination of SNV and MC, four spectral sub-ranges 9100–7900 cm−1, 7600–6500 cm−1, 6400–5600 cm−1, and 4700–4500 cm−1, and seven factors. It has the Rc 0.9780, the Rv 0.9667, the Rcv 0.9491, the RMSEC 0.0154 mg g−1, the RMSEV 0.0207 mg g−1, the RMSECV 0.0232 mg g−1, and the bias 0 mg g−1. Compared with the model 10, the model 12 has the lower Rv 0.9571 and the larger RMSEC 0.0162 mg g−1, RMSEV 0.0215 mg g−1, and RMSECV 0.0235 mg g−1 although it has the higher Rc 0.9790 and Rcv 0.9507. Therefore, the model 12 is not as good as the model 10. Fig. 4a illustrates that the smallest RMSECV 0.0232 mg g−1 of the model 10 is obtained by using seven factors. And Fig. 4b shows the linear relations between reference values and predicted values of tazarotene content in the gel in both calibration and validation of the model 10.
Consequently, the ultra low-content tazarotene in the gel (0.41–0.65 mg g−1) can be sensitively, in time, and nondestructively determined by the PLS model based on the NIR spectra. The specificity of the model is demonstrated by the following experimental results. The ultra low-content tazarotene in the gel can be determined by the PLS model no matter if the gel is inoculated with 50 μl of E. coli DH5a in LB broth, 50 μl of LB broth, or nothing. That is, the determination of tazarotene in the gel is mainly based on the characteristic NIR absorptions of tazarotene. And the precision of the model is described with the relative standard deviations (RSD). The RSDs (n = 3) for the three API levels (0.41, 0.51, and 0.65 mg g−1; that is, 82%, 102%, and 130% of 0.5 mg g−1) are 1.43%, 1.15%, and 1.79%, respectively.
Comparing with the previously reported NIR methods for determining the API in pharmaceutical gel (about 0.5–2% w/w),10–12 the PLS model uses the second overtones of CH3, CH2, and aromatic 9100–7900 cm−1 but does not use 4500–4000 cm−1. The high-frequency regions are less affected by the overlapping bands than low-frequency regions. On the other hand, the low-frequency band, such as the combination band of phenyl near 4050 cm−1, has the very strong absorptions outside the linear range of the detector. We speculate that the combined use of the spectral information at 7600–6500 cm−1 and 6400–5600 cm−1 with the second overtones 9100–7900 cm−1 is the reason for the ultra low-content tazarotene 0.041–0.065% w/w can be determined by the PLS model. The established PLS model is very important in practice because it can be used to determine the ultra low-content API in the pharmaceutical gel.
Detection of E. coli DH5a contamination and determination of tazarotene in real tazarotene gel
9 samples of real tazarotene gel (uncontaminated, API content 0.49–0.52 mg g−1), produced by Chongqing Huapont Pharmaceutical Co., Ltd. (Chongqing, China), were used to verify the practicality of the established NIR spectroscopy. Among them, 6 samples were used as calibration samples and 3 samples as validation samples.In the detection of E. coli DH5a contamination, 60 calibration samples were consisted of 30 positive samples with 50 μl of different concentrations of E. coli DH5a in LB broth (1–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1 of E. coli DH5a in the gel) and 30 negative samples, which include 3 negative samples with 50 μl of LB broth, 21 negative samples with nothing, and 6 real samples uncontaminated. Consequently, 3 real samples uncontaminated were correctly distributed by the CP-ANN model in the zone of negative samples, as shown in Fig. 5a.
CFU g−1 of E. coli DH5a in the gel) and 30 negative samples, which include 3 negative samples with 50 μl of LB broth, 21 negative samples with nothing, and 6 real samples uncontaminated. Consequently, 3 real samples uncontaminated were correctly distributed by the CP-ANN model in the zone of negative samples, as shown in Fig. 5a.
In the determination of tazarotene in real tazarotene gel, 50 calibration samples were consisted of 44 simulated samples (API content 0.41–0.65 mg g−1), which include 20 positive samples with 50 μl of different concentrations of E. coli DH5a in LB broth, 3 negative samples with 50 μl of LB broth, and 21 negative samples with nothing, and 6 real samples (API content 0.49–0.52 mg g−1). Consequently, the tazarotene contents in 3 real samples were determined by the PLS model, as illustrated in Fig. 5b. And the three relative errors (REs) 8%, −8%, and 4% are acceptable.5
Conclusions
A real-time and non-destructive method was developed to sensitively and simultaneously detect E. coli DH5a contamination and determine ultra low-content tazarotene in tazarotene gel (1–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1 of E. coli DH5a; 0.41–0.65 mg g−1 of tazarotene) based on the NIR spectra of tazarotene gel. Using the real-time and multiparametric NIR approach, pharmaceutical gels, typically requiring tedious extractions, can be sensitively and quickly analyzed and released into the next stage. This work can extend the NIR applications in the sensitive and simultaneous monitoring of both microbial contamination and an ultra low-content API in a pharmaceutical gel.
CFU g−1 of E. coli DH5a; 0.41–0.65 mg g−1 of tazarotene) based on the NIR spectra of tazarotene gel. Using the real-time and multiparametric NIR approach, pharmaceutical gels, typically requiring tedious extractions, can be sensitively and quickly analyzed and released into the next stage. This work can extend the NIR applications in the sensitive and simultaneous monitoring of both microbial contamination and an ultra low-content API in a pharmaceutical gel.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We are grateful for the financial supports from the Municipal Science and Technology Committee of Chongqing [grant number cstc2012gg – yyjs10039] and from the District Science and Technology Committee of Yuzhong District of Chongqing [grant number 20120206] and the supports from Chongqing Huapont Pharmaceutical Co., Ltd. and from the Key Laboratory of Molecular Biology on Infectious Diseases, Chinese Ministry of Education, Chongqing Medical University.References
- The United States Pharmacopeia, The United States Pharmacopeial Convention, Rockville, MD, 2015 Search PubMed.
- The European Pharmacopoeia, European Directorate for the Quality of Medicines & Healthcare, Strasbourg, 2013 Search PubMed.
- The British Pharmacopoeia 2015, The British Pharmacopoeia Commission, London, 2014 Search PubMed.
- The Japanese Pharmacopoeia, The Society of Japanese Pharmacopeia, Tokyo, 2016 Search PubMed.
- The Chinese Pharmacopoeia, Chinese Pharmacopoeia Commission, Beijing, 2015, vol. 4 Search PubMed.
- F. Shikata, S. Kimura, Y. Hattori and M. Otsuka, RSC Adv., 2017, 7, 38307–38317 RSC.
- Q. Y. Luo, Y. H. Yun, W. Fan, J. H. Huang, L. X. Zhang, B. C. Deng and H. M. Lu, RSC Adv., 2015, 5, 5046–5052 RSC.
- M. Otsuka, A. Koyama and Y. Hattori, RSC Adv., 2014, 4, 17461–17468 RSC.
- C. Quintelas, D. P. Mesquita, J. A. Lopes, E. C. Ferreira and C. Sousa, Int. J. Pharm., 2015, 492, 199–206 CrossRef PubMed.
- M. Blanco, M. Alcalá and M. Bautista, Eur. J. Pharm. Sci., 2008, 33, 409–414 CrossRef PubMed.
- J. G. Rosas, M. Blanco, J. M. González and M. Alcalá, J. Pharm. Sci., 2011, 100, 4442–4451 CrossRef PubMed.
- M. Donoso and E. S. Ghaly, Pharm. Dev. Technol., 2006, 11, 389–397 CrossRef PubMed.
- M. R. Patel, R. B. Patel, J. R. Parikh and B. G. Patel, Anal. Methods, 2010, 2, 275–281 RSC.
- R. Jogarami, P. Jain and S. Sharma, J. Pharm. Res., 2012, 5, 2273–2275 Search PubMed.
- A. Vintiloiu and J. C. Leroux, J. Controlled Release, 2008, 125, 179–192 CrossRef PubMed.
- R. H. Foster, R. N. Brogden and P. Benfield, Drugs, 1998, 55, 705–711 CrossRef PubMed.
- W. Z. Lu, Modern near infrared spectroscopy analytical technology, China Petrochemical Press, Beijing, 2006 Search PubMed.
- J. Workman and L. Weyer, Practical guide to interpretive near-infrared spectroscopy, CRC Press, USA, 2008 Search PubMed.
- L. E. Rodriguez-Saona, F. M. Khambaty, F. S. Fry and E. M. Calvey, J. Agric. Food Chem., 2001, 49, 574–579 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this article. |
| This journal is © The Royal Society of Chemistry 2018 |