Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Gadolinium-labelled iron/iron oxide core/shell nanoparticles as T1–T2 contrast agent for magnetic resonance imaging†
Kaili Wang,
Lu An,
Qiwei Tian*,
Jiaomin Lin and
Shiping Yang *
*
The Key Laboratory of Resource Chemistry of the Ministry of Education, The Shanghai Key Laboratory of Rare Earth Functional Materials, The Shanghai Municipal Education Committee Key Laboratory of Molecular Imaging Probes and Sensors, Shanghai Normal University, Shanghai 200234, China. E-mail: qiweitian@shnu.edu.cn; shipingy@shnu.edu.cn
First published on 26th July 2018
Abstract
Magnetic resonance imaging (MRI) is indispensable and powerful in modern clinical diagnosis and has some advantages such as non-invasiveness and high penetration depth. Furthermore, dual T1–T2 MR imaging has attracted crucial interest as it can decrease the risk of pseudo-positive signals in diagnosing lesions. And it's worth nothing that the dual-mode MR imaging displays a vital platform to provide relatively comprehensive diagnosis information and receive accurate results. Herein, we report a dual T1–T2 MR imaging contrast agent (CA) grounded on the iron/iron oxide core/shell nanomaterials conjugated with gadolinium chelate. The Gd-labeled Fe@Fe3O4 NPs reveal the feasibility to utilize them to serve as a dual T1–T2 MR imaging CA, and the relaxivity results in a 0.5 T MR system showed a longitudinal relaxivity value (r1) and transverse relaxivity value (r2) of 7.2 mM−1 s−1 and 109.4 mM−1 s−1, respectively. The MTT results demonstrate the Gd-labeled Fe@Fe3O4 NPs have no obvious cytotoxicity and a good compatibility. The in vitro and in vivo MRI generated a brighter effect and darkening in T1-weighted MR imaging and T2-weighted images, respectively. The results clearly indicate that Gd-labeled Fe@Fe3O4 NPs have potential as a magnetic resonance imaging contrast reagent.
1. Introduction
Cancer has become a predominant cause of death across the world and seriously threatens human health.1,2 Hence, exact diagnosis of cancer becomes extremely important. During the past few decades, the representative imaging modalities in either preclinical research or clinical settings are magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET), optical fluorescence imaging (FI), single-photon emission computed tomography (SPECT), ultrasound (US), and photoacoustic imaging (PA).3–5 Even so, when we need precise small animal imaging, such a single imaging mode is defective and more challenges arise in complex environments.6 To settle this problem and achieve accurate molecular imaging, a multi-mode imaging technique is one of the promising ways.7 Therefore, multifunctional CAs are usually indispensable as the complementary information of different imaging modes can be obtained at one time.8As one of the most excellent modern clinical imaging techniques, MRI has become an extremely important part due to its noninvasive character, high soft tissue contrast, large penetration depth and outstanding spatial resolution.9–11 Therefore, MRI integrated multi-mode imaging techniques including MR-PET/SPECT, MR-Optical, MR-Ultrasound and MR-Photoacoustic were investigated widely.12–16 However, it is worth noting that these multi-mode imaging techniques must be performed on different imaging devices, which cost much more. Recently, multi-mode imaging carried out on one device, such as the T1–T2 dual-weighted MRI, has attracted more attention.17–20 Furthermore, the difficulties of image matching in the multiple imaging instruments could be eliminated when a single imaging system was selected, in other words, the accuracy of tumor detection was improved.21,22 Thus, it is necessary to design and synthesis of the dual T1–T2 MRI CAs for realizing dual-modal imaging on a single instrument.6,11,23 To date, a great deal of work in effects have already been carried out to prepare T1–T2 dual-weighted CAs for MRI via the combination of iron materials with Gd elements. For example, Liang et al. prepared the core/shell Fe3O4/Gd2O3 nanocubes and put it into use for heightened contrast effect.24 Zhang et al. applied Fe3O4 as a core, SiO2 as a separating layer, Gd2O(CO3)2 as the outer shell to synthesis core/shell/shell nanoparticles and explored the potential of images.25,26 Chen et al. used hydrophobic interaction between gadolinium with iron oxide to gain a new nanocrystal (GdIO). This novel GdIO nanocluster showed an important MRI monitoring capability.27
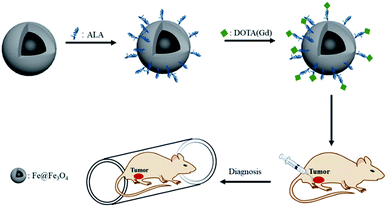
Besides, Long et al. also reported an underlying T1–T2 MRI probe of gadolinium-labelled Fe3O4 nanoparticles but did not further used especially in vivo.28 Inspired by this, in order to get even more in-depth imaging information and expand the application of the probe, we conjugated the Gd chelates onto Fe@Fe3O4 nanoparticles to create a platform for dual modal detection. Because iron has a good biocompatibility, so Fe@Fe3O4 core/shell NPs have been widely treated on T2-weight MRI CAs. Our group have developed several T2-weight MRI guided therapy agents based on the excellent T2-weight MRI properties of the Fe@Fe3O4 core/shell NPs. Gadolinium(III) containing MRI CAs are the commercial and most commonly used T1-MRI agents. The most used injection reagent of the Gd based T1-MRI agents is a chelated compound due to the toxic of the free solubilized aqueous Gd ion.29 Thus, it offers an opportunity for the Gd chelated compound to undergo ligand exchange with Fe@Fe3O4 core/shell NPs, forming a dual T1–T2 MRI CA. Hence, to expand the application of CAs in MRI and increase the sensitivity detection of lesion sites, we fabricate a dual-functional Gd-labeled Fe@Fe3O4 NPs for dual T1–T2 MR images. The formation process of NPs consisted of three procedures (Scheme 1). Firstly, the Fe@Fe3O4, capping with oleic and oleylamine, was formed through the thermal decomposition approach. In this procedure, Fe(CO)5 was utilized as a precursor. Secondly, alendronate-coated Fe@Fe3O4 was prepared via the ligand exchange based on the strong coordination ability of the Fe and phosphonic acid groups by mixing the obtained Fe@Fe3O4 NPs and alendronate solution. Then, the acquired alendronate-coated Fe@Fe3O4 NPs were combined with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), one of the typical Gd chelating agents, by activating with 3-(3-dimethylaminopropyl)-1-ethylcarbodiimide hydrochloride/N-hydroxysuccinimide (EDC/NHS) method. After purification by removing the unreacted reagent, Gd3+ was added to get DOTA(Gd)-Fe@Fe3O4 NPs by chelating with DOTA. The formed multifunctional DOTA(Gd)-Fe@Fe3O4 NPs were characterized by transmission electron microscopy (TEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), relaxivity measurements and MRI. At the same time, their effect of tumor diagnosis on 4T1 cells in vitro and in vivo were further evaluated. Our results indicated that DOTA(Gd)-Fe@Fe3O4 NPs played an important role in tumor diagnosis.
2. Experimental section
2.1. Apparatus and reagents
Oleic acid, 1-octadecene, oleylamine, 3-(3-dimethylaminopropyl)-1-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were supplied from Sigma-Aldrich. GdCl3·6H2O, alendronate sodium (ALA) and DOTA were provided from Adamas-beta. Fe(CO)5 was supplied from Development of Beijing Chemical Technology Co., Ltd. Branch. All the chemicals were used without further purify.XRD of the Fe@Fe3O4 was obtained on a Rigaku DMAX 2000 diffractometer (Cu/Kα radiation, 40 kV, 40 mA, scan range 20–80°). TEM images were measured on a JEOL JEM-2010 microscope with the accelerating voltage in 200 kV. The dynamic light scattering (DLS) and surface potential were collected utilizing a Malvern Zetasizer Nano ZS model ZEN3600 (Worcestershire, U.K.). FTIR spectra were captured by a Nicolet Avatar 370 FTIR spectrometer. The concentration of the metal in the samples was collected by inductively coupled plasma atomic emission spectroscopy (ICP-AES). The hysteresis loops were performed in a superconducting quantum interference device (SQUID) from Lake Shore.
2.2. Synthesis of ALA coated Fe@Fe3O4 NPs
Firstly, as previously reported, 12 nm Fe@Fe3O4 samples were prepared.30 Then, the ALA coated Fe@Fe3O4 was prepared by a ligand exchange method. The details as follow: 100 mg ALA were dispersed in 15 mL mixture of water and DMF (v/v = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in single-necked flask. Then, 8 mL oleic acid-coated NPs in DMF (3 mg mL−1) and 0.4 mL KOH (1 M) was added to the above mixture. After shaking for 12 hours at room temperature, the ALA coated Fe@Fe3O4 NPs was captured by centrifugation and washed with acetone via several times. At last, nanoparticles were dissolved in 10 mL water for next utilize.
1) in single-necked flask. Then, 8 mL oleic acid-coated NPs in DMF (3 mg mL−1) and 0.4 mL KOH (1 M) was added to the above mixture. After shaking for 12 hours at room temperature, the ALA coated Fe@Fe3O4 NPs was captured by centrifugation and washed with acetone via several times. At last, nanoparticles were dissolved in 10 mL water for next utilize.
2.3. Preparation of DOTA-Fe@Fe3O4 NPs
In details, 10 mL of double distilled water containing NHS (4.268 mg) and EDC (4.740 mg) was mixed with DOTA (10 mg, 5 mL) underneath magnetic stirring. After 2 h, the DOTA which activated was appended into ALA coated Fe@Fe3O4 NPs water dispersion, and this mixture was reacted for overnight. After collecting by centrifugation and washed with acetone, the acquired product was dispersed in sodium acetate buffer (0.2 M, pH = 6.5) for subsequent use.2.4. Preparation of DOTA(Gd)-Fe@Fe3O4 NPs
In a typical process, a GdCl3·6H2O aqueous solution (10 mg, 5 mL) was appended to the prepared DOTA-Fe@Fe3O4 sodium acetate buffer solution dispersion. After stirring for 10 h, DOTA(Gd)-Fe@Fe3O4 NPs were obtained by centrifugal separation and washed with acetone via several times. At last, the obtained DOTA(Gd)-Fe@Fe3O4 NPs were dispersed in distilled water and preserved in 4 °C before utilization.2.5. Cytotoxicity assay
Methyl thiazolyl tetrazolium (MTT) measurement was utilized to assess the cytotoxicity of the DOTA(Gd)-Fe@Fe3O4 NPs by means of 4T1 cell line. 4T1 cells were seeded into 96-well culture plates (5 × 104 cells per well) in DMEM assisted by 1% penicillin-streptomycin and 10% fetal bovine serum at 37 °C under 5% CO2. After being cultured for 12 h, the DOTA(Gd)-Fe@Fe3O4 NPs with different concentrations (0, 10, 20, 50, 100, 200 μg mL−1 dispersed in DMEM) were added to culture the cells for a further 12 or 24 h, respectively. Thereafter, the cells were incubated for another 4 h when a certain amount of MTT solution (20 μL 5 mg mL−1) was appended to each well under a similar condition. After that, with the adding of DMSO (150 μL per well), the blue formazan crystals were captured after shaking (150 rpm, 5 min). At last, the absorption was acquired by microplate reader.2.6. Relaxivity measurements in solution
The relaxation characteristics of both DOTA(Gd)-Fe@Fe3O4 NPs and ALA coated Fe@Fe3O4 NPs at various iron concentrations were test to evaluate the dual-model MR performance. All nanoparticles were dispersed in distilled water and carried out on the 0.5T NMI20 Analyst (Shanghai Niumag Electronic Technology Co., Ltd.). As to T1-weighted relaxation time, it was collected by a spin-echo sequence, and parameters were as follows: TW = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ms, NS = 2, P1 = 10.48 μs, SW = 100 KHz, RFD = 0.020 ms, RG1 = 20.0 db, DRG1 = 3, TD = 1024. For T2-weighted relaxation time, TW/TE = 12
000 ms, NS = 2, P1 = 10.48 μs, SW = 100 KHz, RFD = 0.020 ms, RG1 = 20.0 db, DRG1 = 3, TD = 1024. For T2-weighted relaxation time, TW/TE = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/1.5 ms, SF = 18 MHz, P2 = 20.00 μs, NECH = 15
000/1.5 ms, SF = 18 MHz, P2 = 20.00 μs, NECH = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The other parameters are similar to T1-weighted MRI. The acquired fitted curves for 1/T (s−1) against the Fe concentration (mM) were analyzed to calculate the relaxivity values of r.
000. The other parameters are similar to T1-weighted MRI. The acquired fitted curves for 1/T (s−1) against the Fe concentration (mM) were analyzed to calculate the relaxivity values of r.
2.7. MRI in cells
The in vitro dual-model MRI performance of the prepared DOTA(Gd)-Fe@Fe3O4 NPs was test by incubating the DOTA(Gd)-Fe@Fe3O4 NPs with 4T1 cells on 0.5T NMI20 Analyst. In details, 4T1 cells were seeded in a 6-well plate at a cell density of 1 × 106 cells per mL for 12 h. Then, the cells were incubated with DOTA(Gd)-Fe@Fe3O4 NPs for 4 h. After that, the cells were resuspended in xanthan gum (1 × 106 cells per well) after washed with PBS buffer solution for several times. Given the above, the preparation of MRI in cells was completed. For T1-weighted MRI, parameters were determined as follows: D0 = 500 ms, D4 = D5 = 50 ms, RFA1 = 5.5%, RFA2 = 9.5%, RP1Count = 2, RP2Count = 128. For T2-weighted MRI, D0 = 5500 ms, D4 = D5 = 200 ms. The others of parameters were the same.2.8. MRI in vivo
The in vivo dual-model MRI was performed by a 0.5 T system (MiniMR-60; Shanghai Niumag Electronic Technology Co., Ltd.). The prepared DOTA(Gd)-Fe@Fe3O4 NPs was intertumoral injected in 4T1 tumor mouse. Noted, animal experiments were approved by the animal ethics committee of Shanghai Normal University and in strict accordance with the policy of the Institutional Animal Care and Use Committee. Firstly, the 4T1 tumor bearing mice model was prepared by inoculating subcutaneously with 4T1 cells (3 × 107 cells per mL) for female BALB/c mice (6 weeks). After diameter of tumor reached ∼1.2 cm, the DOTA(Gd)-Fe@Fe3O4 NPs (50 μL, 2 mg mL−1) was injected into BALB/c mice via intertumoral injection. As to MRI in vivo, experiments were performed on the 0.5 T MRI system after injected for 30 min. For T1-weighted MRI, a conventional spin-echo sequence was utilized, the parameters were as follows: TR/TE = 300/12 ms, 192 × 256 matrices, 80 × 80 mm field of view, P1 = 5.3, NS = 10, a slice thickness of 4 mm. For T2-weighted MR imaging, TR/TE = 1000/18.125 ms, 256 × 256 matrices, 130 × 130 mm field of view, a slice thickness of 4 mm. All the tests were parallelly carried out for three times. Mouse selection and methods of care, welfare, and killing were authorized by the animal ethics committee of Shanghai Normal University, and were in strict accordance with the policy of the Institutional Animal Care and Use Committee.3. Results and discussion
3.1. Characterization of the prepared NPs
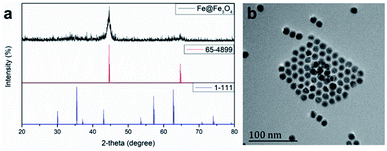
According the previous reports,30 12 nm Fe@Fe3O4 nanoparticles were prepared firstly. To verify the crystal structure of the fabricated samples, XRD studies were performed on the obtained dry powder. The two diffraction peaks at 44.6 and 65.2 degree (2θ) of the obtained sample are a good match with the (110) and (200) of the body-centered cubic iron (JCPDS card no. 65-4899), respectively (Fig. 1). On the contrary, it is difficult to find the diffraction peak to index to the crystal Fe3O4 (JCPDS card no. 1-111) due to the extremely poor crystallization of Fe3O4 in the sample by the natural oxidation method used in the experiment. These results suggest the existence of the body-centered cubic iron, not the crystal Fe3O4.31 In order to demonstrate that the body-centered cubic iron was coated with amorphous Fe3O4, the TEM was used to further characterize the structure of the prepared sample. The TEM image demonstrate that the obtained sample showed obvious core–shell structures with the shell thickness of ∼2 nm and a high uniformity particle size distribution with a diameter of 12 nm. The similar results with previous literature suggest that the Fe@Fe3O4 NPs with a core shell structure were prepared successfully.Then, the gadolinium chelate was conjugated to the Fe@Fe3O4 NPs. In order to obtain the amino group to conjugate the DOTA, the ALA was used to modify the Fe@Fe3O4 NPs via a ligand exchange method at beginning. The results were firstly characterized by the TEM. As shown in Fig. 2, both ALA coated Fe@Fe3O4 NPs and DOTA(Gd)-Fe@Fe3O4 NPs are exhibit clearly core–shell structures and a high uniformity particle size distribution with a little bit increased diameter (∼13 and ∼14 nm respectively) after modification, indicating that the modification will not affect structure and size of the Fe@Fe3O4 NPs. Besides, their DLS results of nanoparticles are shown in Fig. 2c, and it showed that their hydrodynamic diameter were about 49 nm, 92 nm and 258 nm (ESI, Fig. S1†). As a kind of substance with large molecular weight, Gd-DOTA has a strong ability to combine with water, resulting a large size distribution for DOTA(Gd)-Fe@Fe3O4 NPs. Importantly, the zeta potential of the ALA coated Fe@Fe3O4 is −22.8 mV. Notably, the surface potential from measurement of DOTA-Fe@Fe3O4 and DOTA(Gd)-Fe@Fe3O4 is −37.4 mV and 24.6 mV respectively (Fig. 2d, ESI,† Fig. S2†). The great change of the surface potential caused by the follow reasons. For the ALA coated Fe@Fe3O4, the observed potential is negative, which is due to the residual phosphate group in the solution is much than the amino group. After grafting with DOTA, the more negative potential can be attributed to the lot of carboxyl groups of DOTA. Once the DOTA(Gd)-Fe@Fe3O4 formed, the surface potential changed from negative to positive. The result of which advocate the fact that Gd(III) ions is coordination with the carboxylate groups of DOTA ligand. The great change of the surface potential also can demonstrate that the DOTA(Gd)-Fe@Fe3O4 was prepared successfully. In order to confirm the elements content of compound, the obtained nanoparticles were determined by ICP assay. According to the results of three parallel tests, the ratio of Fe to Gd is ∼3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
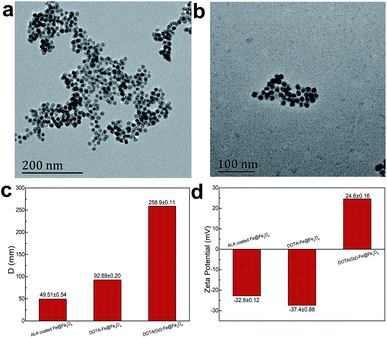 | ||
| Fig. 2 TEM micrograph of (a) ALA coated Fe@Fe3O4 NPs and (b) DOTA(Gd)-Fe@Fe3O4 NPs, the comparison images of (c) DLS and (d) zeta potential. | ||
FTIR was utilized to further characterize the surface ligand of all the obtained NPs, including Fe@Fe3O4, ALA coated Fe@Fe3O4, DOTA-Fe@Fe3O4 and DOTA(Gd)-Fe@Fe3O4 (Fig. 3). Several characteristic peaks (Fig. 3, origin line a) were observed for Fe@Fe3O4: 593 cm−1 (Fe–O stretch), 2925 cm−1 and 2853 cm−1 (–CH2 vibrations) similar with previous ref. 32 and 33. After coated by the ALA, the spectra showed a significantly band of phosphonate resonance in 1100 cm−1 (Fig. 3, origin line b), suggesting the success of ligand exchange. After the attachment of DOTA ligands, two disubstituted amides peaks (3286 cm−1, 3168 cm−1), a monosubstituted amides peak (1562 cm−1) and a strong ester group stretch vibrations (1415 cm−1) were observed (Fig. 3, origin line c). When it comes to the DOTA(Gd)-Fe@Fe3O4 NPs, the vibration absorption peak of the carboxyl group abruptly widens, which should be attributed to the coordination of Gd3+ and water.34 All of the above results demonstrated that the three-step reaction is successful under the reaction conditions.
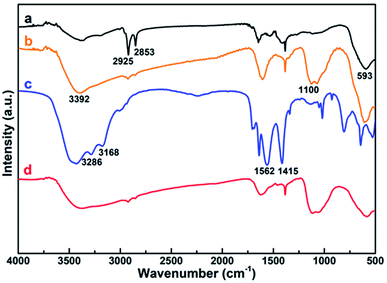 | ||
| Fig. 3 FTIR spectroscopy of (a) Fe@Fe3O4, (b) ALA coated Fe@Fe3O4, (c) DOTA-Fe@Fe3O4 and (d) DOTA(Gd)-Fe@Fe3O4 NPs. | ||
3.2. Magnetic measurement
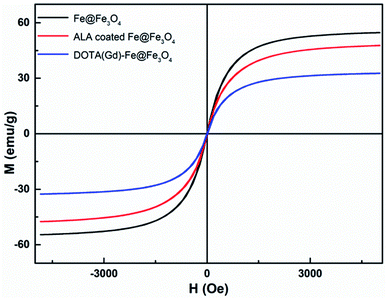
The magnetic properties of bcc-Fe/Fe3O4, ALA coated Fe@Fe3O4, DOTA(Gd)-Fe@Fe3O4 was investigated by a SQUID magnetometer at room temperature (300 K) (Fig. 4). The results of hysteresis loops indicated that all the nanoparticles exhibit typically paramagnetic behaviors without magnetic hysteresis. The saturation magnetization (Ms) values decrease with the gradual modification of materials. The obtained Fe@Fe3O4 NPs exhibit strong paramagnetic behaviors with Ms value of 55 emu g−1, which is less than our previous results (∼100 emu g−1). The decreased Ms value can be assigned to more thickness of the Fe3O4 shell. Once the ALA was utilized to decorate the obtained Fe@Fe3O4 NPs, the Ms value reduced to 48 emu g−1. The reason for the decrease in Ms value of the NPs may be the phosphate disodium salt shell coating, which decreased magnetic anisotropy. Moreover, as for the DOTA(Gd)-Fe@Fe3O4 NPs, the Ms value decreased to 32 emu g−1 which may be because of the content significantly diminishment of iron oxide in the NPs.35 The saturation magnetization calculated by the terms of per gram Fe (ESI, Fig. S3†) suggests that the Ms value increased after conjugating DOTA(Gd) on Fe@Fe3O4, which is similar with the literature.28 Nevertheless, Gd ions can realize the spin order at the same direction with the effect of paramagnetic Fe@Fe3O4 NPs when exert an external magnetic field. Certainly, the aforementioned changes lead to form a local magnetic field. Hence, the relaxivity of Gd ions was augmented and the homogeneity of magnetic field for T2 CA was broke as well, and that is why it is always accompanied by improve the T1 or T2 contrast effect.36 Therefore, we consider that Fe@Fe3O4@ALA-DOTA(Gd) NPs can show excellent T1–T2 dual-modal ability.To further provide more evidences to support the capability of dual mode T1- and T2-weighted contrast, a 0.5 T MRI scanner was performed to detect the relaxivity of the ALA coated Fe@Fe3O4 and DOTA(Gd)-Fe@Fe3O4 NPs in aqueous media. The value of r1 and r2 were determined by analyzing the fitted curves for the reciprocal of relaxation time to the Fe concentration. As shown in the Fig. 5a and b, the r1 relaxivity values of ALA coated Fe@Fe3O4 and DOTA(Gd)-Fe@Fe3O4 are 4.1 and 7.2 mM−1 s−1, while the r2 relaxivity values are 57.7 and 109.4, respectively. Simultaneously, in order to evaluate the effect of Gd element in NPs, we also made a plot of 1/T1 against concentration of Gd ion. As shown in the picture (ESI, Fig. S4†), the r1 of DOTA(Gd)-Fe@Fe3O4 NPs is much higher than DOTA(Gd). The results show that the DOTA(Gd)-Fe@Fe3O4 exhibit better relaxivity both for r1 and r2, which is benefit for MRI. To now, the highest r value for dual mode MR CAs is 836.7 ± 51.1 and 31.6 ± 2.6 for r2 and r1 respectively, which is obtained by Long et al.28 at 1.47 T for DOTA conjugated Fe3O4. Compared with these reported highest values, the r value of DOTA(Gd)-Fe@Fe3O4 is much lower. However, both of the r2 and r1 of DOTA(Gd)-Fe@Fe3O4 are higher than that of the Fe@Fe3O4 and DOTA(Gd) alone. These increased r2 and r1 are assigned to an alignment of the electronic spins of the paramagnetic ion by the induced magnetic field generated by the superparamagnetic particles.28 These results suggest that the prepared DOTA(Gd)-Fe@Fe3O4 shows great potential for MRI contrast.
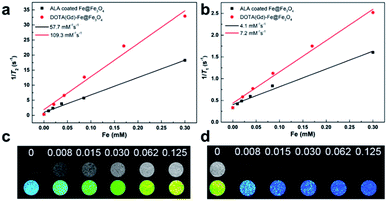 | ||
| Fig. 5 (a) r1 and (b) r2 relaxivity values of ALA coated Fe@Fe3O4 and DOTA(Gd)-Fe@Fe3O4 NPs; (c) T1-weighted and (d) T2-weighted images of DOTA(Gd)-Fe@Fe3O4 NPs in solution at 0.5 T MR system. | ||
Then, the T1-and T2-Weighted images of the DOTA(Gd)-Fe@Fe3O4 was carried out at different Fe concentrations (0 to 0.125 mM) and presented in the Fig. 5c and d. With increasing molar concentration of Fe, the T1 signal became much brighter, while the T2 MR contrast became darker, indicating that the DOTA(Gd)-Fe@Fe3O4 NPs could be utilized as a dual-mode MRI CA. Above all, these results show that the DOTA(Gd)-Fe@Fe3O4 NPs will exhibit excellent MR signal both for T1 and T2.
3.3. Cytotoxicity assay
To assess the cytotoxic potential effects of DOTA(Gd)-Fe@Fe3O4 NPs in vitro and in vivo applications, the particles was evaluated via an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay for two cell lines, 4T1 (a mouse breast cancer cell lines) and HUVEC (a human umbilical vein endothelial cell lines) respectively. As displayed in Fig. 6, the cells that incubated with the NPs (10, 20, 50, 100, 200 μg mL−1) compared to the contrasts hardly show the remarkable difference even for 24 h, which proved the favorable viability of cells. Furthermore, what counts is that the cell viability still remained above 90% when the concentration reaches to 200 μg mL−1. Above all, the high cell viability indicates that DOTA(Gd)-Fe@Fe3O4 NPs exhibit excellent biocompatibility within the experimental concentration (ESI, Fig. S5†). These results establish the foundation of NPs for further biological applications.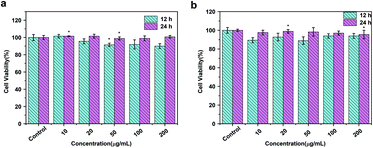 | ||
| Fig. 6 MTT results of (a) 4T1 and (b) HUVEC cells incubated with DOTA(Gd)-Fe@Fe3O4 NPs in different concentrations for 12 h and 24 h at 37 °C (n = 3, *p < 0.05). | ||
3.4. Dual T1–T2 MR imaging in cells
To validate the dual-mode MRI performance of the DOTA(Gd)-Fe@Fe3O4 NPs, various concentrations samples from 10 to 150 μg mL−1 were utilized to incubate the 4T1 cells for 4 h. The T1- and T2-weighted MR images and the corresponding signal value of the cells were measured. As displayed in Fig. 7, with DOTA(Gd)-Fe@Fe3O4 NPs concentration increasing in 4T1 cells, as far as we can see, the positive contrast of T1-weighted MR images demonstrated an obvious enhancement. Similarly, the 4T1 cells cultivated in particles exhibited a marked negative signal drop with dose-dependent. On the other hand, this point was confirmed via collecting the signal intensity changes by quantitative analysis as well. It is worth nothing that DOTA(Gd)-Fe@Fe3O4 NPs (150 μg mL−1) enhance the signal value of T1 by ∼39.87% compared to the blank group. Simultaneously, the MR signal intensity of T2 decreased by ∼63.49% after incubation with DOTA(Gd)-Fe@Fe3O4 NPs even at the lowest concentration. This indicates that the DOTA(Gd)-Fe@Fe3O4 NPs were successfully taken up by 4T1 cells, which indicated that the dipolar interaction of protons and the magnetic moments in the water and cell media. Besides, the MR contrast on both longitudinal (T1) and transverse (T2) proton relaxation time sequences was generated. These results demonstrate that the DOTA(Gd)-Fe@Fe3O4 NPs can be utilized as a platform for dual T1–T2 MRI CAs in vitro.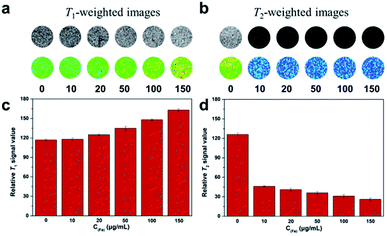 | ||
| Fig. 7 (a) T1- and (b) T2-weighted MR imaging of 4T1 cells with various incubate concentration of DOTA(Gd)-Fe@Fe3O4 NPs. (c) and (d) Signal intensity analysis for MR imaging. | ||
3.5. MRI in vivo
The feasibility of DOTA(Gd)-Fe@Fe3O4 NPs in vivo MRI for tumor was further captured by means of 4T1 murine tumor model. In order to realize the test in vivo, the mice were performed by DOTA(Gd)-Fe@Fe3O4 NPs (50 μL 0.2 mg mL−1, 2 mg kg−1 of mouse bodyweight) PBS dispersion via intratumor injection on a MR imaging scanner. Fig. 8 showed the T1- and T2-weighted images of tumor acquired before and 0.5 h post injection images, respectively. Through the comparison of these images, T1-weighted MR images showed obvious brighter signal. At the same time, T2-weighted MR images signal at 30 min become darker. This phenomenon is presumably because of the fact that the rapid diffusion of reagent in the tumor site. Furthermore, the T1 MR images signal intensity was increased by 70% when compared with pre-injection in tumor. While the T2 MR images signal intensity also exhibited obvious changes, it was decreased by 20% after 30 min post injection. These results declared that DOTA(Gd)-Fe@Fe3O4 NPs can exhibit positive T1 or negative T2 contrast enhancement for tumor MR images simultaneously via selecting r1 or r2 relaxivities. Therefore, the developed multifunctional DOTA(Gd)-Fe@Fe3O4 NPs are quite fit for a platform to dual T1–T2 MR imaging of tumors.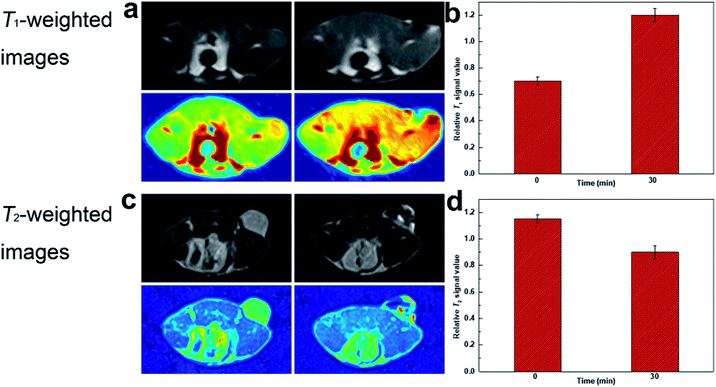 | ||
| Fig. 8 (a) T1-weighted and (c) T2-weighted MR images of tumor injected with DOTA(Gd)-Fe@Fe3O4 NPs; (b) and (d) relative signal intensity for T1- and T2-weighted MR images in tumor. | ||
4. Conclusions
In summary, diagnosis is of necessity in clinical application and plays an important role in treatment to some extent. In this work, we successfully manufactured multifunctional Gd-labeled Fe@Fe3O4 NPs that might act as a dual T1–T2 MR CA to reciprocally enhance images of cancer cells both in vitro and in vivo. These promising NPs have a good biocompatibility and show a high cell viability even at the largest concentration of 200 μg mL−1. The accuracy of diagnosis is significantly improved both in positive T1 and negative T2 enhancement. The low toxicity and excellent contrast of the developed dual model MRI agents give it a bright future in the clinical application for tumor diagnosis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by National Natural Science Foundation of China (No. 21601124 and 21671135), Program of Young Eastern Scholar from Shanghai Institutions of Higher Learning (QD2015038), Shanghai Rising-Star Program (17QA1402600), the Natural Science Foundation of Shanghai (16ZR1424700), Ministry of Education of China (PCSIRT_IRT_16R49), and International Joint Laboratory on Resource Chemistry (IJLRC).Notes and references
- Y. Liu, K. Ai, J. Liu, M. Deng, Y. He and L. Lu, Adv. Mater., 2013, 25, 1353–1359 CrossRef PubMed.
- R. Kumar, W. S. Shin, K. Sunwoo, W. Y. Kim, S. Koo, S. Bhuniya and J. S. Kim, Chem. Soc. Rev., 2015, 44, 6670–6683 RSC.
- J. Estelrich, M. J. Sanchez-Martin and M. A. Busquets, Int. J. Nanomed., 2015, 10, 1727–1741 Search PubMed.
- M. Rudin and R. Weissleder, Nat. Rev. Drug Discovery, 2003, 2, 123–131 CrossRef PubMed.
- S. R. Cherry, Annu. Rev. Biomed. Eng., 2006, 8, 35–62 CrossRef PubMed.
- B. R. Smith and S. S. Gambhir, Chem. Rev., 2017, 117, 901–986 CrossRef PubMed.
- T. H. Shin, Y. Choi, S. Kim and J. Cheon, Chem. Soc. Rev., 2015, 44, 4501–4516 RSC.
- H. Kobayashi, M. R. Longmire, M. Ogawa and P. L. Choyke, Chem. Soc. Rev., 2011, 40, 4626 RSC.
- Y. Cao, L. Xu, Y. Kuang, D. Xiong and R. Pei, J. Mater. Chem. B, 2017, 5, 3431–3461 RSC.
- G. H. Im, S. M. Kim, D.-G. Lee, W. J. Lee, J. H. Lee and I. S. Lee, Biomaterials, 2013, 34, 2069–2076 CrossRef PubMed.
- A. Szpak, S. Fiejdasz, W. Prendota, T. Strączek, C. Kapusta, J. Szmyd, M. Nowakowska and S. Zapotoczny, J. Nanopart. Res., 2014, 16, 2678 CrossRef PubMed.
- X. Gao, G. Ji, R. Cui, J. Liu and Z. Liu, Dalton Trans., 2017, 46, 13686–13689 RSC.
- C.-T. Yang, P. Padmanabhan and B. Z. Gulyás, RSC Adv., 2016, 6, 60945–60966 RSC.
- P. M. Robson, D. Dey, D. E. Newby, D. Berman, D. Li, Z. A. Fayad and M. R. Dweck, JACC: Cardiovascular Imaging, 2017, 10, 1165–1179 CrossRef PubMed.
- B. Zhou, Z. Xiong, P. Wang, C. Peng, M. Shen, S. Mignani, J.-P. Majoral and X. Shi, Drug Delivery, 2018, 25, 178–186 CrossRef PubMed.
- G. Angelovski, Angew. Chem., Int. Ed., 2016, 55, 7038–7046 CrossRef PubMed.
- D. Yoo, J.-H. Lee, T.-H. Shin and J. Cheon, Acc. Chem. Res., 2011, 44, 863–874 CrossRef PubMed.
- T. Tirusew, X. Wenlong, A. Md Wasi, B. Jong Su, C. Yongmin, B. Ji Eun, C. Kwon Seok, K. Tae Jeong and L. Gang Ho, Nanotechnology, 2015, 26, 365102 CrossRef PubMed.
- E.-K. Lim, T. Kim, S. Paik, S. Haam, Y.-M. Huh and K. Lee, Chem. Rev., 2015, 115, 327–394 CrossRef PubMed.
- Z. Yi, X. Li, W. Lu, H. Liu, S. Zeng and J. Hao, J. Mater. Chem. B, 2016, 4, 2715–2722 RSC.
- J.-s. Choi, J.-H. Lee, T.-H. Shin, H.-T. Song, E. Y. Kim and J. Cheon, J. Am. Chem. Soc., 2010, 132, 11015–11017 CrossRef PubMed.
- Z. Gao, T. Ma, E. Zhao, D. Docter, W. Yang, R. H. Stauber and M. Gao, Small, 2016, 12, 556–576 CrossRef PubMed.
- E. Peng, F. Wang and J. M. Xue, J. Mater. Chem. B, 2015, 3, 2241–2276 RSC.
- F. Li, D. Zhi, Y. Luo, J. Zhang, X. Nan, Y. Zhang, W. Zhou, B. Qiu, L. Wen and G. Liang, Nanoscale, 2016, 8, 12826–12833 RSC.
- M. Yang, L. Gao, K. Liu, C. Luo, Y. Wang, L. Yu, H. Peng and W. Zhang, Talanta, 2015, 131, 661–665 CrossRef PubMed.
- T.-H. Shin, J.-s. Choi, S. Yun, I.-S. Kim, H.-T. Song, Y. Kim, K. I. Park and J. Cheon, ACS Nano, 2014, 8, 3393–3401 CrossRef PubMed.
- X. Wang, Z. Zhou, Z. Wang, Y. Xue, Y. Zeng, J. Gao, L. Zhu, X. Zhang, G. Liu and X. Chen, Nanoscale, 2013, 5, 8098–8104 RSC.
- N. A. Keasberry, M. Banobre-Lopez, C. Wood, G. J. Stasiuk, J. Gallo and N. J. Long, Nanoscale, 2015, 7, 16119–16128 RSC.
- Z. Zhou, Y. Sun, J. Shen, J. Wei, C. Yu, B. Kong, W. Liu, H. Yang, S. Yang and W. Wang, Biomaterials, 2014, 35, 7470–7478 CrossRef PubMed.
- J. Wang, H. Zhao, Z. Zhou, P. Zhou, Y. Yan, M. Wang, H. Yang, Y. Zhang and S. Yang, ACS Appl. Mater. Interfaces, 2016, 8, 19872–19882 CrossRef PubMed.
- X. Mu, C. Yan, Q. Tian, J. Lin and S. Yang, Int. J. Nanomed., 2017, 12, 7207–7223 CrossRef PubMed.
- Y. Leng, K. Sato, Y. Shi, J.-G. Li, T. Ishigaki, T. Yoshida and H. Kamiya, J. Phys. Chem. C, 2009, 113, 16681–16685 CrossRef.
- L. Wang, H. Zhang, Z. Zhou, B. Kong, L. An, J. Wei, H. Yang, J. Zhao and S. Yang, J. Mater. Chem. B, 2015, 3, 1433–1438 RSC.
- G. Zhang, R. Du, L. Zhang, D. Cai, X. Sun, Y. Zhou, J. Zhou, J. Qian, K. Zhong, K. Zheng, D. Kaigler, W. Liu, X. Zhang, D. Zou and Z. Wu, Adv. Funct. Mater., 2015, 25, 6101–6111 CrossRef.
- K. H. Bae, Y. B. Kim, Y. Lee, J. Hwang, H. Park and T. G. Park, Bioconjugate Chem., 2010, 21, 505–512 CrossRef PubMed.
- Z. Zhou, C. Wu, H. Liu, X. Zhu, Z. Zhao, L. Wang, Y. Xu, H. Ai and J. Gao, ACS Nano, 2015, 9, 3012–3022 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra04530e |
| This journal is © The Royal Society of Chemistry 2018 |