Open Access Article
Open Access ArticleConstruction of 2D/2D layered g-C3N4/Bi12O17Cl2 hybrid material with matched energy band structure and its improved photocatalytic performance†
Lei Shi *a,
Weiwei Sia,
Fangxiao Wangc and
Wei Qi
*a,
Weiwei Sia,
Fangxiao Wangc and
Wei Qi *b
*b
aCollege of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University, Fushun 113001, China. E-mail: shilei_hit@qq.com
bShenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China. E-mail: wqi@imr.ac.cn; Tel: +86-02456861842
cCollege of Chemistry, Chemical Engineering and Material Science, Shandong Normal University, Jinan 250014, China
First published on 6th July 2018
Abstract
A series of visible-light-induced 2D/2D layered g-C3N4/Bi12O17Cl2 composite photocatalysts were successfully synthesized by a one step chemical precipitation method with g-C3N4, BiCl3 and NaOH as the precursors at room temperature and characterized through XRD, FTIR, XPS, TEM, BET and UV-vis DRS measurements. The results of XRD, FTIR and XPS indicated that g-C3N4 has been introduced in the Bi12O17Cl2 system. The TEM image demonstrated that there was strong surface-to-surface contact between 2D g-C3N4 layers and Bi12O17Cl2 nanosheets, which contributed to a fast transfer of the interfacial electrons, leading to a high separation rate of photoinduced charge carriers in the g-C3N4/Bi12O17Cl2 system. Rhodamine B was considered as the model pollutant to investigate the photocatalytic activity of the resultant samples. The g-C3N4/Bi12O17Cl2 composite showed a clearly improved photocatalytic degradation capacity compared to bare g-C3N4 and Bi12O17Cl2, which was ascribed to the interfacial contact between the 2D g-C3N4 layers and Bi12O17Cl2 sheet with a matched energy band structure, promoting the photoinduced charges' efficient separation. Finally, combined with the results of the trapping experiment, ESR measurements and the band energy analysis, a reasonable photocatalytic mechanism over the 2D/2D layered g-C3N4/Bi12O17Cl2 composite was proposed.
1. Introduction
The photocatalytic technique is considered as one of the applied prospect strategies to rationally utilize solar energy for resolving energy and environmental problems, and has drawn extensive attention in the field of green chemistry and materials science during the past few decades. Conventional semiconductor materials, including TiO2, ZnO, SnO2, etc., have showed admirable photocatalytic properties for splitting water to produce H2 and degrading all kinds of pollutants under ultraviolet (UV) irradiation.1–3 Nevertheless, in view of the sufficient utilization of the abundant solar energy, it is essential to exploit visible-light-induced and efficient semiconductor photocatalysts.Recently, bismuth oxyhalide (BiOX, X = Cl, Br, I) has attracted more and more attention in photocatalytic degradation of environmental pollutants and energy conversion etc.4–7 Due to the unique layered structure and internal static electric fields perpendicular to each layer, BiOX could effectively separate photogenerated electron–hole pairs, achieving enhanced photocatalytic performance.8 Among these BiOX materials, BiOCl has been sufficiently researched,9,10 nevertheless, the relatively wide energy band limits its photocatalytic efficiency. In order to improve the visible light induced photocatalytic performance of BiOCl, some bismuth oxychlorides with non-stoichiometric ratios were developed, such as Bi3O4Cl,11 Bi12O15Cl6 (ref. 12) and Bi24O31Cl10,13 etc., which have been proposed and intensively studied in the field of photocatalysis. Among these materials, Bi12O17Cl2 has revealed superb visible-light photocatalytic properties in selective oxidation and degradation of various organic pollutants.14,15 Nevertheless, its photocatalytic performance was still limited by the low separation rate of photogenerated electrons and holes. Hence, it is interesting to improve its photocatalytic properties through some modified method. For this kind of 2D sheet material, it is an effective method to fabricate interfaces through surface to surface via introducing other two-dimensional (2D) layered semiconductor components, which contribute to promote the charge separation.
As one of the representative layered materials, graphitic carbon nitride (g-C3N4), has shown several advantages as photocatalysts, such as low-cost, high thermal and chemical stability etc.16–18 In addition, the appropriate valence and conduction band position makes the g-C3N4 compound be widely applied in some photocatalytic processes. Several individual research groups have reported that various g-C3N4-based composite photocatalysts have been successfully synthesized and utilized, such as MoS2/g-C3N4,19 BiOCl/C3N4,20 g-C3N4/Ag3PO4,21 g-C3N4/BiPO4,22 CdS/g-C3N4,23 SnO2/g-C3N4,24 Bi2O2CO3/g-C3N4,25 g-C3N4/Bi2MoO6,26 g-C3N4/ZnO,27 BiOBr/g-C3N4,28 g-C3N4/Bi4O5I2,29 and so on. The general concept for the catalyst design among these research work is the rational combination of the two type components yielding hybrid materials with accurate energy band and relatively high photocatalytic performance. Therefore, it seems to be ideal that the combination of g-C3N4 with Bi12O17Cl2 might improve their photocatalytic performance. Zhou et al. prepared the carbon-doped carbon nitride/Bi12O17Cl2 (CCN/Bi12O17Cl2), which had superior photocatalytic performance for degrading antibiotic tetracycline.30 However, this work involved the solvothermal preparation of Bi12O17Cl2 and ultrasonic combination of CCN/Bi12O17Cl2, preparation process was multi-step. Hence, it is necessary to develop the facile strategy for preparing carbon nitride/Bi12O17Cl2 composite.
Therefore, in the present study, we have developed 2D/2D layered g-C3N4/Bi12O17Cl2 composite materials by one step chemical precipitation method at room temperature. The chemical structure, composition, morphology and optical property of the synthesized g-C3N4/Bi12O17Cl2 composites were thoroughly characterized. The related results indicated that g-C3N4 and Bi12O17Cl2 matched complementary potentials of conduction band and valence band in g-C3N4/Bi12O17Cl2 composite, which could effectively separate the photogenerated electron–hole pairs, resulting that g-C3N4/Bi12O17Cl2 heterojunctions revealed excellent photocatalytic activity and recycling stability for degrading rhodamine B (RhB). Finally, the reasonable mechanism for the improved photocatalytic capacity over g-C3N4/Bi12O17Cl2 composite was proposed.
2. Experimental section
2.1 Preparation of the photo-catalyst
g-C3N4 was obtained by pyrolysis of urea (30 g) at 550 °C for 2 h in an oven.g-C3N4/Bi12O17Cl2 composite was prepared at room temperature as following procedures. A given amount of g-C3N4 (9.5, 28.5, 47.5 and 66.6 mg) and 4 mmol BiCl3 were dissolved in 20 mL ethanol under continuous stirring for 30 min, and the pH of solution was about 2. 20 mL of distilled water containing 24 mmol NaOH was added dropwisely into above mixture, then the solution vigorously stirred for 120 min with stirred rate 150rpm, and the pH of solution become to 14, the obtained product was filtered, washed with distilled water and ethanol, and dried at 60 °C in a oven for 12 h. In prepared process of samples, the system did not proceed exothermic. On the basis of the theoretical mass ratio of g-C3N4 and Bi12O17Cl2, the as-made composites were denoted as g-C3N4/Bi12O17Cl2 (1 wt%), g-C3N4/Bi12O17Cl2 (3 wt%), g-C3N4/Bi12O17Cl2 (5 wt%) and g-C3N4/Bi12O17Cl2 (7 wt%), respectively. For comparison, pure Bi12O17Cl2 was obtained according to above method without g-C3N4, and similar method has been reported in previous literatures.31,32 In addition, N-doped TiO2 (N–TiO2) was prepared by heating with TiO2 (P25) and urea as the precursor according to previous reports.33,34 Carbon nanotubes modified Bi12O17Cl2 composite (CNTs/Bi12O17Cl2) and CCN/Bi12O17Cl2 were prepared according to previous reports.30,35
2.2 Characterizations
The X-ray diffraction (XRD) patterns of all as-made samples were measured by an X-ray diffractometer (Bruker D8 Advance). Fourier transform infrared spectra (FTIR) of all as-prepared samples were detected by Thermo Fisher Scientific IS10. The X-ray photoelectron spectroscopy (XPS) of g-C3N4/Bi12O17Cl2 (3 wt%) composite was recorded on Thermo Fisher Scientific Escalab 250. The microtopographies of g-C3N4, Bi12O17Cl2 and g-C3N4/Bi12O17Cl2 (3 wt%) composite were observed using JEM-2100F transmission electron microscope (TEM). The UV-vis diffuse reflectance spectra (DRS) of as-made samples were collected by an UV-vis spectrometer (Agilent Cary 5000). BET surface areas of all as-made samples were collected using a Micromeritics Tristar 3020 analyzer. Samples were outgassed at 150 °C for 12 h prior to measurements. Transient photocurrent properties of g-C3N4, Bi12O17Cl2 and g-C3N4/Bi12O17Cl2 (3 wt%) were measured by a CHI760 electrochemical system (China) in a three-electrode quartz cells. Pt wire was the counter electrode, and the saturated calomel electrode was the reference electrode. The sample films coated on ITO glasses were applied as the working electrode was. A 300 W Xe lamp with 400 nm filters provided the light source, and the electrolyte was 0.1 M Na2SO4. The total organic carbon (TOC) assays were investigated by using a Shimadzu TOC-VCPH analyzer.2.3 Photocatalytic testing
The photocatalytic property of the resultant sample was evaluated using degradation of RhB or methyl orange (MO) as a model reaction, which was performed under visible-light irradiation. A 300 W Xe lamp with a 400 nm cutoff filter was used for the visible-light source. 30 mg resultant sample was dispersed into 50 mL 5 mg L−1 RhB or 10 mg L−1 MO solution and the solution was stirred in dark for 1 h to reach the adsorption–desorption equilibrium. About a certain volume solutions were collected at every given time interval under irradiation, and the catalyst was removed via centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 3 min). The concentration of RhB or MO was detected through a UV-vis spectrophotometer at the wavelength of 552 or 464 nm.
000 rpm, 3 min). The concentration of RhB or MO was detected through a UV-vis spectrophotometer at the wavelength of 552 or 464 nm.
3. Results and discussions
Fig. 1 shows the XRD patterns of all the resultant samples. For pure Bi12O17Cl2, there are some diffraction peaks corresponding to the tetragonal Bi12O17Cl2 (JCPDS No. 37-0702),36 including (113), (115), (117), (0012), (200), (220) and (317). g-C3N4 compounds have two distinct diffraction peaks at 27.4° and 13.1°, which were indexed to the (002) and (100) planes of hexagonal g-C3N4 (JCPDS 87-1526),37 and the two diffraction peaks were in good agreement with the previous report.38–40 However, no diffraction peaks belonged to g-C3N4 were found in as-prepared g-C3N4/Bi12O17Cl2 composite, which might be attributed to the low contents of g-C3N4 or their relatively high dispersions. Hence, to prove that g-C3N4 was introduced in the Bi12O17Cl2 system, the FTIR, XPS and TEM were also carried out.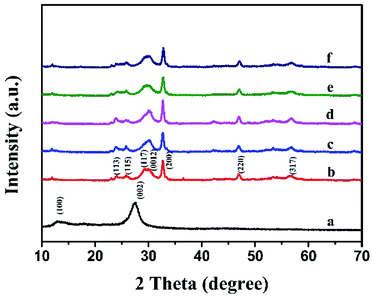 | ||
| Fig. 1 The XRD patterns of (a) g-C3N4, (b) Bi12O17Cl2, (c) g-C3N4/Bi12O17Cl2 (1 wt%), (d) g-C3N4/Bi12O17Cl2 (3 wt%), (e) g-C3N4/Bi12O17Cl2 (5 wt%) and (f) g-C3N4/Bi12O17Cl2 (7 wt%). | ||
Firstly, FTIR analysis was investigated. Fig. 2 presents the FTIR results of the pure g-C3N4, Bi12O17Cl2 and a series of g-C3N4/Bi12O17Cl2 composites. For pure g-C3N4, there were some vibration bands at 810 cm−1 and the scope of 1200–1650 cm−1, which could be assigned as the typical breathing mode of s-triazine and stretching vibration modes of heptazine heterocyclic ring in g-C3N4.41 Meanwhile, these characteristic IR signals could be also observed for as-made g-C3N4/Bi12O17Cl2 composites, suggesting that the 2D g-C3N4 has successfully incorporated with the Bi12O17Cl2 nano-material, verifying that g-C3N4/Bi12O17Cl2 photocatalyst has been synthesised.
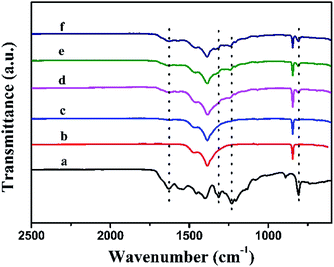 | ||
| Fig. 2 The FTIR spectra of (a) g-C3N4, (b) Bi12O17Cl2, (c) g-C3N4/Bi12O17Cl2 (1 wt%), (d) g-C3N4/Bi12O17Cl2 (3 wt%), (e) g-C3N4/Bi12O17Cl2 (5 wt%) and (f) g-C3N4/Bi12O17Cl2 (7 wt%). | ||
Fig. 3 shows the XPS survey and the corresponding Bi 4f, Cl 2p, O 1s, C 1s and N 1s high-resolution spectra for g-C3N4/Bi12O17Cl2 (3 wt%) composite. The XPS survey spectra (Fig. 3A) indicates that the elements of Bi, Cl, O, C and N exist in the g-C3N4/Bi12O17Cl2 composite. In the Bi 4f high-resolution spectra (Fig. 3B), the peaks at 159.1 and 164.4 eV could be assigned as the Bi 4f7/2 and Bi 4f5/2, respectively.42 For the Cl 2p spectrum in Fig. 3C, the signal is deconvoluted into two peaks at 197.9 and 199.6 eV, attributed to Cl 2p3/2 and Cl 2p1/2 signals, respectively, deriving from the Cl−.43 In Fig. 3D, the O 1s spectrum is deconvoluted into two peaks. The former belonged to the Bi–O bonds in [Bi2O2]2+ slabs, and the latter might correspond to the surface adsorbed hydroxy groups.44 For C 1s signal in Fig. 3E, the peaks at 284.6 and 288.7 eV could be ascribed to carbon atoms and the sp2 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonded, respectively.45 The N 1s spectrum (Fig. 3F) is deconvoluted into three peaks with binding energies at 398.7, 399.8 and 401.3 eV, respectively. The peak at 398.8 eV is typically attributed to C–N
N bonded, respectively.45 The N 1s spectrum (Fig. 3F) is deconvoluted into three peaks with binding energies at 398.7, 399.8 and 401.3 eV, respectively. The peak at 398.8 eV is typically attributed to C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. The two peaks at 399.8 and 401.4 eV could be assigned to N–(C)3 and N–H, respectively.46 The above results revealed the surface property and chemical composition of the g-C3N4/Bi12O17Cl2 composite and further confirmed the successful combination of g-C3N4 and Bi12O17Cl2 species, which agrees with FTIR results.
C. The two peaks at 399.8 and 401.4 eV could be assigned to N–(C)3 and N–H, respectively.46 The above results revealed the surface property and chemical composition of the g-C3N4/Bi12O17Cl2 composite and further confirmed the successful combination of g-C3N4 and Bi12O17Cl2 species, which agrees with FTIR results.
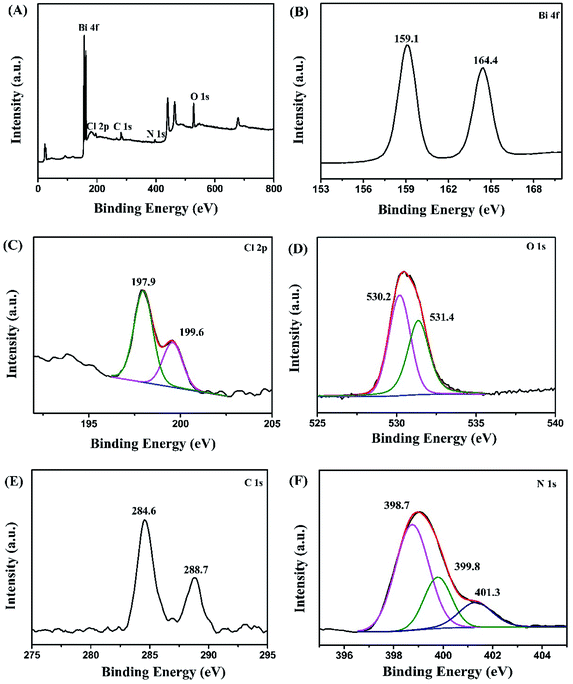 | ||
| Fig. 3 XPS spectra of g-C3N4/Bi12O17Cl2 (3 wt%) composite: (A) survey spectra, (B) Bi 4f, (C) Cl 2p, (D) O 1s, (E) C 1s and (F) N 1s. | ||
The morphology features of g-C3N4/Bi12O17Cl2 composite is revealed via TEM measurements. As can be shown in Fig. 4A, pure g-C3N4 exhibits its typical 2D nanosheet morphology, and there are some pores on the surface. For pure Bi12O17Cl2 seen in Fig. 4B, the typical 2D nanosheets are observed, and the surface of them are normally smooth. Fig. 4C gives the corresponding TEM image of the g-C3N4/Bi12O17Cl2 composite. Bi12O17Cl2 nanosheets were loaded on the surface g-C3N4 via surface-to-surface contact, which could favor the charges transfer between these two components. Moreover, the HRTEM image in Fig. 4D indicates that the lattice fringe spacing of 0.272 and 0.306 nm could be observed respectively, attributing to the (2 0 0) and (1 1 7) plane of Bi12O17Cl2 species, and there is a close combination and the formation of the interface between Bi12O17Cl2 and g-C3N4, which might benefit the transfer of the photogenerated electrons, leading to the improved photocatalytic property. TEM results suggest the successful hybridization of g-C3N4 and Bi12O17Cl2. The layered 2D structure of the two components are well maintained, and the formation of the interfaces between the two species may facilitate the electron transfer process.
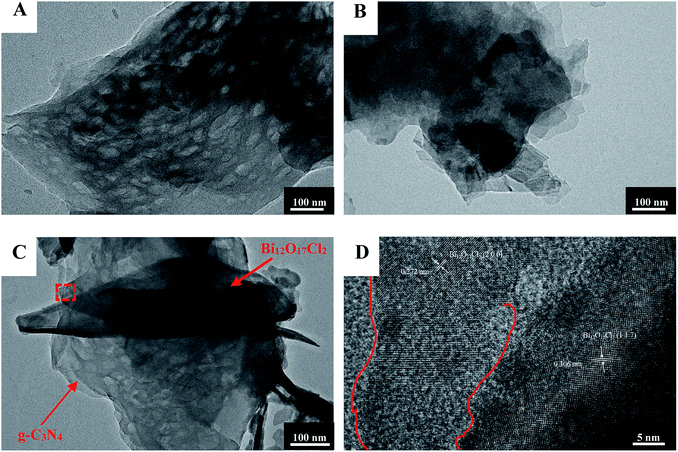 | ||
| Fig. 4 The TEM images of (A) pure g-C3N4, (B) Bi12O17Cl2 and (C and D) g-C3N4/Bi12O17Cl2 (3 wt%) composite. | ||
BET surface area of the as-prepared g-C3N4, Bi12O17Cl2, g-C3N4/Bi12O17Cl2 (1 wt%), g-C3N4/Bi12O17Cl2 (3 wt%), g-C3N4/Bi12O17Cl2 (5 wt%) and g-C3N4/Bi12O17Cl2 (7 wt%) are 45.8, 25.5, 26.7, 28.3, 30 and 31.6 m2 g−1, respectively. The combination of g-C3N4 could increase the surface area of the composites, which could contribute to improve photocatalytic performance of the composite.
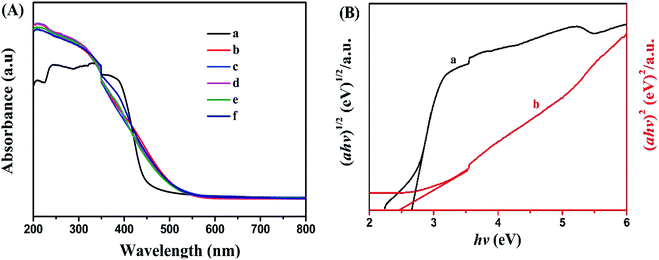
UV-vis diffuse reflectance spectra of g-C3N4, Bi12O17Cl2, g-C3N4/Bi12O17Cl2 (1 wt%), g-C3N4/Bi12O17Cl2 (3 wt%), g-C3N4/Bi12O17Cl2 (5 wt%) and g-C3N4/Bi12O17Cl2 (7 wt%) were also measured to show their light adsorption ability. As illustrated from Fig. 5A, for pure Bi12O17Cl2, the adsorption edge is approximate 500 nm, showing excellent photo-absorptions ability. g-C3N4 displays photoresponse from ultraviolet to visible light region, and its adsorption edge is at approximate 460 nm. After the cooperation of Bi12O17Cl2 and g-C3N4, a series of g-C3N4/Bi12O17Cl2 composites exhibit a similar absorption ability as Bi12O17Cl2, indicating that the introduction of g-C3N4 has little effect on the light absorption of Bi12O17Cl2. In addition, the band gap energies of the as-prepared g-C3N4 and Bi12O17Cl2 are obtained via following equation.47,48
| αhν = A(hv − Eg)n/2 | (1) |
Photocurrent measurement is an effective method for estimating the separation efficiency of photoinduced electrons and holes. Currently, the higher photocurrent intensity indicates the better separated efficiency of photoinduced electrons and holes. As seen in Fig. 6, the visible light irradiation could result pure Bi12O17Cl2, g-C3N4 and g-C3N4 modified Bi12O17Cl2 samples to create photocurrent signals. Clearly, the photocurrent intensity of g-C3N4/Bi12O17Cl2 (3 wt%) composite is higher than that of pure Bi12O17Cl2 and g-C3N4, meaning that the introduction of g-C3N4 modifier could promote in the effective separation of photogenerated electron–hole pairs.
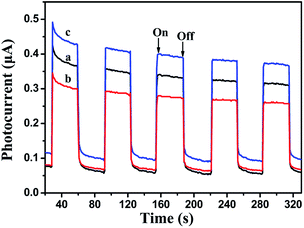 | ||
| Fig. 6 Transient photocurrent property of (a) pure Bi12O17Cl2, (b) g-C3N4 and (c) g-C3N4/Bi12O17Cl2 (3 wt%). | ||
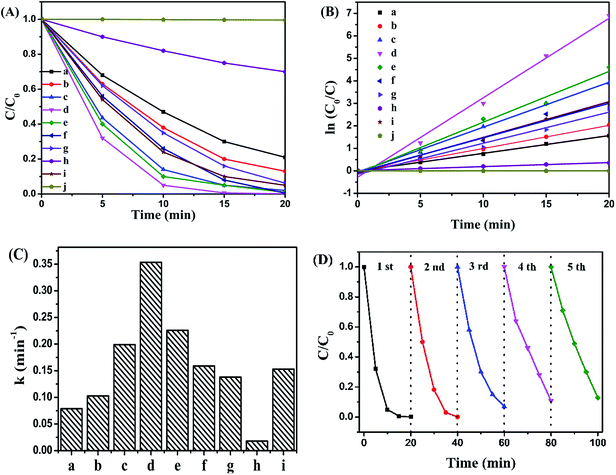
The visible-light photocatalytic activities of the as-prepared samples were estimated by liquid phase degradation of pollutant, and RhB was chose as a model pollutant. As seen in Fig. 7A, the blank experiment revealed that RhB could be hardly decomposed without any photo-catalysts. Single g-C3N4 or Bi12O17Cl2 was employed as photocatalysts, about 76% or 83% of RhB was degraded respectively. After their coordination, the resultant g-C3N4/Bi12O17Cl2 composites revealed obviously enhanced photocatalytic performance under the same condition. In addition, the photocatalytic performance of CNTs/Bi12O17Cl2, N–TiO2 and CCN/Bi12O17Cl2 were also detected as the compared samples and the related results were shown in Fig. 7A. Clearly, their photocatalytic activities were worse than that of g-C3N4/Bi12O17Cl2 (3 wt%) nanocomposite, meaning that as-prepared g-C3N4/Bi12O17Cl2 nanocomposite was an effective photocatalyst. In addition, the photodegradation of various concentration RhB (10 mg L−1, 15 mg L−1 and 20 mg L−1) over g-C3N4/Bi12O17Cl2 (3 wt%) nanocomposite was carried out. As shown in Fig. S1,† RhB was still degraded even if the concentration of RhB increased. And the TOC of RhB solution over as-prepare g-C3N4/Bi12O17Cl2 (3 wt%) after photodegradation 1 h was detected in Fig. S2,† the related result indicated that the TOC decreased 90%, which implied that g-C3N4/Bi12O17Cl2 nanocomposite exhibited excellent photocatalytic activity for removing pollutant. In addition, as a the azo reactive dye, methyl orange (10 mg L−1) was also chose as the model pollutant to access the photocatalytic performance of g-C3N4/Bi12O17Cl2 (3 wt%) nanocomposite. It could be seen that MO can be degraded 85% over g-C3N4/Bi12O17Cl2 (3 wt%) in the 120 min in Fig. S3.†
Following, in Fig. 7B, a pseudo-first-order reaction kinetic model for the photo-catalytic degradation of RhB could be described as the following rate equation:
| ln(C0/C) = kt | (2) |
Moreover, the sustainable used ability of a photocatalyst is a significant factor for its assessment and potential applications. Hence, the recycling runs for the photocatalytic degradation of RhB over g-C3N4/Bi12O17Cl2 (3 wt%) composite were performed to evaluate its photocatalytic stability. After reaction was over, g-C3N4/Bi12O17Cl2 (3 wt%) composite was separated, and washed with abundant water and ethanol, then dried at 60 °C, subsequently, dispersed in another fresh 5 mg L−1 RhB aqueous solution for next run. The experimental result was presented in Fig. 7D. It could be found that the photocatalytic activity remained nearly 85% of the first run after five times recycling, indicative of the acceptable stability of g-C3N4/Bi12O17Cl2 catalysts.
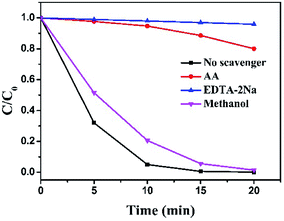
Generally, several active species, such as electrons (e−), holes (h+), superoxide radicals (˙O2−) and hydroxyl radicals (˙OH), etc., are generated in the process of photo-degradation under light irradiation in the presence of photo-catalysts. To identify and quantify the dominating reactive species in the photo-degradation process over g-C3N4/Bi12O17Cl2 composite and propose the reasonable photocatalytic mechanism, reactive specie trapping experiments were performed. Here, ascorbic acid (AA, 1 mmol L−1), ethylenediaminetetraacetic acid disodium salt (EDTA-2Na, 1 mmol L−1) and methanol (1 mmol L−1) were respectively added into photocatalytic system, which served as scavenger to quench ˙O2−, h+ and ˙OH.49–52 As shown in Fig. 8, after AA and EDTA-2Na were added into the photocatalytic reaction system, respectively, it was clearly observed that the photodegraded activity of the g-C3N4/Bi12O17Cl2 composite was sharply declined, meaning that ˙O2− and h+ radical were the key active species in this reaction. On the contrast, the introduction of methanol only resulted in a small inhibition for the photo-degradation of RhB, indicating that ˙OH radical may have limited activity in the present photocatalytic reaction.
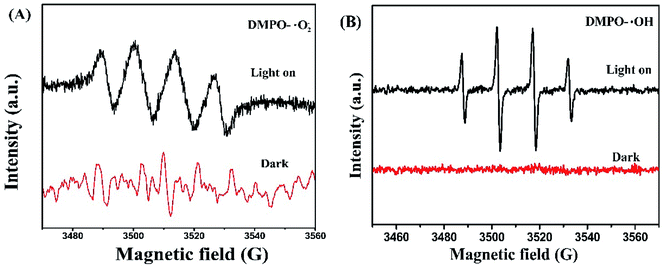
To further identify the radical species during the photo-catalytic process, electron spin resonance (ESR) spectra technique was also applied using 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) as scavengers in the presence of methanol (forming DMPO-·O2−) and deionized water (forming DMPO-˙OH), respectively, on a Germany A300-10/12 spectrometer. As represented in Fig. 9, when the system was in dark, no any signals could be detected. After visible light was irradiated for several minutes, evident signals belonging to ·O2− and ·OH radicals were observed, which proved the generation of ·O2− and ·OH radicals.
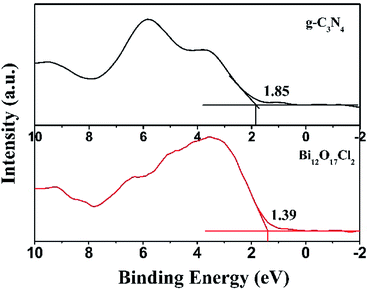
To better explain the photocatalytic mechanism and the enhanced activity of g-C3N4/Bi12O17Cl2 hybrid materials, the valence band (VB) structure of g-C3N4 and Bi12O17Cl2 were measured in Fig. 10. It could be clearly observed that the VB maxima of g-C3N4 and Bi12O17Cl2 located at 1.85 and 1.39 eV, respectively. Hence, the conduction band (CB) potentials (ECB) of g-C3N4 and Bi12O17Cl2 could be calculated according to eqn (3).53
| ECB = EVB − Eg | (3) |
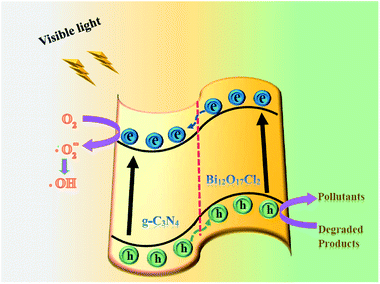
Therefore, combining with the above experimental results, Fig. 11 illustrates a reasonable photocatalytic mechanism for degrading organic pollutants over g-C3N4/Bi12O17Cl2 composite. Electrons (e−) and holes (h+) were easily generated after g-C3N4/Bi12O17Cl2 composite was irradiated by visible light. The e− in the CB of Bi12O17Cl2 could transfer to the CB of g-C3N4, because the CB and VB of g-C3N4 lie below those of Bi12O17Cl2, at the same time, h+ left in the VB of g-C3N4 would transfer to the VB of Bi12O17Cl2. Then e− could reduce the adsorbed O2 to form superoxide radicals (˙O2−), which might react with H2O to further generate the active ˙OH species.54,55 Because the VB level of Bi12O17Cl2 was less positive than the standard redox potential of ˙OH/H2O (2.68 eV vs. SHE),56 abundant h+ in the VB of Bi12O17Cl2 would be directly involved into the degradation of pollutants. This result might explain why ˙OH radical had limited influence on the photodegraded efficiency of pollutant. Finally, the obtained main active species (h+ and ˙O2−) reacted with the pollutants to generate the degradation products. Thus, during the photo-catalytic process, the recombined rate of the photogenerated electron–hole pairs was limited to achieve the efficient charge separation, promoting the photocatalytic performance.
4. Conclusions
In conclusion, a novel 2D/2D g-C3N4/Bi12O17Cl2 composite photo-catalyst has been successfully fabricated by a one step chemical precipitation method. Compared with pristine g-C3N4 and Bi12O17Cl2, g-C3N4/Bi12O17Cl2 composites exhibited better photocatalytic performance for the degradation of organic pollutants under visible light irradiation, and kept good recyclability during the photo-degradation process, which could be attributed to the surface-to-surface interfaces between g-C3N4 and the Bi12O17Cl2 sheet with a matched energy band structure, promoting the efficient charge separation. The results of the trapping experiment and ESR measurements indicated that ˙O2− and h+ radicals were the key active species in this reaction. The present work sheds light on the rational design and fabrication of highly efficient photo-catalysts for solving the environmental and energy issues, such as pollutant degradations etc.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We sincerely acknowledge the financial supported by talent scientific research fund of LSHU (No. 2016XJJ-080), basic research projects of Liaoning Provincial Education Department (L2017LQN004) and NSFC of China (21761132010, 91645114 and 21573256).References
- H. Wang, L. Zhang, Z. Chen, J. Hu, S. Li, Z. Wang, J. Liu and X. Wang, Chem. Soc. Rev., 2014, 43, 5234–5244 RSC.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahneman, Chem. Rev., 1995, 95, 69–96 CrossRef.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef PubMed.
- L. Ye, Y. Su, X. Jin, H. Xie and C. Zhang, Environ. Sci.: Nano, 2014, 1, 90–112 RSC.
- J. Li, H. Li, G. Zhan and L. Zhang, Acc. Chem. Res., 2017, 50, 112–121 CrossRef PubMed.
- D. S. Bhachu, S. J. A. Moniz, S. Sathasivam, D. O. Scanlon, A. Walsh, S. M. Bawaked, M. Mokhtar, A. Y. Obaid, I. P. Parkin, J. Tang and C. J. Carmalt, Chem. Sci., 2016, 7, 4832–4841 RSC.
- S. L. Wang, L. L. Wang, W. H. Ma, D. M. Johnson, Y. F. Fang, M. K. Jia and Y. P. Huang, Chem. Eng. J., 2015, 259, 410–416 CrossRef.
- M. Ji, J. Di, Y. Ge, J. Xia and H. Li, Appl. Surf. Sci., 2017, 413, 372–380 CrossRef.
- J. Jiang, K. Zhao, X. Xiao and L. Zhang, J. Am. Chem. Soc., 2012, 134, 4473–4476 CrossRef PubMed.
- L. Ye, K. Deng, F. Xu, L. Tian, T. Peng and L. Zan, Phys. Chem. Chem. Phys., 2012, 14, 82–85 RSC.
- X. Lin, T. Huang, F. Huang, W. Wang and J. Shi, J. Phys. Chem. B, 2006, 110, 24629–24634 CrossRef PubMed.
- C. Y. Wang, X. Zhang, X. N. Song, W. K. Wang and H. Q. Yu, ACS Appl. Mater. Interfaces, 2016, 8, 5320–5326 CrossRef PubMed.
- B. Yin, Z. Fang, B. Luo, G. Zhang and W. Shi, Catal. Lett., 2017, 147, 2167–2172 CrossRef.
- C. Y. Wang, X. Zhang, H. B. Qiu, W. K. Wang, G. X. Huang, J. Jiang and H. Q. Yu, Appl. Catal., B, 2017, 200, 659–665 CrossRef.
- X. Xiao, J. Jiang and L. Zhang, Appl. Catal., B, 2013, 142–143, 487–493 CrossRef.
- W. J. Ong, L. L. Tan, Y. H. Ng, S. T. Yong and S. P. Chai, Chem. Rev., 2016, 116, 7159–7329 CrossRef PubMed.
- Y. Wang, X. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68–89 CrossRef PubMed.
- Z. Zhao, Y. Sun and F. Dong, Nanoscale, 2015, 7, 15–37 RSC.
- Q. Li, N. Zhang, Y. Yang, G. Wang and D. H. L. Ng, Langmuir, 2014, 30, 8965–8972 CrossRef PubMed.
- X. J. Wang, Q. Wang, F. T. Li, W. Y. Yang, Y. Zhao, Y. J. Hao and S. J. Liu, Chem. Eng. J., 2013, 234, 361–371 CrossRef.
- S. Kumar, T. Surendar, A. Baruah and V. Shanker, J. Mater. Chem. A, 2013, 1, 5333–5340 RSC.
- C. Pan, J. Xu, Y. Wang, D. Li and Y. Zhu, Adv. Funct. Mater., 2012, 22, 1518–1524 CrossRef.
- L. Ge, F. Zuo, J. Liu, Q. Ma, C. Wang, D. Sun, L. Bartels and P. Feng, J. Phys. Chem. C, 2012, 116, 13708–13714 CrossRef.
- R. Yin, Q. Luo, D. Wang, H. Sun, Y. Li, X. Li and J. An, J. Mater. Sci., 2014, 49, 6067–6073 CrossRef.
- Y. Ma, Y. Bian, P. Tan, Y. Shang, Y. Liu, L. Wu, A. Zhu, W. Liu, X. Xiong and J. Pan, J. Colloid Interface Sci., 2017, 497, 144–154 CrossRef PubMed.
- H. Li, J. Liu, W. Hou, N. Du, R. Zhang and X. Tao, Appl. Catal., B, 2014, 160–161, 89–97 CrossRef.
- J. X. Sun, Y. P. Yuan, L. G. Qiu, X. Jiang, A. J. Xie, Y. H. Shen and J. F. Zhu, Dalton Trans., 2012, 41, 6756–6763 RSC.
- L. Ye, J. Liu, Z. Jiang, T. Peng and L. Zan, Appl. Catal., B, 2013, 142–143, 1–7 Search PubMed.
- N. Tian, Y. Zhang, C. Liu, S. Yu, M. Li and H. Huang, RSC Adv., 2016, 6, 10895–10903 RSC.
- C. Zhou, C. Lai, P. Xu, G. Zeng, D. Huang, Z. Li, C. Zhang, M. Cheng, L. Hu, J. Wan, F. Chen, W. Xiong and R. Deng, ACS Sustainable Chem. Eng., 2018, 6, 6941–6949 CrossRef.
- C. Bi, J. Cao, H. Lin, Y. Wang and S. Chen, Mater. Lett., 2016, 166, 267–270 CrossRef.
- C. Bi, J. Cao, H. Lin, Y. Wang and S. Chen, Appl. Catal., B, 2016, 195, 132–140 CrossRef.
- L. Yao, L. Shi and F. Wang, Mater. Res. Express, 2018, 5, 045042 CrossRef.
- K. Kobayakawa, Y. Murakami and Y. Sato, J. Photochem. Photobiol., A, 2005, 170, 177–179 CrossRef.
- L. Shi, J. Ma, L. Yao, L. Cui and W. Qi, J. Colloid Interface Sci., 2018, 519, 1–10 CrossRef PubMed.
- X. Y. Chen, H. S. Huh and S. W. Lee, J. Solid State Chem., 2007, 180, 2510–2516 CrossRef.
- L. Shi, F. Wang, J. Zhang and J. Sun, Ceram. Int., 2016, 42, 18116–18123 CrossRef.
- Y. Kang, Y. Yang, L. C. Yin, X. Kang, G. Liu and H. M. Cheng, Adv. Mater., 2015, 27, 4572–4577 CrossRef PubMed.
- Y. Zhou, L. Zhang, W. Huang, Q. Kong, X. Fan, M. Wang and J. Shi, Carbon, 2016, 99, 111–117 CrossRef.
- S. Cao, Q. Huang, B. Zhu and J. Yu, J. Power Sources, 2017, 351, 151–159 CrossRef.
- L. Shi, L. Liang, F. Wang, M. Liu, K. Chen, K. Sun, N. Zhang and J. Sun, ACS Sustainable Chem. Eng., 2015, 3, 3412–3419 CrossRef.
- F. Chang, X. Wang, J. Luo, J. Wang, Y. Xie, B. Deng and X. Hu, J. Mol. Catal. A: Chem., 2017, 427, 45–53 CrossRef.
- G. He, C. Xing, X. Xiao, R. Hu, X. Zuo and J. Nan, Appl. Catal., B, 2015, 170–171, 1–9 Search PubMed.
- H. Huang, K. Xiao, Y. He, T. Zhang, F. Dong, X. Du and Y. Zhang, Appl. Catal., B, 2016, 199, 75–86 CrossRef.
- L. Zhang, Y. Zhang, R. Shi, S. Bao, J. Wang, A. Amini, B. N. Chandrashekar and C. Cheng, Materials Today Energy, 2017, 5, 91–98 CrossRef.
- Y. Yang, Y. Guo, F. Liu, X. Yuan, Y. Guo, S. Zhang, W. Guo and M. Huo, Appl. Catal., B, 2013, 142–143, 828–837 CrossRef.
- L. Shi, L. Liang, J. Ma, Y. N. Meng, S. F. Zhong, F. X. Wang and J. M. Sun, Ceram. Int., 2014, 40, 3495–3502 CrossRef.
- M. Zhang, C. Bi, H. Lin, J. Cao and S. Chen, Mater. Lett., 2017, 191, 132–135 CrossRef.
- J. Xu, Y. Hu, C. Zeng, Y. Zhang and H. Huang, J. Colloid Interface Sci., 2017, 505, 719–727 CrossRef PubMed.
- L. Shi, L. Liang, J. Ma, F. X. Wang and J. M. Sun, Dalton Trans., 2014, 43, 7236–7244 RSC.
- C. Hu, T. Peng, X. Hu, Y. Nie, X. Zhou, J. Qu and H. He, J. Am. Chem. Soc., 2010, 132, 857–862 CrossRef PubMed.
- A. R. Upreti, Y. Li, N. Khadgi, S. Naraginti and C. Zhang, RSC Adv., 2016, 6, 32761–32769 RSC.
- J. Ma, L. Shi, L. Yao, Z. Wang, C. Lu, W. Qi and D. Su, ChemistrySelect, 2017, 2, 8535–8540 CrossRef.
- S. He, Q. Rong, H. Niu and Y. Cai, Chem. Commun., 2017, 53, 9636–9639 RSC.
- S. G. Kumar and K. S. R. K. Rao, RSC Adv., 2015, 5, 3306–3351 RSC.
- N. Wang, L. Shi, L. Yao, C. Lu, Y. Shi and J. Sun, RSC Adv., 2018, 8, 537–546 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra03981j |
| This journal is © The Royal Society of Chemistry 2018 |