Open Access Article
Open Access ArticleA combined experimental and DFT mechanistic study for the unexpected nitrosolysis of N-hydroxymethyldialkylamines in fuming nitric acid†
Yu Zhang,
Po Zou,
Yingbin Han,
Yongliang Geng,
Jun Luo * and
Baojing Zhou*
* and
Baojing Zhou*
School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China. E-mail: luojun@njust.edu.cn; bzhou@njust.edu.cn
First published on 24th May 2018
Abstract
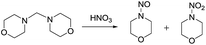
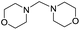
The reaction of dimorpholinomethane in fuming HNO3 was investigated. Interestingly, the major product was identified as N-nitrosomorpholine and a key intermediate N-hydroxymethylmorpholine was detected during the reaction by 1H-NMR tracking which indicates that the reaction proceeds via an unexpected nitrosolysis process. A plausible nitrosolysis mechanism for N-hydroxymethyldialkylamine in fuming nitric acid involving a HNO3 redox reaction is proposed, which is supported by both experimental results and density functional theory (DFT) calculations. The effects of ammonium nitrate and water on the nitrosolysis were studied using different ammonium salts as additives and varying water content, respectively. Observations show the key role of ammonium ions and a small amount of water in promoting the nitrosolysis reaction. Furthermore, DFT calculations reveal an essential point that ammonia, merged from the decomposition of the ammonium salts, acts as a Lewis base catalyst, and the hydroxymethyl group of the substrate participates in a hydrogen-bonding interaction with the NH3 and H2O molecules.
Introduction
N-Nitramines and N-nitrosamines are present in a wide range of drug molecules, functional organic chemicals and energetic materials.1 For example, some N-nitrosamines have biological activity and can be used in various treatments for illnesses including cancer, cardiovascular diseases, central nervous, and diseases related to immunity and physiological disorders.2 N-Nitrosamines are valuable intermediates in organic synthesis3 such as in application for the preparation of biologically important α-disubstituted hydrazines4 and mesoionic-heterocyclic compounds like sydnones.5 N-Nitrosamines can also be applied as a nitroso source to prepare aryl C-nitroso compounds in good yield through a Fischer-Hepp rearrangement.6 Recently, N-nitroso functionality has emerged as a traceless directing group for the activation of inert C–H bonds in aryl rings by associating with transition metals.7 Furthermore, some widely used explosives with excellent performances such as 1,3,5-trinitro-1,3,5-triazacyclohexane (RDX), 1,3,5,7-tetranitro-1,3,5,7-tetraazacyclooctane (HMX) and 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20) belong to the N-nitramine group, which can be prepared from acetamides and N-nitrosamines through nitrolysis or oxidation.8Interestingly, when nitryl chloride, nitrogen pentoxide, nitryl fluoride, nitronium fluoroborate and tetranitromethane were used for the N-nitration of secondary or tertiary amines at low temperatures, nitrosamine products were obtained with low yields.9 Our group made efforts to synthesize HMX by nitrolyzing 3,7-dinitro-1,3,5,7-tetraazabicyclo[3.3.1]nonane (DPT) with traditional nitration systems such as fuming HNO3, HNO3–H2SO4, HNO3–N2O5, and HNO3–Ac2O below 0 °C, but 1-nitroso-3,5,7-trinitro-1,3,5,7-tetraazacyclooctane (MNX) was achieved as the main product. Furthermore, NH4NO3 can inevitably improve the yield of MNX.8f Recently, the so called “small-molecule route” using urea as the starting material to prepare HMX via DPT has been thought to be a promising method for scale-up.10 Although the development of various techniques has enhanced the yield of HMX by the nitrolysis of DPT, no significant progress in practical potential has been achieved.11 The main reason is attributed to the ambiguity of the nitrolysis mechanism.8e,8f It’s widely considered that the bridging methylene of the DPT is cleaved to produce an extremely active intermediate 1-hydroxymethyl-3,5,7-trinitro-1,3,5,7-tetraazacyclooctane.12 The addition of NH4NO3 can hinder esterification (O-acetylation in HNO3–Ac2O and N-nitraton in fuming HNO3) but promote dehydroxymethylation to improve the HMX yield.8e,8f,11,12 However, there is no sufficient or direct experimental evidence to support the above mechanism because of the rapid reaction characteristics of the nitrolysis process, and the production of a complex by-product, and a short-lived intermediate.12
In this work, we selected a bridging methylene diamine dimorpholinomethane as a model substance and investigated in detail its reaction in fuming nitric acid to mimic the nitrolysis of DPT. Interestingly, nitrosomopholine was obtained as the main product and the key intermediate N-hydroxymethylmorpholine was detected by 1H-NMR tracking during the reaction. Based on experimental observations, a plausible mechanism for the nitrosolysis of N-hydroxymethyldialkylamines in fuming nitric acid is proposed. We further provide computational results for the possible reaction mechanism at the molecular level using the density functional theory (DFT), which is one of the popular routes to understand the course of an organic reaction.13
Results and discussion
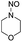
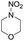
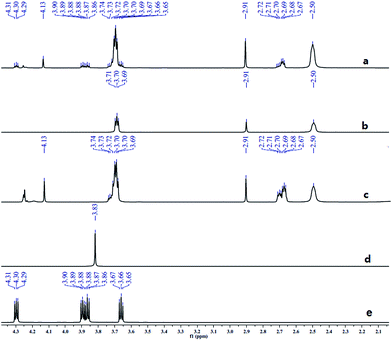
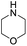
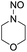
Similar to DPT, dimorpholinomethane has the N,N′-methylene motif. Therefore, we first examined the reaction of the dimorpholinomethane with fuming nitric acid. To our surprise, N-nitrosomorpholine was obtained rather than N-nitromorpholine when the reaction was carried out at room temperature (Table 1, entry 1). Prolonging the reaction time increased the yield of N-nitrosomorpholine (entries 2, 3). A small amount of N-nitromorpholine was isolated after reacting for 12 hours (entry 3). When the reaction temperature was elevated from 25 °C to 40 °C, both of them were obtained with higher yields (entry 4). A further increase of the reaction temperature to 70 °C afforded a lower yield of N-nitrosomorpholine but a higher yield of N-nitromorpholine (entry 5). It is well known that NH4NO3 plays a key role in the nitrolysis of DPT.11 Here, similarly, the yield of N-nitrosomorpholine increased dramatically when NH4NO3 was used as an additive to the reaction mixture (entry 6).It is generally accepted that 1-hydroxymethyl-3,5,7-trinitro-1,3,5,7-tetraazacyclooctane is a key intermediate during the nitrolysis of DPT in fuming nitric acid. Accordingly, we hypothesized that N-hydroxymethylmorpholine is the intermediate in the reaction of dimorpholinomethane in fuming nitric acid. To verify this, the reaction was performed at 0 °C for 10 minutes, then the reaction mixture was poured into ice water, extracted with cold dichloromethane, and washed with ice water to neutralise it. A yellow oily liquid was obtained by rotary evaporation at room temperature. According to the 1H-NMR spectrum, three compounds can be identified (Fig. 1a). As shown in Fig. 1b, the chemical shifts of the dimorpholinomethane occur at 3.70–3.69, 2.91, 2.50 ppm.14 Clearly, the mixture mainly consists of dimorpholinomethane. As shown in Fig. 1c, hydrogen atoms observed at 4.13, 3.74–3.69 and 2.72–2.67 ppm are assigned to N-hydromethylmorpholine.14 Comparing Fig. 1a with 1c, we can distinguish N-hydromethylmorpholine from the mixture. However, N-nitromorpholine, whose chemical shift is at 3.83 ppm (Fig. 1d), is not found in the mixture. Moreover, comparing Fig. 1a with 1e, we found that the chemical shifts at 44.31–4.29, 3.91–3.86 and 3.68–3.66 ppm in the former spectrum belong to N-nitrosomorpholine.14a Thus, N-hydroxymethylmorpholine is formed during the reaction and nitrosolysis is predominant at room temperature in classical nitration systems.
Although N-hydroxymethylmorpholine was observed in the reaction, it is not clear whether it is an intermediate or a by-product. To shed some light on this, we prepared a mixture of N-hydroxymethylmorpholine and dimorpholinomethane as the substrate for the reaction. As shown in Table 2, N-nitrosomorpholine is still the major product in fuming nitric acid, and only 3% of N-nitromorpholine is obtained (entry 1). The yields of N-nitrosomorpholine and N-nitromorpholine increase with the elongation of the reaction time (entries 1–3). In contrast, with an elevation in the reaction temperature, the yield of N-nitrosomorpholine first increases and then decreases (entries 1, 4, 5). Once again, NH4NO3 significantly promotes nitrosolysis (entry 6). These observations are similar to those made in the nitrosolysis of dimorpholinomethane. Thus, N-hydroxymethylmorpholine should be an intermediate in the reaction, rather than a by-product. The nitrosolysis of the mixture of N-hydroxymethylmorpholine and dimorpholinomethane was further carried out in fuming nitric acid/ammonium salts containing (NH4)2SO4, NH4Cl, and CH3COONH4. Similar to NH4NO3, these ammonium salts can also improve the yield of N-nitrosomorpholine (entries 7–9).
| Substrateb | Entry | Reactant system | Nitrosamine product | Nitrosamine yieldh/% | Nitramine product | Nitramine yieldh/% |
|---|---|---|---|---|---|---|
a Unless otherwise noted, fuming HNO3 (200 mmol), T = 25 °C, t = 1 h.b Substrate (5 mmol), n(N-hydroxymethylmorpholine)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(dimorpholinomethane) = 1 n(dimorpholinomethane) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1, n(N-hydroxymethylpiperidine) 1.1, n(N-hydroxymethylpiperidine)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(dipiperidinemethane) = 1 n(dipiperidinemethane) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25, n(N-hydroxymethyldibutylamine) 1.25, n(N-hydroxymethyldibutylamine)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(dibutylamine) = 1 n(dibutylamine) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.17.c t = 4 h.d t = 12 h.e T = 40 °C.f T = 70 °C.g NH4NO3 (12.5 mmol); NH4Cl (12.5 mmol); CH3COONH4 (12.5 mmol); (NH4)2SO4 (6.25 mmol).h Isolated yield. 1.17.c t = 4 h.d t = 12 h.e T = 40 °C.f T = 70 °C.g NH4NO3 (12.5 mmol); NH4Cl (12.5 mmol); CH3COONH4 (12.5 mmol); (NH4)2SO4 (6.25 mmol).h Isolated yield. |
||||||
 |
1 | HNO3 | 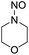 |
21 | 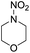 |
3 |
| 2c | HNO3 | 30 | 6 | |||
| 3d | HNO3 | 35 | 12 | |||
| 4e | HNO3 | 33 | 9 | |||
| 5f | HNO3 | 25 | 18 | |||
| 6 | HNO3/NH4NO3g | 51 | 2 | |||
| 7 | HNO3/(NH4)2SO4g | 46 | 3 | |||
| 8 | HNO3/NH4Clg | 49 | 2 | |||
| 9 | HNO3/CH3COONH4g | 47 | 2 | |||
 |
10 | HNO3 | 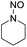 |
21 | 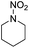 |
0 |
| 11 | HNO3/NH4NO3g | 39 | 0 | |||
 |
12 | HNO3 | 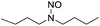 |
19 | 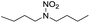 |
4 |
| 13 | HNO3/NH4NO3g | 38 | 4 | |||
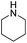
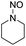
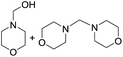
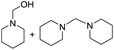
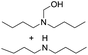
We further examined the nitrosolysis of the mixture of N-hydroxymethylpiperidine with dipiperidinemethane and that of N-hydroxymethyldibutylamine with dibutylamine in the fuming HNO3, respectively. As shown in Table 2, the main products are identified to be N-nitrosamines15 (entries 10, 12). To estimate the interference of dibutylamine, we calculated its amount in the mixture according to the proton NMR test. Then an equal amount of dibutylamine was used as the substrate for a reaction under common reaction conditions, which was monitored by gas chromatography. Only less than 4% N-nitrosodibutylamine (gas chromatography yield) was detected over 2 h. So we conclude that the N-nitrosodibutylamine is formed predominantly by the nitrosolysis of N-hydroxymethyldibutylamine. The achieved yield of N-nitrosopiperidine and N-nitrosodibutylamine in the fuming HNO3/NH4NO3 system was 39% and 38% respectively, much higher than for the reactions without NH4NO3 (entries 10–13).
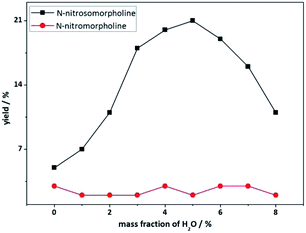
The influence of water on the nitrosolysis of the mixture of N-hydroxymethylmorpholine and dimorpholinomethane was evaluated by performing the reaction in water-containing nitric acid, which was prepared by the addition of different amounts of water into freshly prepared 100% HNO3. As shown in Fig. 2, the yield of N-nitrosomorpholine first increases and then decreases with the increase of water content. The highest yield of 21% was obtained when ca. 5% water was added. Meanwhile, a small amount of N-nitromopholine was observed in all cases. These results suggest that ca. 5% water is optimal for the nitrosolysis of the mixture of N-hydroxymethylmorpholine and dimorpholinomethane.
Considering the fact that only very little HNO2 and its analogues are present in fuming nitric acid, it is puzzling that the product is mostly N-nitrosamine in the above reactions. It is natural to surmise that a redox reaction has occurred. It is plausible that N-hydroxymethyldialkylamines react with NO+ to afford the N-nitrosoamines and release formaldehyde, which reduces nitric acid to nitrous acid. Then nitrous acid can be viewed as a catalyst. To further verify this viewpoint, three secondary amines were reacted in the fuming nitric acid with or without paraformaldehyde (Table 3). It turned out that N-nitrosamines were obtained in less than 6% yield in the absence of paraformaldehyde (entries 1, 3, 5). The addition of paraformaldehyde greatly increases the yield (entries 2, 4, 6). These results suggest that N-hydroxymethyldialkylamines are formed as the more active intermediates under the acidic conditions when paraformaldehyde is present.13
In the above reaction, some N-nitromorpholine was observed and its amount increased with the reaction time (Table 2, entries 1–3). To investigate whether it was formed from the nitrolysis of N-nitrosomorpholine, we monitored the reaction of N-nitrosomorpholine in fuming nitric acid at 40 °C by 1H-NMR spectroscopy. As shown in Fig. 3a, no N-nitromorpholine was observed at the end of the feeding process (Fig. 3a). However, N-nitromorpholine gradually formed with an increase in the reaction time as indicated by the chemical shift of 3.83 ppm in Fig. 3b and c15 and its proportion increased with the disappearance of N-nitrosomorpholine. After 6 hours, almost all of the N-nitrosomorpholine had been converted to N-nitromorpholine (Fig. 3d). On the other hand, some N-nitroamines may have been generated by the oxidation of the N-nitrosamines.8 To examine this possibility, the reactions of nitrosomorpholine in 90% HNO3, 80% HNO3, 70% HNO3, 60% HNO3, and 50% HNO3 were investigated. Almost no product, however, was detected even when the reaction temperature changed from 20 °C to 80 °C. Thus, we conclude that N-nitromorpholine is mainly formed through the nitrolysis of N-nitrosomorpholine in fuming nitric acid.
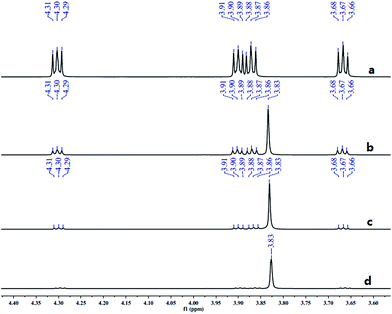 | ||
| Fig. 3 1H-NMR spectra of samples taken from the reaction mixture at: (a) the end of the feeding process; (b) 2 hours; (c) 4 hours (d) 6 hours. | ||
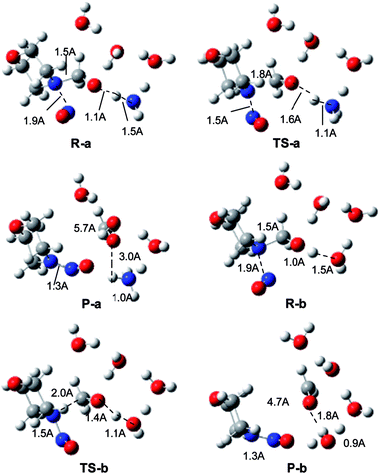
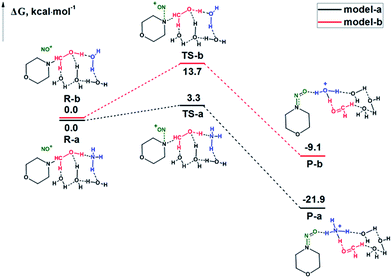
We further performed a computational study to investigate the reaction mechanism at the atomic level. Two reaction models were constructed. The first reactant model contained one N-hydroxymethyldialkylamine, one NH3 and three H2O molecules. In another reaction model, the NH3 molecule was replaced by a H2O molecule. Interestingly, we found that the free ammonia, formed from the unfavourable balance with NH4+ in an acidic environment, is a Lewis base catalyst, while water molecules are essential for the formation of the hydrogen bond (HB) adduct. A bicyclic ring was inferred from the bond angle of H2O molecules. The optimized structures of the reactants, transition states (TSs), and products from the two reaction models in the presence (model a) or absence (model b) of NH3 are compared in Fig. 4.
For both models, we found that NO+ is not involved in the bicyclic ring formed from the HB interactions and it approaches the N atom in the substrate from the other side. In the TS, the N–N bond forms, and the C–N bond is breaking, while the H is transferring from the hydroxyl group to the NH3 (model a) or H2O molecule (model b), which suggests a synergistic mechanism. The computed relative Gibbs free energy profiles for models a and b are summarized in Fig. 5. For model a, the predicted Gibbs free energy of activation is 3.3 kcal mol−1. For model b, the reaction barrier is 13.7 kcal mol−1, which is 10.4 kcal mol−1 higher than that of model a. These results indicate that the NH3 molecule plays the role of a catalyst. Therefore, the addition of NH4+ can significantly promote the nitrosolysis of N-hydroxymethyldialkylamines.
 | ||
| Fig. 5 Energy profile for the nitrosolysis of N-hydroxymethylmorpholine in the presence or absence of NH3. | ||
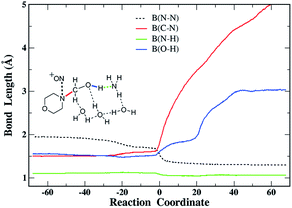
The gradual changes of the four chemical bonds along the reaction coordinate are illustrated in Fig. 6. First, the hydroxyl H is extracted by the N of ammonia, which leaves the negative charge on the N-hydroxymethyldialkylamine substrate. Then the electrophilic attack is initiated by NO+ as indicated by its decreasing separation from the N atom of the substrate. When the N–N bond is half formed, the C–N and O–H bonds break, which leads to the formation of the products, i.e. nitrosamine, formaldehyde, and ammonium. The highly synchronous variations further indicate the concerted reaction mechanism for the nitrolysis of N-hydroxymethylmorpholine.
 | ||
| Fig. 6 Bond cleavages and formations along the IRC path of the nitrosolysis of N-hydroxymethylmorpholine. | ||
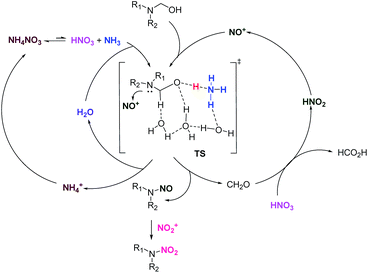
Based on our experimental and theoretical results and the related reports,8e,8f,11,12 we propose a plausible mechanism for the formation of N-nitrodialkylamines from N-hydroxymethyldialkylamines in fuming nitric acid and an ammonium nitrate system (Scheme 1). First, a small amount of HNO2 in fuming HNO3 is converted to NO+ and some free NH3 emerges through the equilibrium reaction with NH4NO3. The substrate is conjoined to one ammonia and three water molecules by HB interactions to form the pre-reactive complex in situ. Then, the NH3 abstracts the hydrogen from the hydroxyl group to increase the electron density of the tertiary N atom, which facilitates the attack of NO+. Then N-nitrosamine forms along with the departure of formaldehyde. Subsequently, formaldehyde is oxidized by HNO3 to form HCOOH.16 Simultaneously, HNO3 is reduced to HNO2 to complete one nitrosolysis cycle. N-Nitramine is afforded from N-nitrosamine by the replacement of the nitroso group with a nitro group via a nitrolysis process.
Conclusions
We synthesized N-nitrosodialkylamines from N-hydroxymethyldialkylamines in fuming HNO3 via nitrosolysis. The reaction mechanism at the molecular level was established based on experimental observations and DFT calculations and involves a redox reaction of HNO3 to afford HNO2. The DFT calculations indicate that the nitrosolysis reaction proceeds very smoothly with a rigid bicyclic transition state consisting of N-hydroxymethyldialkylamine, ammonia and water.Experimental section
General procedure for the nitrosolysis of the mixture of N-hydroxymethyldialkamines in fuming nitric acid
The mixture of N-hydroxymethyldialkamines (5 mmol) was added in portions to fuming nitric acid (200 mmol) under vigorous stirring at 0 °C, and the reaction mixture was then warmed to a certain temperature. The reaction was quenched by being poured into ice water (20 mL). The resulting mixture was extracted with dichoromethane (3 × 30 mL). The combined organic layer was washed with saturated NaHCO3 and dried with Na2SO4. After filtration, the mixture was concentrated under reduced pressure to afford the crude product, and the desired product was obtained by column chromatography.The same procedure as described above was used for the nitrosolysis of dimorpholinomethane.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was sponsored by Qing Lan Project of Jiangsu Province, P. R. China.Notes and references
-
(a) R. N. Leoppky and J. R. Outram, N-Nitroso Compounds: Occurrence and Biological Effects, IARC Scientific Publishers, Lyon, 1982 Search PubMed
; (b) R. N. Loeppky and C. J. Michjda, Nitrosamines and Related N-Nitroso Compounds: Chemistry and Biochemistry, ACS Symposium Series, American Chemical Society, Washington DC, 1994 Search PubMed
; (c) S. Gupta, N. Muniyappan and S. Sabiah, Green Chem., 2016, 18, 2323–2330 RSC
; (d) J. Zhang, J. Jiang and Y. Li, J. Org. Chem., 2013, 78, 11366–11372 CrossRef PubMed
; (e) J. P. Agrawal and R. Hodgson, Organic chemistry of explosives, Wiley-VCH, New York, 2007 Search PubMed
; (f) B. Wang, X. Qi and W. Zhang, J. Mater. Chem. A, 2017, 5, 20867–20873 RSC
; (g) P. Yin and J. M. Shreeve, Angew. Chem., Int. Ed., 2016, 54, 14513–14517 CrossRef PubMed
; (h) P. Yin, D. A. Parrish and J. M. Shreeve, Angew. Chem., Int. Ed., 2014, 53, 12889–12892 CrossRef PubMed
.
-
(a) P. G. Wang and M. Xian, Chem. Rev., 2002, 102, 1091–1134 CrossRef PubMed
; (b) H. Kakeya, M. Imoto, Y. Takahashi, H. Naganawa, T. Takeuchi and K. Umezawa, J. Antibiot., 1993, 46, 1716–1719 CrossRef PubMed
; (c) M. Li, S. M. Shandilya and M. A. Carpenter, ACS Chem. Biol., 2012, 7, 506–517 CrossRef PubMed
; (d) C. T. Gnewuch and G. Sosnovsky, Chem. Rev., 1997, 97, 829–1013 CrossRef PubMed
; (e) A. A. Rossini, A. A. Like and W. L. Chick, Proc. Natl. Acad. Sci. U. S. A., 1977, 74, 2485–2489 CrossRef PubMed
; (f) H. Iimura, T. Takeuchi, S. Kondo and M. Matsuzaki, J. Antibiot., 1972, 25, 497–500 CrossRef PubMed
; (g) W. D. Kumler and P. P. Sah, J. Pharm. Sci., 1952, 41, 375–379 CrossRef
; (h) F. D. Popp, J. Pharm. Sci., 1973, 62, 679–680 CrossRef PubMed
.
- J. E. Saavedra, Org. Prep. Proced. Int., 1987, 19, 85–159 CrossRef
.
-
(a) I. D. Entwistle, R. A. W. Johnstone and A. H. Wilby, Tetrahedron, 1982, 38, 419–423 CrossRef
; (b) F. W. Schueler and C. Hanna, J. Am. Chem. Soc., 1951, 73, 4996 CrossRef
; (c) W. W. Hartman and L. J. Roll, Org. Synth., 1933, 13, 66–67 CrossRef
; (d) R. L. Hinmap and K. L. Hamm, J. Org. Chem., 1958, 23, 529–531 CrossRef
.
-
(a) D. L. Browne and J. P. A. Harrity, Tetrahedron, 2010, 66, 553–556 CrossRef
; (b) F. H. C. Stewart, Chem. Rev., 1964, 64, 129–147 CrossRef
.
-
(a) D. L. H. Williams, Tetrahedron, 1975, 31, 1343–1349 CrossRef
; (b) I. Cikotiene, M. Jonusis and V. Jakubkiene, Beilstein J. Org. Chem., 2013, 9, 1819–1825 CrossRef PubMed
.
-
(a) B. Liu, Y. Fan, Y. Gao, C. Sun, C. Xu and J. Zhu, J. Am. Chem. Soc., 2013, 135, 468–473 CrossRef PubMed
; (b) B. Q. Liu, C. Song, C. Sun, S. G. Zhou and J. Zhu, J. Am. Chem. Soc., 2013, 135, 16625–16631 CrossRef PubMed
; (c) C. Wang and Y. Huang, Org. Lett., 2013, 15, 5294–5297 CrossRef PubMed
; (d) J. Chen, P. Chen, C. Song and J. Zhu, Chem.–Eur. J., 2014, 20, 14245–14249 CrossRef PubMed
; (e) T. Gao and P. Sun, J. Org. Chem., 2014, 79, 9888–9893 CrossRef PubMed
; (f) Y. Wu, L. J. Feng, X. Lu, F. Y. Kwong and H. B. Luo, Chem. Commun., 2014, 50, 15352–15354 RSC
; (g) F. Xie, Z. S. Qi, S. J. Yu and X. W. Li, J. Am. Chem. Soc., 2014, 136, 4780–4787 CrossRef PubMed
; (h) S. Yu and X. Li, Org. Lett., 2014, 16, 1200–1203 CrossRef PubMed
; (i) D. D. Li, Y. X. Cao and G. W. Wang, Org. Biomol. Chem., 2015, 13, 6958–6964 RSC
; (j) J. Dong, Z. Wu, Z. Liu, P. Liu and P. Sun, J. Org. Chem., 2015, 80, 12588–12593 CrossRef PubMed
; (k) Y. Liang and N. Jiao, Angew. Chem., Int. Ed., 2016, 55, 4035–4039 CrossRef PubMed
; (l) L. Zhang, Z. Wang and P. Y. Guo, Tetrahedron Lett., 2016, 57, 511–2514 Search PubMed
.
-
(a) F. J. Brockman, D. C. Downing and G. F. Wright, Can. J. Res., 1949, 27, 469–474 CrossRef
; (b) T. G. Bonner, R. A. Hancock and J. C. Robert, J. Chem. Soc., Perkin Trans. 1, 1974, 5, 653–658 RSC
; (c) W. E. Bachmann and E. L. Jenner, J. Am. Chem. Soc., 1951, 73, 2773–2775 CrossRef
; (d) J. R. Wu, Chin. J. Explos. Propellants, 1989, 4, 1–5 Search PubMed
; (e) W. J. Liu, Z. B. Xu, K. J. Cui and Z. H. Meng, Propellants, Explos., Pyrotech., 2015, 40, 645–651 CrossRef
; (f) Y. Zhang, P. Zou and J. Luo, Propellants, Explos., Pyrotech., 2017, 42, 1208–1213 CrossRef
; (g) N. V. Latypov, A. U. Wellmar and P. Goede, Org. Process Res. Dev., 2000, 4, 156–158 CrossRef
; (h) Y. X. Ou, Z. Meng and J. Q. Liu, Chem. Ind. Eng. Prog., 2007, 26, 762–768 Search PubMed
.
-
(a) D. L. H. Williams, Adv. Phys. Org. Chem., 1983, 19, 381–428 CrossRef
; (b) M. A. Ilyushin, E. L. Golod and B. V. Gidaspov, Zh. Org. Khim., 1977, 13, 11–17 Search PubMed
; (c) T. C. Bruice and S. L. Walters, J. Am. Chem. Soc., 1971, 93, 2269–2282 CrossRef
; (d) A. Castonguay and H. V. Vunakis, Toxicol. Lett., 1979, 4, 475–480 CrossRef
; (e) J. C. Bottaro, R. T. Schmitt and C. D. Bedford, J. Org. Chem., 1987, 52, 2292–2294 CrossRef
; (f) O. V. Anikin, G. V. Pokhvisneva and D. L. Lipilin, Russ. Chem. Bull., 2009, 58, 2043–2046 CrossRef
; (g) Y. D. Park, H. K. Kim and J. J. Kim, J. Org. Chem., 2003, 68, 9113–9115 CrossRef PubMed
; (h) X. F. Chen, K. Yang and B. Z. Wang, J. Phys. Chem. A, 2013, 117, 5007–5014 CrossRef PubMed
; (i) Y. D. Liu and R. G. Zhong, Chin. J. Struct. Chem., 2010, 29, 421–431 Search PubMed
; (j) Z. Mao, H. Jiang and Z. Li, Chem. Sci., 2017, 8, 4533–4538 RSC
; (k) J. Miao, Y. Y. Huo and X. Lv, Biomaterials, 2016, 78, 11–19 CrossRef PubMed
; (l) R. Azadi and K. Kolivand, Tetrahedron Lett., 2015, 56, 5613–5615 CrossRef
.
-
(a) S. Radhakrishnan, M. B. Talawar and S. Venugopalan, J. Hazard. Mater., 2008, 152, 1317–1324 CrossRef PubMed
; (b) S. Radhakrishnan, K. S. Kumar and T. Soman, J. Energ. Mater., 2008, 26, 102–114 CrossRef
; (c) H. Y. Song, P. Wang, Z. X. Ge and Z. H. Meng, Chin. J. Org. Chem., 2010, 30, 414–418 Search PubMed
; (d) H. Qian, D. B. Liu and C. X. Lv, Lett. Org. Chem., 2011, 42, 184–187 CrossRef
; (e) F. S. Toosi and M. Jadidoleslami, Synth. React. Inorg. Met.-Org. Chem., 2016, 46, 159–162 CrossRef
.
-
(a) L. Chen, Z. M. Chen and X. H. Chen, Chin. J. Explos. Propellants, 1986, 3, 1–5 Search PubMed
; (b) X. C. Huang, Z. H. Meng and Z. X. Ge, Chin. J. Appl. Chem., 2013, 42, 299–303 Search PubMed
; (c) M. H. Xi, Chin. J. Energ. Mater., 1996, 4, 57–61 Search PubMed
; (d) Q. L. Li, J. Chen and J. L. Wang, Chin. J. Energ. Mater., 2007, 15, 509–510 Search PubMed
; (e) Z. Y. He, J. Luo and C. X. Lv, Bull. Korean Chem. Soc., 2011, 32, 2677–2682 CrossRef
; (f) H. Z. Zhi, J. Luo and G. A. Feng, Chin. Chem. Lett., 2009, 20, 379–382 CrossRef
; (g) Z. Y. He, J. Luo and C. X. Lv, Chin. J. Explos. Propellants, 2010, 33, 1–4 Search PubMed
.
-
(a) X. C. Huang, T. Yu and Z. X. Ge, Chin. J. Energ. Mater., 2015, 23, 1151–1154 Search PubMed
; (b) A. F. McKay, G. F. Wright and H. H. Richmond, Can. J. Res., 1949, 27, 462–468 CrossRef
; (c) Z. Y. He, J. Luo and C. X. Lv, Chin. J. Energ. Mater., 2012, 20, 5–8 Search PubMed
; (d) S. Epstein and C. A. Winkler, Can. J. Chem., 1952, 30, 734–742 CrossRef
; (e) Z. M. Si, S. H. Jin and L. J. Li, Chin. J. Explos. Propellants, 2011, 34, 21–25 Search PubMed
; (f) L. Shi, Z. Gang and Y. Zhang, Chin. J. Chem., 2010, 28, 1553–1558 CrossRef
; (g) S. L. Zhang, X. F. Xiong and Y. U. Tao, Chem. J. Chin. Univ., 2012, 33, 1444–1449 Search PubMed
; (h) Z. Y. He, J. Luo and C. X. Lv, J. Explos. Propellants, 2010, 33, 1–4 Search PubMed
.
-
(a) S. Li, H. Tang and Y. Wang, J. Mol. Catal. A: Chem., 2015, 407, 137–146 CrossRef
; (b) Y. Wang, L. Zheng, D. Wei and M. Tang, Org. Chem. Front., 2015, 2, 874–884 RSC
; (c) Y. Wang, B. Wu, L. Zheng, D. Wei and M. Tang, Org. Chem. Front., 2016, 3, 190–203 RSC
; (d) Y. Wang, M. Tang and D. Wei, J. Org. Chem., 2016, 81, 5370–5380 CrossRef PubMed
.
-
(a) M. Turlington and L. Pu, Org. Synth., 2010, 87, 59–67 CrossRef
; (b) T. Netscher, F. Mazzini and J. Roselyne, Eur. J. Org. Chem., 2007, 7, 1176–1183 CrossRef
.
-
(a) J. Zhang, J. Jiang and Y. Li, J. Org. Chem., 2013, 78, 11366–11372 CrossRef PubMed
; (b) I. V. Kuchurov, I. V. Fomenkov and S. G. Zlotin, Russ. Chem. Bull., 2010, 59, 2147–2150 CrossRef
.
- R. G. Pearson and J. Songstad, J. Am. Chem. Soc., 1967, 89, 1827–1836 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra03268h |
| This journal is © The Royal Society of Chemistry 2018 |