Open Access Article
Open Access ArticleDirect reaction between silicon and methanol over Cu-based catalysts: investigation of active species and regeneration of CuCl catalyst
Aili Wang a,
Mingming Zhanga,
Hengbo Yin
a,
Mingming Zhanga,
Hengbo Yin *a,
Shuxin Liub,
Mengke Liua and
Tongjie Hua
*a,
Shuxin Liub,
Mengke Liua and
Tongjie Hua
aFaculty of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, China. E-mail: yin@ujs.edu.cn; Tel: +86-511-88787591
bSchool of Chemistry and Chemical Engineering, Mianyang Normal University, Mianyang 621000, China
First published on 24th May 2018
Abstract
When a CuCl/Si mixture was pretreated at 200–240 °C in a N2 atmosphere, trimethoxysilane was predominantly formed in the direct reaction of silicon with methanol. When the pretreatment temperatures were raised to 260–340 °C, tetramethoxysilane was favorably formed. The CuxSiyClz species catalyzed the reaction between silicon and methanol to trimethoxysilane. Chlorination of the spent CuCl/Si mixture promoted the reaction between silicon and methanol to form both trimethoxysilane and tetramethoxysilane due to the recovery of the CuCl phase and the exposure of the metallic Cu0 phase. When Cu2O, CuO, and Cu0 were used as the catalysts, tetramethoxysilane was formed as the main product.
1. Introduction
Direct synthesis of alkoxysilanes and alkylalkoxysilanes via the reaction between silicon and an alcohol or alkene has attracted great interest from researchers.1 Among the products, trimethoxysilane and tetramethoxysilane as coupling agents instead of organochlorosilanes, are widely used for the synthesis of organosilicon products.2–4Synthesis of trimethoxysilane via the direct reaction between silicon and methanol can be catalyzed by cuprous chloride (CuCl) in a fixed-bed or a slurry phase reactor. Pretreatment temperature can significantly affect the formation of trimethoxysilane.5,6 It was suggested that the Cu3Si phase formed at the pretreatment temperature of 350 °C was the active site for the formation of trimethoxysilane.5,7 However, it was found that although the Cu3Si phase was not detected at pretreatment temperatures below 280 °C, a high selectivity of trimethoxysilane (ca. 98%) was obtained. Recently, researchers suggested that the Cu3Si phase itself was not a reactive intermediate, but the precursor composed of Cu–Si complex played an important role in the reaction.8,9 For the direct reaction between silicon and methanol over CuCl catalyst, the active species are still contradictory. On the other hand, the reaction rate between silicon and methanol over a CuCl catalyst in a fixed-bed reactor rapidly decreases in a short reaction time period. The practical use of a CuCl catalyst is limited. Therefore, direct reaction between silicon and methanol over a CuCl catalyst is still worthy of investigation.
Tetramethyoxysilane was formed as a byproduct in the direct reaction between silicon and methanol over a CuCl catalyst.5,7 It was also formed as the main product when the reaction was carried out over metallic Cu0 and Cu2O catalysts.8,10 It was suggested that the formation of tetramethoxysilane originated from the reaction between methanol and trimethoxysilane in series catalyzed by metallic Cu0 active sites.8 The detailed catalytic process is unclear and worthy of further investigation.
In the present work, the reactions between silicon and methanol over CuCl, Cu2O, CuO, and bulk metallic Cu0 catalysts and the regeneration of CuCl catalyst were investigated. The chemical structures of these catalysts after pretreatment and reaction were investigated by XRD, XPS, and XAES techniques. The active sites and reaction mechanisms over these catalysts were discussed below.
2. Experimental
2.1. Materials
Methanol (99.5%), cuprous chloride (97%), cuprous oxide (95%), copper oxide (99%), and bulk metallic copper powder (150 μm) were of reagent grade and were purchased from Sinopharm Chemical Reagent Co., Ltd. Silicon powder (>99.5%) was supplied by Jiangsu Hongda New Materials Co., Ltd. The materials were used as received without further purification.2.2. Characterization
XRD patterns of the samples were recorded on a XRD-6100Lab diffractometer with a graphite monochromator using the Cu Kα radiation (λ = 1.54056 Å) at the scanning speed of 2° min−1. X-ray photoelectron spectra (XPS) and X-ray Auger Electron Spectra (XAES) of the samples were obtained on an X-ray photoelectron spectroscopy/ESCA spectrometer (K-ALPHA, Thermo Fisher Scientific) using Al Kα radiation (1486.68 eV). The binding energies were calculated with respect to C 1s peak of contaminated carbon at 284.6 eV.2.3. Catalytic text
Given amounts of silicon powder and catalyst (CuCl, Cu2O, CuO or bulk metallic Cu0) were mixed in a high-speed, multi-function crusher (2000 rpm) for 2 min. The particle sizes of the catalyst/Si mixture ranged from 50 to 200 mesh. The reaction was carried out in a fixed-bed reactor with diameter and length of 1.8 cm and 50 cm. 20 mL of glass beads (diameter, 3 mm) were added on the top of catalyst/Si bed to evaporate methanol feed into gas phase. A N2 stream (99.999%) with a flow rate of 20 mL min−1 was introduced when the catalyst/Si mixture was pretreated at different temperatures. For the catalytic reaction between silicon and methanol, methanol with a flow rate of 6 mL h−1 was pumped into the reactor with a constant flow pump (TBP 1010, Tauto). The resulting reaction products were condensed and collected in an ice-water trap. The reaction products were analyzed by gas chromatography (Agilent 7890A) with a capillary column (SE-54, 0.32 × 30) and an FID detector.3. Results and discussion
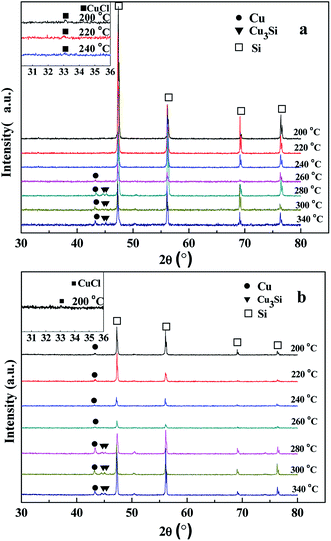
3.1. Structures of CuCl/Si mixtures after pretreatment and reaction
| CuCl + Si → Cu–Si–Cl complex + SiCl4 (200–240 °C) | (1) |
There was no CuCl peak at (2θ) 33.1° detected at the pretreatment temperatures of 260–340 °C. The XRD peak appearing at 43.3° ascribed to the characteristic peak of the standard metallic Cu0 (JCPDs 04-0836) was detected at 260–340 °C. The intensities of the metallic Cu0 peaks slightly increased with pretreatment temperature. The intensity ratios of metallic Cu0 (111) peak to Si (220) peak were in a range of 5.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 to 12.7
100 to 12.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. When the pretreatment temperatures were 280–340 °C, the XRD peaks at 44.5 (012) and 45.1° (300) ascribed to those of the Cu3Si phase (JCPDS 51-0916) were observed. The intensity ratios of Cu3Si (012) and (300) peaks to Si (220) peak ranged from 2.5
100. When the pretreatment temperatures were 280–340 °C, the XRD peaks at 44.5 (012) and 45.1° (300) ascribed to those of the Cu3Si phase (JCPDS 51-0916) were observed. The intensity ratios of Cu3Si (012) and (300) peaks to Si (220) peak ranged from 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 to 4.8
100 to 4.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and from 2.8
100 and from 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 to 6.6
100 to 6.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, respectively. The results revealed that high pretreatment temperature caused the reaction between CuCl and silicon to form metallic Cu0 and Cu3Si phases as follows.11
100, respectively. The results revealed that high pretreatment temperature caused the reaction between CuCl and silicon to form metallic Cu0 and Cu3Si phases as follows.11
| 4CuCl + Si = 4Cu + SiCl4 (260–340 °C) | (2) |
| 12CuCl + 7Si = 4Cu3Si + 3SiCl4 (280–340 °C) | (3) |
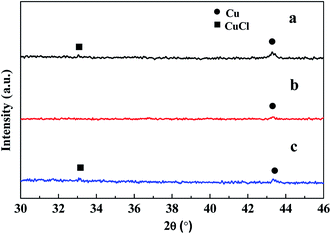
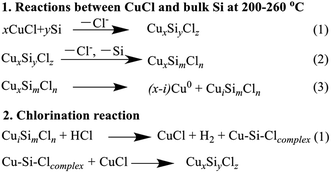
After taking part in the reaction between silicon and methanol, the CuCl phase was only observed in the CuCl/Si mixture pretreated at 200 °C (Fig. 1b, inset). The metallic Cu0 phase (JCPDs 04-0836) appeared in the mixtures pretreated at 200–340 °C, whereas the Cu3Si phase (JCPDs 51-0916) appeared in the mixtures pretreated at 280–340 °C (Fig. 1b). The results revealed that CuCl took part in the reaction and the metallic Cu0 phase was formed in the reaction process. The Cu3Si phase was formed due to the pretreatment at high temperature rather than via the reaction.
When the CuCl/Si mixture was pretreated at 200 °C for 2 h in a N2 atmosphere and then reacted at 220, 240, and 260 °C for 1 h, respectively, the metallic Cu0 phase appeared and the CuCl phase remained (Fig. 2a). After reacting at 240 °C for a longer time period of 12 h, metallic Cu0 was formed, but the CuCl phase disappeared (Fig. 2b), indicating that the CuCl phase took part in the reaction. When the spent CuCl/Si mixture was chlorinated in an HCl stream, in addition to the presence of the metallic Cu0 phase, the CuCl phase appeared again (Fig. 2c). The chlorination converted the copper species to CuCl.
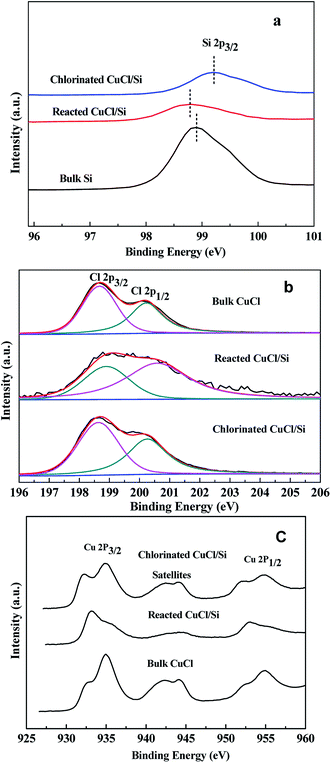
The binding energies of Si 2p3/2 of the bulk Si, reacted CuCl/Si, and chlorinated CuCl/Si samples were 98.9, 98.8, and 99.2 eV, respectively (Fig. 3a). The Si 2p3/2 peak of the reacted CuCl/Si mixture shifted by 0.1 eV to a lower binding energy as compared to that of the bulk Si, revealing that there was an interaction between bulk Si and CuCl during the reaction process. However, chlorination caused a positive shift of the Si 2p3/2 peak of the chlorinated CuCl/Si mixture, probably due to surface-interacted Cu+ being chlorinated to CuCl.
The binding energies of Cl 2p3/2 and Cl 2p1/2 of Cl− of the bulk CuCl, reacted CuCl/Si, and chlorinated CuCl/Si samples were ca. 198.7 and 200.3 eV, respectively (Fig. 3b). According to the deconvolution of the Cl 2p3/2 and Cl 2p1/2 peaks, the area ratios of the Cl 2p3/2 peak to the Cl 2p1/2 peak for the three samples were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8, 1
0.8, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9, and 1
1.9, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8, respectively, revealing that there was an interaction between Si and Cl during the reaction process. After chlorination, the CuCl phase was recovered because the bulk CuCl and chlorinated CuCl/Si samples had the same area ratios of Cl 2p3/2 to Cl 2p1/2.
0.8, respectively, revealing that there was an interaction between Si and Cl during the reaction process. After chlorination, the CuCl phase was recovered because the bulk CuCl and chlorinated CuCl/Si samples had the same area ratios of Cl 2p3/2 to Cl 2p1/2.
The Cu 2p3/2 and Cu 2p1/2 peaks of the bulk CuCl, reacted CuCl/Si, and chlorinated CuCl/Si samples were doublet (Fig. 3c). The presence of a satellite peak at ca. 942.5 eV for the three samples indicated the presence of Cu2+ species.12 The Cu 2p3/2 and Cu 2p1/2 peaks at ca. 935 and 955 eV were ascribed to those of Cu2+ species, whereas the Cu 2p3/2 and Cu 2p1/2 peaks at 932.4 and 952.3 eV were ascribed to those of Cu+ and/or metallic Cu0 species.13–16 The XPS analysis revealed that the three samples contained Cu2+, Cu+ and/or Cu0 species. The presence of the Cu2+ species is observed because CuCl is easily oxidized when it is exposed to air during the ex situ sample characterization process.17
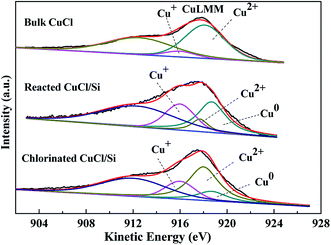
Considering that Cu+ and metallic Cu0 have similar binding energies, it is difficult to distinguish them based on Cu 2p spectra. X-ray excited Auger Electron Spectroscopy (XAES) was used to determine the surface compositions of the Cu2+, Cu+, and Cu0 species.13,16,18–20 The Cu LMM XAES of the bulk CuCl, reacted CuCl/Si, and chlorinated CuCl/Si samples are shown in Fig. 4. Literatures report that the XAES peaks of Cu+, Cu2+, and Cu0 species are centered at 916, 918, and 918.7 eV, respectively.13,16,18–20 Therefore, we chose Gaussian–Lorentzian bands with peak positions at 916, 917.7–918, and 918.7 eV corresponding to Cu+, Cu2+, and Cu0 species, and the XAES peaks were deconvoluted into three symmetrical peaks of Cu+, Cu2+, and Cu0 species by using a XPSPEAK41 software. Considering that a shoulder peak appeared at 912 eV, an extra Gaussian–Lorentzian band with the peak position at ca. 912 eV was used to eliminate the effect of other orbital electrons on the XAES.
According to the deconvolution results, the surface copper species of the bulk CuCl were composed of Cu2+ and Cu+ components (Fig. 4). The surface copper species of the reacted CuCl/Si and chlorinated CuCl/Si mixtures were composed of Cu2+, Cu+, and Cu0 components. After taking part in the reaction, in addition to the formation of the metallic Cu0 component, the surface Cu2+ content of the reacted CuCl/Si mixture was obviously less than that of the bulk CuCl, but the surface Cu+ content of the reacted CuCl/Si mixture was larger than that of the bulk CuCl, indicating that Cu2+ was reduced to copper species with lower valence states (Table 1). After chlorination, the total content of surface Cu2+ and Cu+ species of the chlorinated CuCl/Si mixture increased as compared to that of the reacted CuCl/Si mixture. Considering that the surface Cu2+ species of the chlorinated CuCl/Si mixture could be attributed to the oxidation of Cu+ during the sample characterization process in air,17 it was reasonable to suggest that chlorination increased the surface Cu+ content.
| Samples | Kinetic energies (eV) | Peak area ratiosa, Cu2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu+ Cu+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu0 Cu0 |
Atomic ratiosb, Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu Cu |
||
|---|---|---|---|---|---|
| Cu2+ | Cu+ | Cu0 | |||
| a Peak area ratios among Cu2+, Cu+, and Cu0 were calculated by the deconvolution of Cu LMM XAES.b Atomic ratios of Cl to Cu were obtained by XPS analysis. | |||||
| Bulk CuCl | 918 | 916 | — | 7.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Reacted CuCl/Si | 917.7 | 916 | 918.7 | 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Chlorinated CuCl/Si | 918 | 916 | 918.7 | 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 0.7 |
0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
The XPS analysis revealed that the surface atomic ratio of Cl to Cu in the reacted CuCl/Si mixture was lower than that in the bulk CuCl (Table 1). Chlorination of the reacted CuCl/Si mixture increased the surface atomic ratio of Cl to Cu. It could be explained as follows. During the reaction process, CuCl crystallites reacted with silicon to form CuxSiyClz species. Furthermore, Cl− from the CuxSiyClz species reacted with Si and/or methanol, removing it from the active species. This process caused the decrease in Cl− content of CuxSiyClz active species. With the consumption of Cl−, metallic Cu0 was formed. However, chlorination treatment caused the formation of CuCl and probably recovered the CuxSiyClz active species. The reactions are suggested in Scheme 1.
3.2. Chemical structures of Cu2O/Si, CuO/Si, and Cu0/Si mixtures after pretreatment and reaction
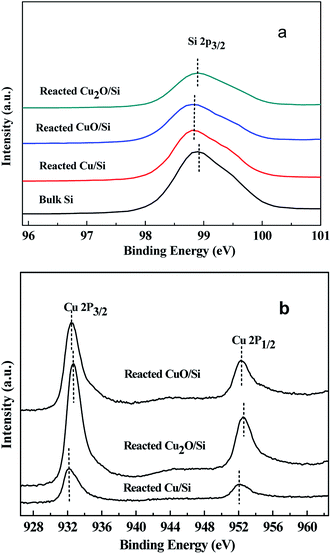 | ||
| Fig. 6 The XPS of (a) Si 2p and (b) Cu 2p of the reacted Cu2O/Si, reacted CuO/Si, and reacted Cu/Si mixtures. | ||
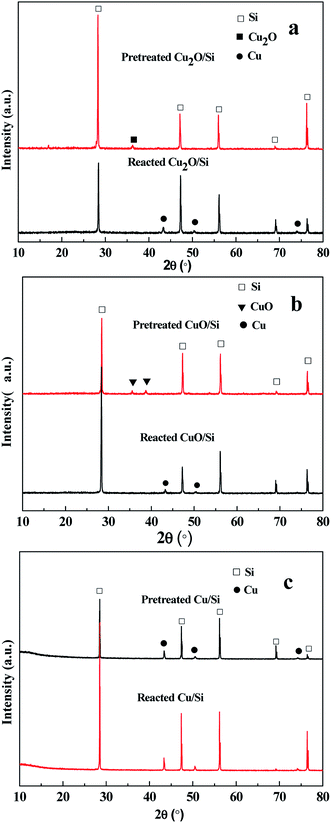
The binding energies of Cu 2p3/2 and Cu 2p1/2 of the reacted Cu2O/Si, reacted CuO/Si, and reacted Cu/Si mixtures were ca. 932.4 and 952.3 eV, respectively, indicating that metallic Cu0 and/or Cu+ species were present (Fig. 6b). The presence of a weak satellite peak at 942.5 eV for the reacted Cu2O/Si and reacted CuO/Si mixtures indicated that there was a trace amount of Cu2+ species present. However, the absence of the satellite peak in the reacted Cu/Si mixture indicated that no Cu2+ species existed.
To ascertain the chemical states of the surface copper species, the XAES peaks were deconvoluted (Fig. 7). Using Gaussian–Lorentzian bands with the peak positions at ca. 916 and 918.7 eV for Cu+ and Cu0 and at ca. 912 eV for other orbital electrons, the XAES peaks were deconvoluted into three symmetrical peaks.13,16,18–20
According to the deconvolution results, the surface copper species of the reacted Cu/Si, reacted Cu2O/Si, and reacted CuO/Si mixtures were mainly composed of Cu+ and metallic Cu0 components. During the reaction process, Cu+ and Cu2+ species in the Cu2O/Si and CuO/Si mixtures were reduced to copper species with lower valence states. The ratios of surface Cu+ to Cu0 were influenced by their copper precursors (Table 2).
3.3. Direct reaction of silicon with methanol over CuCl catalyst
| Pretreatment temperatures (°C) | Reaction temperatures (°C) | Methanol conversions (%) | Trimethoxysilane selectivities (%) | Tetramethoxysilane selectivities (%) |
|---|---|---|---|---|
a The reaction mixtures of silicon and CuCl were pretreated at different temperatures for 2 h in a N2 stream with a flow rate of 20 mL min−1. The mixture of CuCl and silicon was 40 g, the CuCl/Si weight ratio was 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, and the methanol flow rate was 6 mL h−1. 100, and the methanol flow rate was 6 mL h−1. |
||||
| 200 | 220 | 0.5 | 94.2 | 5.8 |
| 240 | 89.3 | 91.6 | 8.4 | |
| 260 | 97.2 | 81.5 | 18.5 | |
| 220 | 220 | 1.1 | 91.3 | 8.7 |
| 240 | 90.7 | 88.7 | 11.3 | |
| 260 | 94.5 | 82.6 | 17.4 | |
| 240 | 220 | 92.4 | 90.4 | 9.6 |
| 240 | 97.1 | 87.7 | 12.3 | |
| 260 | 94.1 | 88.3 | 11.7 | |
| 260 | 220 | 96.8 | 52.9 | 47.1 |
| 240 | 98.0 | 48.0 | 52.0 | |
| 260 | 99.1 | 42.6 | 57.4 | |
| 280 | 220 | 95.6 | 31.1 | 68.9 |
| 240 | 97.6 | 42.5 | 57.5 | |
| 260 | 99.6 | 46.9 | 53.1 | |
| 300 | 220 | 96.0 | 30.3 | 69.7 |
| 240 | 99.1 | 36.9 | 63.1 | |
| 260 | 99.7 | 44.7 | 55.3 | |
| 340 | 220 | 97.0 | 5.8 | 94.2 |
| 240 | 100 | 6.8 | 93.2 | |
| 260 | 99.9 | 9.5 | 90.5 | |
| CuCl/Si weight ratios | Reaction temperatures (°C) | Methanol conversions (%) | Trimethoxysilane | Tetramethoxysilane | ||
|---|---|---|---|---|---|---|
| Selectivities (%) | Yields (%) | Selectivities (%) | Yields (%) | |||
| a The CuCl/Si mixtures were pretreated at 200 °C for 2 h in a N2 stream with a flow rate of 20 mL min−1. The CuCl/Si mixture was 40 g and the methanol flow rate was 6 mL h−1. | ||||||
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
220 | 0.1 | 87.1 | 0.1 | 12.9 | 0.01 |
| 240 | 60.5 | 79.8 | 48.3 | 20.2 | 12.2 | |
| 260 | 81.4 | 62.3 | 50.7 | 37.7 | 30.7 | |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
220 | 0.5 | 93.2 | 0.5 | 6.8 | 0.03 |
| 240 | 90.4 | 89.6 | 81.0 | 10.4 | 9.4 | |
| 260 | 95.2 | 84.0 | 80.0 | 16.0 | 15.2 | |
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
220 | 0.5 | 94.2 | 0.5 | 5.8 | 0.03 |
| 240 | 89.3 | 91.6 | 81.8 | 8.4 | 7.5 | |
| 260 | 97.2 | 81.5 | 79.2 | 18.5 | 18.0 | |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
220 | 0.1 | 91.7 | 0.1 | 8.3 | 0.01 |
| 240 | 90.2 | 87.7 | 79.1 | 12.3 | 11.1 | |
| 260 | 98.4 | 84.0 | 82.7 | 16.0 | 15.7 | |
The reaction results showed that upon increasing the CuCl loading and reaction temperature, the conversion of methanol increased. When the CuCl loadings were 5–10% at the reaction temperatures of 240–260 °C, the trimethoxysilane yield of ca. 80% was obtained.
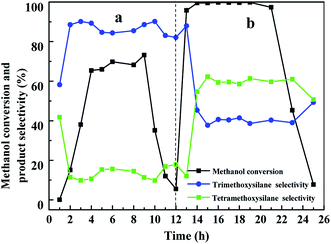
When the spent CuCl/Si mixture was chlorinated with HCl, the conversion of methanol increased to 95% during the first 1 h and remained at above 99% for 9 h (Fig. 8b). Then the methanol conversion decreased to 7.7% in 3 h. The selectivities of trimethoxysilane and tetramethoxysilane remained at ca. 40% and 60%, respectively.
The results revealed that without pretreatment, the reaction rate between silicon and methanol increased gradually with reaction time. The main product was trimethoxysilane. When the spent CuCl/Si mixture was chlorinated with HCl, the reaction rate between silicon and methanol rapidly increased, probably due to the formation of more CuxSiyClz active sites and the exposure of the metallic Cu0 active sites. However, after chlorination with HCl, the selectivity of trimethoxysilane was one half that using a fresh CuCl/Si mixture, indicating that the exposed metallic Cu0 probably promoted the formation of tetramethoxysilane.
3.4. Direct reaction of silicon with methanol over Cu2O, CuO, and metallic Cu0 catalysts
When Cu2O, CuO, and bulk metallic Cu0 were used as the catalysts, tetramethoxysilane was formed as the main product. The Cu2O and CuO catalysts exhibited higher catalytic activities for the reaction between silicon and methanol than the bulk metallic Cu0 catalyst (Table 5). This suggested that the surface Cu+/Cu0 species co-catalyzed the reaction between silicon and methanol to form tetramethoxysilane.| Catalysts | Cu0 (111) crystallite sizesb (nm) | Reaction temperatures (°C) | Methanol conversions (%) | Tetramethoxysilane selectivities (%) | Methyl formate selectivities (%) | Trimethoxysilane selectivities (%) | Activitiesc (molmethanol molCu−1 h−1) |
|---|---|---|---|---|---|---|---|
a The reaction mixtures of silicon and copper-based catalyst were pretreated at 240 °C for 2 h in a N2 stream with a flow rate of 20 mL min−1. The reaction conditions: the amount of catalyst/Si was 40 g; weight ratios of Cu2O, CuO, and bulk Cu0 to silicon were 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, 5 100, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, and 8 100, and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, respectively; methanol flow rate was 6 mL h−1.b Cu0 (111) crystallite sizes were calculated according to the Scherrer equation. The size for bulk Cu powder was the average particle size.c The catalytic activities were based on metallic copper amount in the catalysts. 100, respectively; methanol flow rate was 6 mL h−1.b Cu0 (111) crystallite sizes were calculated according to the Scherrer equation. The size for bulk Cu powder was the average particle size.c The catalytic activities were based on metallic copper amount in the catalysts. |
|||||||
| Cu2O | 27.1 | 220 | 64.4 | 100 | 0 | — | 3.4 |
| 240 | 93.8 | 98.2 | 1.8 | — | 5.0 | ||
| 260 | 97.0 | 90.9 | 9.1 | — | 5.2 | ||
| CuO | 31.7 | 220 | 55.0 | 99.2 | 0.8 | — | 3.3 |
| 240 | 70.3 | 92.3 | 7.7 | — | 4.2 | ||
| Bulk Cu0 | 150 μm | 220 | 11.1 | 96.9 | — | 3.1 | 0.3 |
| 240 | 45.2 | 90.8 | — | 9.2 | 1.3 | ||
| 260 | 54.2 | 87.9 | — | 12.1 | 1.6 | ||
It was interesting to discover that a small amount of methyl formate was formed as the by-product over the Cu2O and CuO catalysts. While using the bulk metallic Cu0 as the catalyst, a small amount of trimethoxysilane was formed as the by-product. According to the crystallite sizes of the metallic Cu0, the high catalytic activities over the Cu2O and CuO catalysts were due to the formation of small-sized metallic Cu0 crystallites in the reaction. The small-sized metallic Cu0 crystallites also catalyzed the formation of methyl formate via the intermolecular dehydrogenation reaction of methanol.21
| 2CH3OH = CH3OOCH + 2H2 | (4) |
4. Conclusions
In the direct reaction of silicon with methanol over a CuCl catalyst, pretreatment temperature affected the evolution of the CuxSiyClz species, metallic Cu0, and Cu3Si phases. The CuxSiyClz species appeared at a lower pretreatment temperature, whereas the Cu3Si phase appeared at a pretreatment temperature of 280 °C or higher.After pretreating CuCl/Si (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–10
100–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) mixtures at the pretreatment temperature of 200 °C, trimethoxysilane was predominantly formed with a yield ca. 80% at methanol conversion of more than 89% and reaction temperatures of 240–260 °C. The CuxSiyClz species catalyzed the formation of trimethoxysilane. After pretreating the CuCl/Si mixture at a pretreatment temperature of above 280 °C, tetramethoxysilane was favorably formed. The surface Cu+ and metallic Cu0 phases catalyzed the formation of tetramethoxysilane.
100) mixtures at the pretreatment temperature of 200 °C, trimethoxysilane was predominantly formed with a yield ca. 80% at methanol conversion of more than 89% and reaction temperatures of 240–260 °C. The CuxSiyClz species catalyzed the formation of trimethoxysilane. After pretreating the CuCl/Si mixture at a pretreatment temperature of above 280 °C, tetramethoxysilane was favorably formed. The surface Cu+ and metallic Cu0 phases catalyzed the formation of tetramethoxysilane.
Chlorination of the spent CuCl/Si mixture promoted the reaction between silicon and methanol to the formation of trimethoxysilane and tetraethoxysilane, probably due to the recovery of the CuxSiyClz active species and the exposure of metallic Cu0 species.
When the direct reaction was catalyzed over Cu2O and CuO catalysts, the Cu2O and CuO phases were reduced to the metallic Cu0 phase during the reaction process. Tetramethoxysilane was formed as the main product with a selectivity of more than 90% at the reaction temperatures of 220–260 °C. Cu2O and CuO catalysts exhibited higher catalytic activities for the direct reaction than the bulk metallic Cu0 catalyst. The surface Cu+ and metallic Cu0 co-catalyzed the formation of tetraethoxysilane.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21506078) and China Postdoctoral Science Foundation (2016M601739).References
- M. Okamoto, J. Komai, M. Uematsu, E. Suzuki and Y. Ono, J. Organomet. Chem., 2001, 619, 235–240 CrossRef.
- M. Okamoto, J. Komai, M. Uematsu, E. Suzuki and Y. Ono, J. Assoc. Arab Univ. Basic Appl. Sci., 2012, 12, 27–32 Search PubMed.
- J. S. Han, J. H. Cho, M. E. Lee and B. R. Yoo, Bull. Korean Chem. Soc., 2009, 30, 683–686 CrossRef.
- L. Zhang, J. Li, K. Yang, C. Hu, S. Ge and C. Yang, Adv. Mater. Res., 2011, 233–235, 1534–1539 Search PubMed.
- M. Okamoto, M. Osaka, K. Yamamoto, E. Suzuki and Y. Ono, J. Catal., 1993, 143, 64–85 CrossRef.
- E. Suzuki, M. Okamoto and Y. Ono, Chem. Lett., 1991, 199–202 CrossRef.
- E. Suzuki and Y. Ono, J. Catal., 1990, 125, 390–400 CrossRef.
- M. Okamoto, E. Suzuki and Y. Ono, J. Catal., 1994, 145, 537–543 CrossRef.
- N. Y. Adonin, S. A. Prikhod’ko, A. Y. Shabalin, I. P. Prosvirin, V. I. Zaikovskii, D. I. Kochubey, D. A. Zyuzin, V. N. Parmon, E. A. Monin, I. A. Bykova, P. O. Martynov, S. L. Rusakov and P. A. Storozhenko, J. Catal., 2016, 338, 143–153 CrossRef.
- M. Okamoto, N. Mimura, E. Suzuki and Y. Ono, Catal. Lett., 1995, 33, 421–427 CrossRef.
- B. Gillot, G. Weber, H. Souha and M. Zenkouar, J. Alloys Compd., 1998, 270, 275–280 CrossRef.
- R. Zhang, H. Yin, D. Zhang, L. Qi, H. Lu, Y. Shen and T. Jiang, Chem. Eng. J., 2008, 140, 488–496 CrossRef.
- W.-L. Dai, Q. Sun, J.-F. Deng, D. Wu and Y.-H. Sun, Appl. Surf. Sci., 2001, 177, 172–179 CrossRef.
- O. Baghriche, S. Rtimi, C. Pulgarin and J. Kiwi, Catal. Today, 2017, 284, 77–83 CrossRef.
- H. Liu, J. Xie, P. Liu and B. Dai, Catalysts, 2016, 6, 120 CrossRef.
- C. L. Aravinda, P. Bera, V. Jayaram, A. K. Sharma and S. M. Mayanna, Mater. Res. Bull., 2002, 37, 397–405 CrossRef.
- K. V. Rajani, S. Daniels, P. J. McNally and S. Krishnamurthy, J. Phys.: Condens. Matter, 2013, 25, 285501–285506 CrossRef PubMed.
- K. L. Deutsch and B. H. Shanks, J. Catal., 2012, 285, 235–241 CrossRef.
- K. Sun, W. Lu, F. Qiu, S. Liu and X. Xu, Appl. Catal., A, 2003, 252, 243–249 CrossRef.
- Z. He, H. Lin, P. He and Y. Yuan, J. Catal., 2011, 277, 54–63 CrossRef.
- Z. Lu, D. Gao, H. Yin, A. Wang and S. Liu, J. Ind. Eng. Chem., 2015, 31, 301–308 CrossRef.
| This journal is © The Royal Society of Chemistry 2018 |