Open Access Article
Open Access ArticleDevelopment and evaluation of pymetrozine controlled-release formulation to control paddy planthopper
Wei-Ming Xu *,
Ming Zhang,
Kun Wei,
Yan Chen†
,
Qin Liu,
Wei Xue,
Lin-Hong Jin,
Ming He,
Zuo Chen and
Song Zeng
*,
Ming Zhang,
Kun Wei,
Yan Chen†
,
Qin Liu,
Wei Xue,
Lin-Hong Jin,
Ming He,
Zuo Chen and
Song Zeng
State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Center for Research and Development of Fine Chemicals, Guizhou University, Guiyang 550025, P. R. China. E-mail: xuweiming2009@163.com; Fax: +86 851 8362 0521; Tel: +86 851 8829 2090
First published on 20th June 2018
Abstract
Continuous outbreaks of rice planthoppers in rice-growing regions in China indicates the importance of redesigning several planthopper management programs. Chemical control remains the main strategy for planthopper control in China and other subtropical and temperate regions. Most common chemical insecticides are emulsifiable concentrates, suspension concentrates, soluble concentrates, and wettable powders. These insecticides are applied by dusting or spraying using simple equipment. The active ingredient, with short effectiveness time, is degraded rapidly in natural paddy ecosystems. Thus, repeated pesticide applications are required to control rice planthoppers. Altering the short-term effect formulation of pesticides to a long-acting formulation may be an alternative solution. A pymetrozine controlled-release granule (CRG; 1%) was developed by loading the pesticide on bentonite and coating the solid pesticide with resin. Analysis of pymetrozine release indicated that the 1% pymetrozine CRG release was more than 80% for 60 days. In the field trial screening, the 1% pymetrozine CRG showed a controlled effect of 61.96–78.87% at 48 days after CGR application. Application of 1% pymetrozine CRG at the recommended dosage and 1.5 times the recommended dosage resulted in terminal residues on brown rice below the maximum residue limit (0.1 mg kg−1) of China and Japan. Moreover, the pesticide granules showed low toxicity against all tested beneficial organisms in the environment. Pymetrozine CRG (1%) showed good controlled release and efficacy for controlling paddy planthoppers. The compound exhibited a low terminal residue and low toxicity against all tested beneficial organisms. Pymetrozine CRG (1%) showed great potential for field applications to control paddy planthoppers, because it overcame the rapid loss of biological function during treatment.
1 Introduction
Laodelphax striatellus Fallen (small brown planthopper, SBPH), Nilaparvata lugens Stål (brown planthopper, BPH), and Sogatella furcifera Horvath (whitebacked planthopper, WBPH) are major pests in rice-growing areas in Asia.1 Continuous outbreaks of rice planthoppers in such regions in China in 2006 (damage of 9.4 million ha), 2007 (damage of 9.4 million ha), and 2011 indicated the importance of redesigning several planthopper management programs.2 Moreover, SBPH caused serious rice yield losses by transmitting rice stripe virus disease3 and rice black-streaked dwarf virus disease.4 A novel viral disease caused by the southern rice black-streaked dwarf virus has recently spread in rice throughout East and Southeast Asia since the mid-2000s. This pathogen has become one of the most important rice pathogens in these regions and is efficiently transmitted by the WBPH in a persistent circulative propagative manner.5–7 Chemical control remains the main strategy for planthopper control in subtropical and temperate regions, such as in China, Japan, and South Korea. Although field experiments have demonstrated that unreasonable use of insecticides could also cause planthopper resurgence in subtropical and temperate areas, insecticides are still extensively used in these regions.8–11The most common formulations of chemical, dustable powder, emulsifiable concentrates, suspension concentrate, soluble concentrate, and wettable powders (WP), are applied by dusting or spraying using simple equipment.12 These formulations have several disadvantages, such as the rapid release. Moreover, high dosage should be applied, leading to the initial very high concentration of pesticide in the soil, but the efficacy decreases rapidly to a low ineffective level for pest control.13 Consequently, these pesticides are applied at much higher doses than needed to overcome losses of the active compound [denoted as active ingredient (a.i)] at the uptake site by dissipation and degradation mechanisms and extend the effectiveness of the pesticide for a longer period. Multiple pesticide applications are required to control rice planthoppers. Moreover, runoff and leaching down the soil of the dusted and sprayed formulations has become serious environmental problems and primary sources of surface and groundwater pollution. The total environment of a treated area is exposed to the toxicant, although the pest organism inhabits only a small fraction.14 Moreover, common formulations have short residual activity time. Thus, the agent is applied at very higher doses, causing harmful environmental problem. Consequently, long-acting and environment-friendly control programs should be developed to deal with planthopper.15
Controlled-release (CR) technology is very important in many fields. CR pesticide formulations can be used to gradually deliver the active substance over time for efficient control of pests. These formulations are combinations of pesticide active agent with inert materials that protect and release the active agent over the required time16–18 or coating the pesticide active agent with capsules or other organic materials. CR formulation have numerous benefits, including protection of active ingredients from environmental degradation, manipulation of bioavailability and persistence, reduction of toxicity and operator hazards, reduction of phytotoxicity to seeds and crops, reduced agent application rates, and less labor requirement.19–21
The present study was performed to propose a pesticide formulation, CR granule (CRG), to control paddy planthopper. The CRG can preserve pesticide stability for long efficacy and guarantee the initial release of the effective dose. The potential of this approach was investigated by encapsulating the model insecticide 1,2,4-triazin-3(2H)-one-4,5-dihydro-6-methyl-4-[(3-pyridinylmethylene)amino] (pymetrozine).22 This compound is a novel insecticide with selective activity against homopteran insects unrelated to neonicotinoids with a unique mode of action.23 The effects of various processing parameters, such as curing time and pesticide content, were investigated. The release rule of the CRG was determined, and field experiment was conducted to study the insecticidal efficacy, the terminal residues and acute toxicity on several beneficial organisms were evaluated too.
2 Experimental
2.1 General
Unless indicated otherwise, all common reagents and solvents were used as obtained from commercial supplies without further purifications. Epoxy resin (E-44) was procured from Shenzhen Golden Longsheng Technology Co. Ltd. (China). Polyamide resin (605) was procured from Yuanda Chemicals Co. Ltd. (China). Pymetrozine was obtained from Jiangsu Kwin Group Co., Ltd. The core particle of bentonite was obtained from Henan province (China), and bentonite was obtained from Guizhou province (China). The particle strength was determined on a KQ-3 instrument (Yunnan Chemical Research Institute). The pan granulator with heating function was manufactured by the Changzhou Huaxia Drying & Granulation Equipment Co., Ltd. (China). The compounds were analyzed by HPLC using the Agilent 1100 series apparatus composed of a quarternary pump, an autosampler, a diode array detector, a vacuum degasser, a column oven, and Agilent Chemstation software. The columns employed reversed-phase column Kromasil ODS-1 C18 (250 mm × 4.6 mm i. d., 5 μm; Daicel Chemical Industries Ltd.). The injection volume of the analytical samples was 20 μL. The mobile phases were composed of acetonitrile/water (20/80, v/v). Flow rate was set to 1.0 mL min−1, and the detection wavelength was fixed at 298 nm, the temperature was kept at 25 °C. The terminal residues of pymetrozine was separated on a Waters ACQUITY ultra-performance liquid chromatography system fitted with a sample manager, a quaternary solvent manager, a PDA detector, and a BEH C18 column (50.0 mm × 2.1 mm i. d., 1.7 μm film thickness) from Waters corporation (Massachusetts, USA). 1 μL sample solution was injected and the pymetrozine was detected by measurement of absorbance at 298 nm on a PDA detector. A mixture of methanol, acetonitrile and purified water were used as the mobile phase for gradient elution. The following gradient elution was employed: 10% methanol and 90% purified water at the start 1 min, then 10% acetonitrile and 90% purified water fort 1.1 min, then 15% acetonitrile and 85% purified water for 3.1 min, then 10% acetonitrile and 90% purified water fort 1.1 min, then 10% methanol and 90% purified water for 1.3 min.2.2 Preparation of pymetrozine CRG
The coated CRG was produced by a pan granulator, and the pan was rotated constantly at approximately 30 rpm all the time, as follows:(1) Pymetrozine (132.6 g, 95%), bentonite (3738.6 g), 1-dodecanesulfonic acid sodium salt (10 g), and calcium lignosulfonate (10 g) were mixed and kneaded well.
(2) Epoxy resin (E-44, 118.8 g) and polyamide resin (605, 118.8 g) were diluted by ethanol to 50% content.
(3) Core particles (7990 g) were charged into the pan granulator. The pan was rotated constantly at approximately 30 rpm. The mixed powder in step (1) was added to the core particle controlled by pan granulation, the particles with pymetrozine were used in subsequent processing.
(4) Epoxy resin (E-44, 118.8 g, 50%) and polyamide resin (605, 118.8 g, 50%) were mixed and homogeneously sprayed over the pymetrozine solid pesticide particles by a spraying nozzle. After resin curing at approximately 95 °C for 25 min, 4080 g of the particles were taken off from the pan and obtaining the first part of pymetrozine CRG with 1% resin content.
(5) Then, the rest 7920 g pymetrozine CRG were continuously sprayed on epoxy resin (E-44, 79.2 g, 50%) and polyamide resin (605, 79.2 g, 50%). After resin curing at approximately 95 °C for 25 min, 4080 g of the particles were taken off from the pan and obtaining the second part of pymetrozine CRG with 2% resin content.
(6) Then, the rest 3919 g pymetrozine CRG was continuously sprayed on epoxy resin (E-44, 39.6 g, 50%) and polyamide resin (605, 39.6 g, 50%). After resin curing at approximately 95 °C for 25 min, obtaining the third part of pymetrozine CRG with 3% resin content (about 3960 g).
(7) Uniform mixing the 3 parts of CRG (the first part of 4080 g CRG taken from step 4, the second part of 4080 g CRG taken from step 5, the first part of 3960 g CRG taken from step 6) to finally obtain 1% pymetrozine CRG. Thus, this process is inexpensive and convenient for application formulation.
2.3 Pymetrozine content in granule
Uncoated and resin-coated granule samples (10 mg) were ground to fine powder and quantitatively transferred to a 25 mL volumetric flask. The volume was made up to 25 mL with methanol, and the contents were stirred in an ultrasonic bath for 10 min to completely disintegrate/dissolve the soluble material. After 2 h at room temperature, the methanolic sample was filtered quantitatively through a 0.45 μm millipore filter, and 5 μL was injected into the chromatograph column. Analyses were performed in triplicate, and calibration standards were analyzed on the same day as the samples.2.4 Analysis of pymetrozine release from CRG
We adopted two methods to analyze the release rule of 1% pymetrozine CRG.Method one is the dissolution test by section water. Pymetrozine CRG (1%, 30 g) and pure water (1000 mL, pH 7.0–7.2) were added into a 1000 mL jar at 25 °C. The sample water (10 mL) of the solution was taken from the middle of the jar, the rest of the water (990 mL) was abandoned, and pure water (1000 mL) was added. The sample water (10 mL) ware extracted by dichloromethane (15 × 3 mL) and dried by anhydrous sodium sulfate, filtered, and removed the solvent. The residue was dried at 25 °C and reconstituted by methanol (2 mL) for HPLC analysis. All experiments were performed in triplicate, the release analyze of 1% pymetrozine CRG were take on day 1, 3, 5, 7, 14, 21, 28, 42, and 60 after sampling.
Method two tests the remaining particle. Pymetrozine CRG (1%, 30 g) was sealed into a millipore nylon net (150 μm) and put into a wide-mouthed jar. Then, 1000 mL of pure water (pH 7.0–7.2) was added to the jar under constant temperature of 25 °C. The samples in the nylon net were taken out on days 1, 3, 5, 7, 14, 21, 28, 42, and 60 and naturally dried at 25 °C. Then, the samples were ground to fine powder and quantitatively transferred to a 25 mL volumetric flask. The volume was made up to 25 mL with methanol. The contents were stirred in an ultrasonic bath for 5 min to completely disintegrate/dissolve the soluble material. After 2 h at room temperature, the methanolic sample was then filtered quantitatively through a millipore filter (0.45 μm). Then, 5 μL of the sample was injected into the chromatograph column. Analyses were performed in triplicate.
2.5 Insecticide field trials of 1% pymetrozine CRG
Field tests were conducted at Libo country, Guizhou province, China, in July and August of 2012. Twenty-four field plots (20 m2) with medium fertility were planted to test the insecticide activity. The plots were isolated from each other to avoid cross-contamination. Prior to the application of 1% pymetrozine CRG, the rice field had a water layer, which was 4–5 cm deep. The water layer was kept for 2–4 days after pesticide application. Four insecticide treatments and a blank water control were performed as follows: (1) 1% pymetrozine CRG at 75 g ai per ha, broadcasted uniformly to the field surface with 0.25 kg of urea fertilizer; (2) 1% pymetrozine CRG at 150 g ai per ha, broadcasted uniformly to the field surface with 0.25 kg of urea fertilizer; (3) 1% pymetrozine CRG at 300 g ai per ha, broadcasted uniformly to the field surface with 0.25 kg of urea fertilizer; (4) 1% pymetrozine CRG at 450 g ai per ha, broadcasted uniformly to the field surface with 0.25 kg of urea fertilizer; (5) control group, 25% pymetrozine WP at 93.75 g ai per ha, sprayed by a manual sprayer (Shandong Wish Plant Protection Machinery Co., LTD, China) operated at a pressure of 2 kg cm−2; (6) only water as blank control. Randomized block design with four replications was used. Diagonal sampling was adopted at five positions, and 25 rice clusters were investigated for each position. The planthoppers were collected with a basin while the rice clusters were flapped gently. Insecticide efficacy was calculated as follows: [(sum of planthoppers in the blank control area − sum of planthopper in the pesticide application area)/sum of planthopper in the blank control area] × 100.2.6 Terminal residues of pymetrozine in the soil, rice straw, rice husk, and brown rice
Based on previously described methods (NY/T788-2004, Guideline on pesticide residue trials),24 the field trials were conducted in three experimental fields in Guizhou (red soil, pH 5.5), Guangxi (yellow brown soil, pH 6.8), and Heilongjiang (black mud, pH 6.7) during the agricultural season in 2012 and 2013. Each field was divided into 30 m2 blocks for the control and treatment groups in the dissipation rate study. To investigate the distribution of terminal residue of pymetrozine in the soil, rice straw, rice husk, and brown rice, 1% pymetrozine CRG was applied at doses of 450 g ai per ha and 675 g ai per ha, which were the recommended dosage and 1.5 times the recommended dosage, respectively. Soil samples were collected from soil layer depths of 0–15 cm at harvest time. Rice straw, rice husk, and brown rice samples were collected into polyethylene bags at harvest time, transported to the laboratory, and stored at −20 °C until analysis.Portions of the homogenized soil (10 g), rice straw (10 g), and rice husk (5 g) samples were weighed into a 150 mL conical flask and extracted with 60 mL of acetone/water (v/v = 8/2, by volume, and containing 1% ammonia). The mixture was vibrated for 60 min on a reciprocating shaker. The mixture was filtered through Celite® 545 and washed with 20 mL of acetone/water (v/v = 8/2, by volume, and containing 1% ammonia). The extract was collected and pooled. Acetone was removed under reduced pressure. Potassium carbonate (3 g) and sodium chloride (3 g) were added, and the mixture was extracted with dichloromethane (2 × 50 mL). The dichloromethane layer was dried with anhydrous Na2SO4, and organic solvent was removed under reduced pressure. The residue was dissolved in 1 mL of methanol, after purification by PSA (0.05 g) and anhydrous magnesium sulfate (0.1 g). The solution was filtered with a 0.22 μm nylon filter (Millipore, Billerica, MA, USA) and subjected to UPLC.
A portion of the chopped brown rice (10 g) was weighed into a 50 mL polytetrafluoroethylene tube and extracted with acetonitrile (20 mL) and ammonium hydroxide (6 mL, 0.1 mol L−1). After vortex oscillation for 4 min, sodium chloride (4.0 g) was added to the solution. The sample was again vortex oscillated for 2 min and centrifuged for 5 min at 6000 rpm to obtain 10 mL of the supernatant. The solvent was removed under reduced pressure. For future determination, the residue was dissolved in 1 mL of methanol. After purification by PSA (0.05 g) and anhydrous magnesium sulfate (0.1 g), the solution was filtered with a 0.22 μm nylon filter (Millipore, Billerica, MA, USA) and subjected to UPLC.
2.7 Acute toxicity of 1% pymetrozine CRG on some beneficial organisms in the environment
Acute toxicity was determined based on previously described methods (GB/T31270, Test guidelines on environmental safety assessment for chemical pesticides)25 recommended by the Standardization Administration of the People's Republic of China and General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China and proposed by the Ministry of Agriculture of the People's Republic of China. Acute toxicity tests were performed according to GB/T31270 (Part 9, avian acute toxicity test; Part 10, honeybee acute toxicity test; Part 12, fish acute toxicity test; Part 13, Daphnia sp. acute immobilization test; Part 14, alga growth inhibition test; Part 15, earthworm acute toxicity test) on Apis mellifera L., Coturnix coturnix japonica, Brachydanio rerio, Daphnia magna Straus, Selenastrum capricornutum, and Eisenia foetida. Toxicity grades of pesticides on silkworm were defined as extremely toxic, highly toxic, moderately toxic, or lowly toxic according to the LC50, EC50, or LD50 values (GB/T31270-9, 10, 12, 13, 14, and 15).2.8 Statistical analysis
Pymetrozine content and release were analyzed using Excel. Data from the field tests were statistically analyzed by ANOVA using SPSS (PASW Statistic 18). Duncan's multiple tests were applied to calculate the significant differences among the CR of the blends at 5% level (P = 0.05).3 Results and discussion
3.1 Preparation of 1% pymetrozine CRG
Pymetrozine is a fat-soluble pesticide, in the water, surfactant 1-dodecanesulfonic acid sodium salt and calcium lignosulfonate ensure that pymetrozine reaches the crop. The characteristics of the 1% pymetrozine CRG are presented in Table 1. The core granules were generally spherical in shape. The white powder technical-grade pymetrozine was readily mixed with the bentonite matrix and then added to the core particle. This process resulted in larger and heavier granules with more spherical, higher weight and less aggregation.| Entry | T/°C | Time/min | Hardness/N | Curing quality |
|---|---|---|---|---|
| a 30 CRG particles (with 2% resin content) were randomly selected for hardness test. | ||||
| 1 | 70 | 90 | 7.62 ± 0.32 | Sticky |
| 2 | 80 | 60 | 7.9 ± 0.57 | A little sticky |
| 3 | 90 | 30 | 8.39 ± 0.45 | Solidifying |
| 4 | 100 | 25 | 9.02 ± 0.46 | Solidifying |
When the epoxy resin (E-44) and polyamide resin (605) were mixed in one system, single molecules (monomers) of the resins combined to form long chains of molecules (polymers). As the mixture was cured, the sample became a hard polymer. The hardened, finished polymers were almost nontoxic, and the insecticide ingredient pymetrozine was sealed. We optimized the curing conditions, and Table 1 shows that, out of the four entries, entry 4 afforded the best result in terms of quality.
3.2 Pymetrozine content in granule
First, we tested the accuracy and precision of the method adopted. Pymetrozine standard was dissolved in absolute methanol, and pymetrozine samples (0.099, 0.198, 1.98 μg mL−1) were prepared. The accuracy and precision of the determination were tested, y = 71.128x − 919.91, γ = 0.9996. The average recovery rate was 90.32%, 90.37% and 90.97%, the RSD were 1.27, 0.92 and 2.30, Therefore, this method is suitable to test the pymetrozine content of CRG.A controlled-drug-release carrier should have the capacity to encapsulate a large amount of drug to prolong the release and reduce the quantity of carrier required for application. The hollow structure of bentonite facilitates its entrapment of more pymetrozine. To determine the amount of pymetrozine entrapped and resin encapsulated, as well as test the uniform distribution, we investigated the pymetrozine content of uncoated granule sample and cured, resin-coated granule sample. The results showed that the initial pymetrozine content was 1.10%, which decreased to 1.03% after coating and curing. The results indicated that bentonite could entrap pymetrozine, and the resins did not adsorb pymetrozine. Therefore, resin can be used to develop CR formulation of pymetrozine.
3.3 Analysis of pymetrozine release from 1% pymetrozine CRG
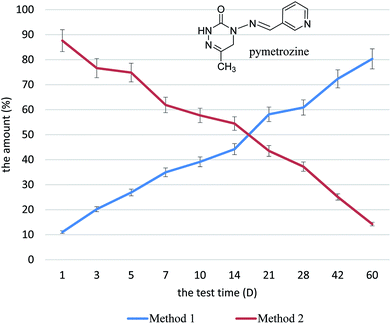
The Pesticide Fact Sheet of pymetrozine by the United States Environmental Protection Agency (EPA) showed that the hydrolysis time (half-life) of pymetrozine is <14 days (pH 5, 25 °C), >80 days (pH 7), and >86 days (pH 9). Solubility of this compound in water is 0.29 g L−1 (pH 6.4–6.5, 25 °C). Thus, pymetrozine hydrolyses readily at low pH, and the water qualities have strong influence on the stability of pymetrozine.Li et al. studied the residue behavior of pymetrozine in the paddy field in the main rice production region of China (Hunan and Zhejiang province), the results showed that the dissipation rates of pymetrozine in rice water were fast with half-life of 7–9 days.24 Yang developed a systematic study on pymetrozine residues in rice and environmental media by combining laboratory and field trials. The results showed that the half-life of pymetrozine was less than two days in Henan and Hunan province.25 The degradation of pymetrozine was generally fast, and this pesticide belongs to the easily degraded pesticide in Chinese main rice production region. The degradation of 1% pymetrozine CRG cannot be ignored because of its long-acting formulation. To avoid the influence of degradation, we tested the release condition of 1% pymetrozine CRG in two methods. Method 1 was designed to measure the amount of cumulative release amount from the granule, by testing the content of pymetrozine in water. Method 2 was designed to measure the amount of pymetrozine remaining in the granule and calculate the release amount of pymetrozine (Fig. 1).
On days 1, 3, 5, 7, 14, 21, 28, 42, and 60 after preparation of the test system, we tested the released amount of pymetrozine. For method 1, the released amount at certain days were as follows: day 1, 11.04%; day 5, 26.89%, day 14, 44.21%, which was almost half of the total amount; and day 28, 60.87%; The release rate of pymetrozine decreased with prolonged time. The cumulative released amounts on days 42 and 60 were 72.33% and 80.32%, respectively. For method 2, on day 1, the remaining and released amounts were 87.6% and 12.4%, respectively. On day 5, the remaining particles were 74.84%. On day 14, the remaining particles were 54.45%, while the corresponding release was 45.55%, which was almost half of the total amount. Meanwhile, the remaining particles on day 28 were 37.22%. The release rate of pymetrozine decreased with prolonged time. On days 42 and 60, the remaining particles were 25.1% and 14.23%, respectively, and the corresponding released amounts were 74.9% and 85.77%, respectively.
The release test demonstrated that the release period of the CRG formulation lasted for more than 60 days. The release period could be divided into three stages with the fastest release on days 1–2, during which the release amount was more than 15%. The medium-release stage was from 3 days to 28 days, with cumulative release amount of approximately 50%. The remaining pymetrozine was released during the slow stage. These results demonstrate that the CR formulation could release the active ingredient from the composition at desired timing and desired properties with extraordinarily good CR property of active ingredients. Application of pymetrozine CRG in the paddy during the “before-heading period” could effectively control planthopper by quickly increasing the pymetrozine concentration in the paddy water during the fastest release period. The long-term releasing properties of pymetrozine could control the subsequent periods.
3.4 Insecticide field trials of 1% pymetrozine CRG
In the field trial screening, the insecticidal efficacy of 1% pymetrozine CRG against S. furcifera in Guizhou was evaluated. The CR formulations were broadcasted to the field surface after 9 days of rice transplanting. The results are provided in Table 2 in terms of control effect values.| Entry | 14 days | 21 days | 28 days | 38 days | 48 days | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effecta | Sigb | Effect | Sig | Effect | Sig | Effect | Sig | Effect | Sig | |
| a Efficiency (%).b Sig = significance of difference, the statistical analysis was conducted by DMRT method at the condition of equal variances assumed (p = 0.05). Entry 1, 2, 3, and 4 mean 75 g ai per ha, 150 g ai per ha, 300 g ai per ha, and 450 g ai per ha of pymetrozine in CRG formulation, entry 5 means 25% pymetrozine WP at 93.75 g ai per ha. | ||||||||||
| 1 | 65.83 | Ab | 72.71 | Bb | 75.68 | Aa | 76.07 | Bb | 61.96 | Aa |
| 2 | 69.73 | Ab | 82.39 | Ab | 82.72 | Aa | 83.62 | Aa | 73.93 | Aa |
| 3 | 79.41 | Aab | 83.63 | Aa | 84.14 | Aa | 86.38 | Aa | 77.44 | Aa |
| 4 | 84.40 | Aab | 86.62 | Aa | 87.02 | Aa | 88.21 | Aa | 78.87 | Aa |
| 5 | 90.80 | Aa | 86.27 | Aa | 46.17 | Bb | 23.09 | Cc | 12.48 | Bb |
At doses of 75, 150, 300, and 450 g ai per ha, the control effects changed with time, with the control effects on certain days after CRG application as follows: day 14, 65.83–84.40%; day 21, 72.71–96.8%; day 28, 72.71–86.62% control effects; days 38, 75.68–87.02%; and day 48, 61.96–78.87%. At 450 g ai per ha, the effect ranged from 78.87% to 88.21% at 14–48 days after CRG application. By contrast, dose of 300 g ai per ha resulted in 77.44% to 86.38% effect for the same period. The persistence period of 1% pymetrozine CRG was apparently approximately 48 days. For 25% pymetrozine WP at 375 g ai per ha, the persistence period was approximately 21 days, and the effect ranged from 12.48% to 46.17% at 28–48 days after spraying. Moreover, the effects of 300 g ai per ha and 450 g ai per ha showed no significant difference on day 48. However, the effects of these doses showed significant difference with those of 75 g ai per ha and 150 g ai per ha at all investigated times of 48 days after CRG application.
3.5 Terminal residues of pymetrozine in the soil, rice straw, rice husk, and brown rice
Terminal residue levels of pymetrozine in the soil, rice straw, rice husk, and brown rice are listed in Table 3.| Matrix | Location | RSD | 2012 | 2013 | ||
|---|---|---|---|---|---|---|
| 450 g ai per ha | 675 g ai per ha | 450 g ai per ha | 675 g ai per ha | |||
| a RSD: relative standard deviation. | ||||||
| Soil | Guiyang | 3.25 ± 0.60 | 0.0168 ± 0.0015 | 0.0256 ± 0.009 | 0.0148 ± 0.0036 | 0.0287 ± 0.0076 |
| Nanning | 0.0195 ± 0.0095 | 0.0538 ± 0.0051 | <0.0116 | <0.0116 | ||
| Heilongjiang | <0.0116 | 0.0225 ± 0.0054 | 0.0305 ± 0.0138 | 0.1063 ± 0.036 | ||
| Rice straw | Guiyang, Nanning, Heilongjiang | 4.68 ± 1.3 | <0.058 | <0.058 | <0.058 | <0.058 |
| Rice husk | Guiyang, Nanning, Heilongjiang | 4.89 ± 0.9 | <0.0464 | <0.0464 | <0.0464 | <0.0464 |
| Brown rice | Guiyang, Nanning, Heilongjiang | 4.65 ± 2.25 | <0.0116 | <0.0116 | <0.0116 | <0.0116 |
We have mix pymetrozine standard with the matrix material, such as soil, rice straw, rice husk, brown rice, the RSD 3.25 ± 0.60, 4.68 ± 1.3, 4.89 ± 0.9, 4.65 ± 2.25, respectively. At one time application of 450 g ai per ha (the recommended dosage), the terminal residue levels of pymetrozine in the soil at harvest ranged from <0.0116 mg kg−1 to <0.058 mg kg−1. When applied at 675 g ai per ha (1.5 times the recommended dosage), the terminal residue levels ranged from <0.0116 mg kg−1 to 0.1063 mg kg−1 in the soil. Terminal residue levels of pymetrozine in the rice straw, rice husk, and brown rice were <0.058, <0.0464, and <0.0116 mg kg−1, respectively, when pymetrozine was applied at 450 and 675 g ai per ha. In China and Japan, the MRL on brown rice was 0.1 mg kg−1. Hence, at the recommended dosage and 1.5 times the recommended dosage, the terminal residue were below the MRL. This result indicated the safety of 1% pymetrozine CRG application on rice at the recommended dosage.
3.6 Acute toxicity of 1% pymetrozine CRG on several beneficial organisms in environment
Under standard procedures, we tested the acute inhaling toxicity, acute oral toxicity, acute immobilization toxicity, and growth inhibition toxicity against the beneficial organisms in the environment. These organisms included A. mellifera L., C. coturnix japonica, B. rerio, D. magna Straus, S. capricornutum, and E. foetida, and the results are listed in Table 4.| Living organism | Test guidelines | The result at exposure time |
|---|---|---|
| Coturnix coturnix japonica | 9, acute oral toxicity | >66.8 mg ai per kg bw, 168 (h) (LD50) |
| Apis mellifera L. | 10, acute inhaling toxicity | >11.0 μg ai per bee, 48 (h) (LD50) |
| Apis mellifera L. | 10, acute oral toxicity | >2000 mg ai per L, 48 (h) (LD50) |
| Brachydanio rerio | 12, acute oral toxicity | >100 mg ai per L, 96 (h) (LD50) |
| Daphnia magna Straus | 13, acute immobilisation test | >100 mg ai per L, 48 (h) (EC50) |
| Selenastrum capricornutum | 14, growth inhibition test | >100 mg ai per L,72 (h) (EC50) |
| Eisenia foetida | 15, acute oral toxicity | >100 mg ai per kg dry soil, 14 (d) (LC50) |
The acute inhaling toxicity against A. mellifera L indicated LD50 of >11.0 μg ai per bee. The acute oral toxicity against A. mellifera L. C. coturnix japonica, B. rerio, and E. foetida showed LD50 values of >2000 mg ai per L, >66.8 mg ai per kg bw, >100 mg ai per L and >100 mg ai per kg dry ground, respectively. The acute immobilization test against D. magna Straus showed EC50 of >100 mg ai per L. The growth inhibition test against Selenastrum capricornutum showed EC50 of >100 mg ai per L. In the “Fact Sheet for Pymetrozine” published by the United States Environmental Protection Agency, demonstrate that pymetrozine has been determined to be of low acute toxicity to humans, birds, aquatic organisms, mammals. For example, the acute inhaling toxicity against northern bobwhite quail (Colinus virginianus) indicated LD50 of >2000.0 mg kg−1, and categorized to practically nontoxic. Furthermore, the pymetrozine content in CRG is only 1%. These results indicated that 1% pymetrozine CRG had low toxicity against all tested beneficial organisms in the environment.
4 Conclusions
The effectiveness time of most chemical pesticides are not long enough to control rice planthoppers. Altering traditional agrichemical formulations into formulations with longer efficacy may be an alternative solution. We prepared 1% pymetrozine CRG using bentonite and resin. Analysis of the pymetrozine release indicated that the CR formulation of pymetrozine had good release property. In the field trial screening, 1% pymetrozine CRG showed good efficacy for controlling paddy planthopper, with control effect of 61.96–78.87% at 48 days. At the recommended dosage and the 1.5 times of the recommended dosage, the terminal residues in brown rice remained below the MRL (0.1 mg kg−1) in China and Japan. Moreover, 1% pymetrozine CRG showed low toxicity against all tested beneficial organisms in the environment. Thus, 1% pymetrozine CRG demonstrated great potential for field applications to control paddy planthopper, because it overcame the rapid loss of biological function during treatment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support of the National Key Research and Development Plan (No. 2016YFD0200707), National Natural Science Foundation of China (No. 31760532), Special Fund for Agro-Scientific Research in the Public Interest (No. 201203022). Guizhou S&T Project (No. 201462, 20113071, 2013137), for the financial support. The authors thank Mr Changyu Shao (plant protection and quarantine station, Guizhou province) who carried out the field trials experiments in this study. The authors thank National Center for the Quality Supervision & Test of Pesticides (Beijing) for acute toxicity on several beneficial organisms.References
- J. A. Cheng, in Planthoppers: new threats to the sustainability of intensive rice production systems in Asia, ed. K. Heong and B. Hardy, International Rice Research Institute, Los Baños (Philippines), 2009, pp. 157–178 Search PubMed.
- J. A. Cheng, J. L. Zhu, Z. R. Zhu and L. G. Zhang, J. Environ. Entomol., 2008, 2, 016 Search PubMed.
- L. J. Cai, X. Z. Ma, L. Kang, K. J. Deng, S. Y. Zhao and C. B. Li, J. Virol. Methods, 2003, 112, 115–120 CrossRef.
- X. C. He, H. X. Xu, X. S. Zheng, Y. J. Yang, G. C. Gao, J. H. Pan and Z. X. Lu, Rice Sci., 2012, 19, 335–338 CrossRef.
- Z. Chen, C. J. Yin, J. J. Liu, M. J. Zeng, Z. C. Wang, D. D. Yu, L. Bi, L. H. Jin, S. Yang and B. A. Song, Arch. Virol., 2012, 157, 2327–2333 CrossRef PubMed.
- D. D. Yu, Z. C. Wang, J. Liu, M. M. Lv, J. J. Liu, X. Y. Li, Z. Chen, L. H. Jin, D. Y. Hu, S. Yang and B. A. Song, J. Agric. Food Chem., 2013, 61, 8049–8055 CrossRef PubMed.
- G. H. Zhou, D. L. Xu, D. G. Xu and M. X. Zhang, Front Microbiol., 2013, 4, 270 Search PubMed.
- T. Nagata, T. Kamimuro, Y. C. Wang, S. G. Han and N. M. Noor, J. Asia-Pac. Entomol., 2002, 5, 113–116 CrossRef.
- S. Y. Li, X. Liu, C. F. Gao, X. P. Bo, J. Y. Su, Y. H. Wang, L. Yu, X. Yan, J. L. Shen and J. Yang, Chin. J. Rice Sci., 2009, 23, 79–84 CrossRef.
- H. B. Bao, S. H. Liu, J. H. Gu, X. Z. Wang, X. L. Liang and Z. W. Liu, Pest Manage. Sci., 2009, 65, 170–174 CrossRef PubMed.
- S. F. Jin, M. G. Feng, S. H. Ying, W. J. Mu and J. Q. Chen, Pest Manage. Sci., 2011, 67, 36–43 CrossRef PubMed.
- F. Quaglia, F. Barbato, G. De Rosa, E. Granata, A. Miro and M. I. La Rotonda, J. Agric. Food Chem., 2001, 49, 4808–4812 CrossRef PubMed.
- J. I. Perez-Martinez, E. Morillo, C. Maqueda and J. M. Gines, Pest Manage. Sci., 2001, 57, 688–694 CrossRef PubMed.
- L. X. Wen, Z. Z. Li, H. K. Zou, A. Q. Liu and J. F. Chen, Pest Manage. Sci., 2005, 61, 583–590 CrossRef PubMed.
- T. Sanchez-Verdejo, T. Undabeytia, S. Nir, C. Maqueda and E. Morillo, Environ. Sci. Technol., 2008, 42, 5779–5784 CrossRef PubMed.
- M. D. Lopez, A. Maudhuit, M. J. Pascual-Villalobos and D. Poncelet, J. Agric. Food Chem., 2012, 60, 1187–1192 CrossRef PubMed.
- Z. J. Zhou, Y. Z. Shen, C. W. Du, J. M. Zhou, Y. S. Qin and Y. J. Wu, Land Degrad. Dev., 2017, 28, 2370–2379 CrossRef.
- A. Rashidzadeh, A. Olad and M. J. Hejazi, Adv. Polym. Technol., 2017, 36, 177–185 CrossRef.
- R. Gil-Ortiz, Pest Manage. Sci., 2015, 71, 1685–1693 CrossRef PubMed.
- J. X. Liu, Q. Zhao and X. G. Zhang, Appl. Clay Sci., 2017, 145, 44–52 CrossRef.
- A. Watanabe, Y. Takebayashi, T. Ohtsubo and M. Furukawa, Pest Manage. Sci., 2009, 65, 1233–1240 CrossRef PubMed.
- B. Sechser, B. Reber and F. Bourgeois, J. Pestic. Sci., 2002, 75, 72–77 Search PubMed.
- H. Mercan, E. Yilmaz and R. Inam, J. Hazard. Mater., 2007, 141, 700–706 CrossRef PubMed.
- Ministry of Agriculture and Rural Affairs, PRC, standard, 2004, NY/T 788, 13.
- Ministry of Agriculture and Rural Affairs, PRC, standard, 2014, GB/T31270/9-15, 68.
Footnote |
| † Present work unit: Shangqiu polytechnic, Shangqiu 476000, Henan, P. R. China. |
| This journal is © The Royal Society of Chemistry 2018 |