Open Access Article
Open Access ArticleThe effect of the morpholinium ionic liquid anion on the catalytic activity of Rh (or Pt) complex–ionic liquid systems in hydrosilylation processes
Magdalena Jankowska-Wajda a,
Izabela Dąbekb,
Ryszard Fiedorowa and
Hieronim Maciejewski
a,
Izabela Dąbekb,
Ryszard Fiedorowa and
Hieronim Maciejewski *ab
*ab
aFaculty of Chemistry, Adam Mickiewicz University in Poznań, Umultowska 89B, 61-614 Poznań, Poland. E-mail: maciejm@amu.edu.pl
bPoznań Science and Technology Park, A. Mickiewicz University Foundation, Rubież 46, 61-612 Poznań, Poland
First published on 30th July 2018
Abstract
Studies were performed on the catalytic activity for olefin hydrosilylation shown by three rhodium complexes, [{Rh(μ-OSiMe3)(cod)}2] (I), [{Rh(μ-Cl)(cod)}2] (II) and [RhCl(PPh3)3] (III), and three platinum complexes, [Pt(PPh3)4] (IV), [Pt(PPh3)2Cl2] (V) and PtCl4 (VI), immobilized in a series of different anion-containing morpholinium ionic liquids. The effect of the kind of anion (its nucleophilic character) on the activity, stability and possibility of a catalytic system with multiple uses in the hydrosilylation process has been established. In the case of the best systems it was possible to reuse the same catalyst sample 10 times almost without any decrease in the activity and a TON value over 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was obtained.
000 was obtained.
Introduction
The application of ionic liquids (ILs) as solvents in chemical reactions is well-documented in the literature and makes one of the oldest directions of studies on ionic liquids.1–3 The interest in ionic liquids has been focused mainly on their use as a green alternative to volatile organic solvents.4 Due to the unique properties of ILs, first of all their high polarity, non-volatility and high thermal stability, they enable conduction of high temperature and pressure processes and often act not only as solvents but also as catalysts, catalyst immobilizers and initiators. Recently, a number of excellent review papers as well as books were devoted to these applications, particularly to the role played by ionic liquids in catalysis.5–12 Over 100 types of chemical reactions are known in which ionic liquids were successfully applied. Among them are reactions catalyzed by transition metal complexes, mainly hydrogenation, oxidation, hydroformylation, carbonylation and metathesis, as well as coupling reactions.13 Moreover, reactions of hydrosilylation, in which ILs immobilize platinum or rhodium catalysts, should be mentioned here.14–31 In most cases, ILs can well dissolve and immobilize metal complexes and, at the same time, they are insoluble in reactants thus enabling to perform processes in biphasic systems and to easily isolate products (after a reaction) with the possibility of reusing the catalytic system. Organic molten alkylpyridinium and dialkylimidazolium salts were also used as thermoregulated supports for rhodium complexes. The catalyst phase is liquid under reaction conditions, whereas it is solid at room temperature so it can be reused by simple decantation.32–34Very effective catalytic systems based on the use of different ILs were developed for many processes, however, the effect of the kind of ionic liquid on the course of reactions catalyzed by transition metal complexes is still poorly recognized. This fact is a result of the complexity of such systems and the possibility of different interactions. The course of reactions can be influenced both by the kind of metal complex and ionic liquid and by the kind of parent compounds and products formed (their hydrophilic–hydrophobic properties). Moreover, impurities associated with a catalyst can also affect the reaction course. All these factors have an effect on the final catalytic result and considerably impede studies on direct interactions between a metal complex and an ionic liquid as well as on the determination of the real influence of ionic liquids on a catalytic reaction course.10 For this reason, the more information we obtain on the Ils-containing systems and different metal complexes, the easier will be the determination of their interactions.
Up to the present, the main interest in ionic liquids was focused on imidazolium, tetraalkylammonium, phosphonium, sulfonium, piperidinium and pyridinium derivatives combined with different inorganic and organic anions. On the other hand, morpholinium ILs were employed relatively rarely, and to the best of our knowledge, they were never applied in hydrosilylation processes. Taking into consideration the fact that the ecological effect of ionic liquids is discussed more and more frequently, morpholinium ILs that are characterized by a low ecotoxicity, can be highly desirable as a green alternative to conventional solvents.25 Although quite a number of papers have been published on hydrosilylation carried in the presence of ILs, most of them were focused on a narrow scope of catalyst/IL systems, namely they presented results of in-depth study of one particular catalyst. This is why we have compared in this study the catalytic activity of rhodium and platinum complexes, where platinum was at different oxidation states. Moreover, the aim of the study included the determination of the effect of anion (from morpholinium ionic liquids) on the catalytic activity, stability and possibility of reusing Rh and Pt complex–ionic liquid systems in hydrosilylation reactions.
Experimental
Materials and methods
Rhodium complexes, [{Rh(μ-OSiMe3)(cod)}2] and [{Rh(μ-Cl)(cod)}2], were prepared as described in ref. 35 Other Rh and Pt complexes as well as heptamethyltrisiloxane and octene were purchased from Aldrich. Ionic liquids: [Mor][HSO4], [Mor][TolSO3], [Mor][CH3SO3], [Mor][NO3], [Mor][NO2] were purchased from Iolitec GmbH, Germany. All reagents were dried under vacuum for 24 h followed by degassing by repeated freeze–pump–thaw cycles.NMR spectra (1H, 13C and 29Si) were recorded on a Varian Gemini 300 VT and Varian Mercury 300 VT spectrometers. C6D6, CDCl3 or CD3OD were used as solvents. GC analyses were carried out on a Varian 3800 chromatograph equipped with a 30 meter DB-1 capillary column and TCD detector using the following temperature program: 60 °C (3 min) – 100 °C min−1 – 300 °C (10 min). GC-MS analyses were carried out on a Bruker 450-GC gas chromatograph (30 m capillary column DB-5) equipped with a Bruker 320-MS Triple Quad mass spectrometer. Analysis of the post-reaction mixture for a possible presence of rhodium or platinum was carried out on a Varian Vista-MPX inductively coupled plasma-optical emission spectrometer (ICP-OES).
Real time FT-IR spectroscopy
Real-time infrared spectroscopy has been applied to monitor hydrosilylation of octene with heptamethyltrisiloxane. The measurements were performed on a Mettler-Toledo ReactIR 15 spectrometer equipped with 9.5 mm AgX DiComp (diamond) probe and a liquid nitrogen cooled MCT detector. The spectra were taken with the resolution of 4 cm−1 collecting 50 scans for each spectrum at 15 s intervals. The reaction progress in the studied systems of parent compounds and catalysts was quantified by observing the rate of changes occurring with time in the area of the band at 904 cm−1 originating from stretching vibrations of Si–H bond.Catalytic tests
All manipulations were carried out under argon using Schlenk techniques. Appropriate amounts of a catalyst (in the ratio of 10−5 mole per 1 mole of Si–H) and morpholinium ionic liquid (1% based on the total weight of combined substrates) were placed into the reaction vessel and heated to 120 °C for 0.5 h in order to dissolve the catalyst in the ionic liquid (IL). Then the reaction system was cooled down and the mixture of 1,1,1,3,5,5,5-heptamethyltrisiloxane (HMTS) and 1.1 equivalent of 1-octene were added and the reaction vessel was heated again to 120 °C. After 1 h, the reaction vessel was cooled down to room temperature and then the reaction mixture was separated from the catalytic system phase by decantation. The post-reaction mixture was analyzed by gas chromatography. The conversion of substrates and the product yields were calculated on the basis of decane internal standard which was added to the reactant mixture prior to the reaction (10% by volume in relation to the total amount of substrates). The isolated product, 3-octyl-1,1,3,5,5,5-heptamethyltrisiloxane, was characterized by NMR spectroscopy:1H NMR: (CDCl3, 298 K, 300 MHz), δ (ppm): 0.04 (18H, s, –OSi(CH3)3), 0.23 (3H, s, –OSi(CH3)O–), 0.91 (3H, (t, J = 7.0 Hz), –CH3), 1.16–1.37 (10H, –CH2–, 1.23 (tt, J = 7.3, 6.5 Hz), 1.28 (h, J = 7.0 Hz), 1.24 (tt, J = 7.0, 6.9 Hz), 1.23 (tt, J = 6.9, 6.5 Hz), 1.23 (quint, J = 6.5 Hz)), 1.47 (2H, tt, J = 7.3, 7.0 Hz, –SiCH2–).
13C NMR: (CDCl3, 298 K, 75.4 MHz), δ (ppm): −0.25 (–OSi(CH3)O–), 1.86 (–OSi(CH3)3), 14.13 (–SiCH2–), 17.69 (CH3), 23.14 (–CH2–).
29Si NMR: (CDCl3, 298 K, 59.6 MHz), δ (ppm): −20.87 (–OSiCH3), 7.08 (OSi(CH3)3).
Results and discussion
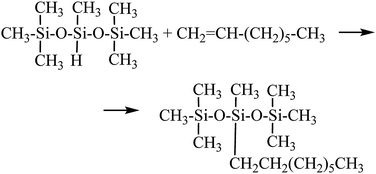
The reaction employed in this study was hydrosilylation of 1-octene with 1,1,1,3,5,5,5-heptamethyltrisiloxane (see Scheme 1) that can be regarded as a model reaction for the polymeric system because lower boiling points of products made it possible to determine conversion of substrates as well as selectivity and yields of products by means of GC analysis.Studies of catalytic activity for the above reaction were carried out using three rhodium complexes: [{Rh(μ-OSiMe3)(cod)}2] (I), [{Rh(μ-Cl)(cod)}2] (II), [RhCl(PPh3)3] (III), and three platinum complexes: [Pt(PPh3)4] (IV), [Pt(PPh3)2Cl2] (V) and PtCl4 (VI) that contained platinum at different oxidation states. All the reactions resulted in the formation of one product only that was the β-adduct as seen in a post-reaction mixture chromatogram shown by way of illustration (Fig. 1b). However, in the case of platinum catalysts, particularly in that of PtCl4, an undesirable process of olefin isomerization was also observed as results from Fig. 1c. Conversions of substrates and yields of products were calculated on the basis of GC analyses using an internal standard method. The products were confirmed by GC-MS analysis.
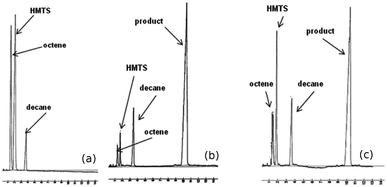 | ||
| Fig. 1 Exemplary chromatograms: a mixture of substrates (a), a post-reaction mixture from Rh complex-catalyzed process (b), a post-reaction mixture from Pt complex-catalyzed process (c). | ||
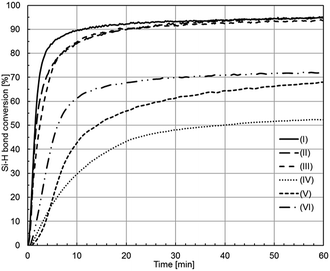
To compare catalytic activity of rhodium and platinum complexes, analyses were carried out of reaction mixtures (in the presence of studied complexes) in real time using in situ FT-IR spectroscopy. Fig. 2 shows the increase in the concentration of hydrosilylation product in time for the reaction catalyzed by all complexes studied (in the absence of ionic liquids).
A comparison of activities of all catalysts employed in the reaction studied shows that rhodium complexes are characterized by considerably higher activity than platinum ones, which manifests itself not only by higher yields, but also by considerably faster reaching the final yield. For instance, the product yield after 10 minutes of the reaction was almost 90% in the case of all rhodium complexes, whereas in that of catalysis by platinum complexes the product yield ranged between 30 and 60%. From among rhodium complexes, the highest activity was shown by rhodium siloxide complex (I), albeit activities of remaining rhodium complexes were very similar. On the other hand, activities of platinum catalysts were more diversified and one can notice that platinum complexes with higher oxidation state were more active than those with low oxidation state.
All the metal complexes appeared to be very well soluble in morpholinium ILs that were compounds of 4-benzyl-4-methylmorpholinium with different anions (see Scheme 2). Such catalytic systems were employed in the model reaction of hydrosilylation.
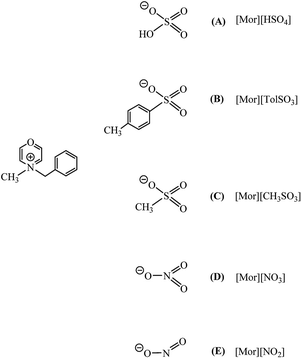 | ||
| Scheme 2 Morpholinium ionic liquids employed for the immobilization of rhodium and platinum complexes. | ||
In all cases biphasic systems were formed, which enabled easy separation of the catalytic system from the post-reaction mixture (by decantation) and created the possibility of its reusing in subsequent catalytic runs. Taking into account diversified activity of initial complexes, all reactions were conducted for one hour, followed by analysis of the composition of post-reaction mixtures using GC and GC-MS techniques. Product yields obtained in reactions studied are presented in Table 1.
| Transition metal complex | Yield of hydrosilylation product [%] | |||||
|---|---|---|---|---|---|---|
| No IL | [Mor][HSO4] (A) | [Mor][TolSO3] (B) | [Mor][CH3SO3] (C) | [Mor][NO3] (D) | [Mor][NO2] (E) | |
| [{Rh(μ-OSiMe3)(cod)}2] (I) | 95 | 99 | 99 | 99 | 99 | 90 |
| [{Rh(μ-Cl)(cod)}2] (II) | 95 | 97 | 97 | 93 | 95 | 90 |
| [RhCl(PPh3)3] (III) | 94 | 90 | 87 | 90 | 89 | 85 |
| [Pt(PPh3)4] (IV) | 53 | 71 | 89 | 75 | 78 | 65 |
| [Pt(PPh3)2Cl2] (V) | 68 | 90 | 93 | 85 | 80 | 80 |
| PtCl4 (VI) | 72 | 95 | 90 | 86 | 82 | 74 |
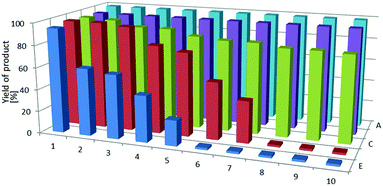
The results presented in Table 1 show that immobilization of complexes is not the only advantage obtained from the application of ionic liquids in the studied process. In the case of platinum complexes, the introduction of ionic liquid resulted in an increase of catalytic activity as indicated by higher product yields. Moreover, the reactions investigated were not accompanied by olefin isomerization and this fact also contributed to the increase in hydrosilylation product yield. The only complex that showed a bit lower activity in the presence of ionic liquids was Wilkinson catalyst, [RhCl(PPh3)3] (III). Taking into consideration the possibility of easy separation of the catalytic system from the reaction mixture and its multiple use in subsequent runs, it is important to determine if the catalytic activity is stable. For this reason in the further part of this study we have conducted the reaction of hydrosilylation with the reuse of the same catalytic system. By way of illustration, yields of the hydrosilylation product in 10 subsequent runs carried out in the presence of a system consisting of [Rh(PPh3)3Cl] complex dissolved in ionic liquids (A–E) are shown in Fig. 3. One can expect that if the above complex, whose catalytic performance in the first run in the presence of ionic liquids was worse than that of other rhodium complexes, maintains stable activity in the next runs, then other complexes will also perform well and this was why we chose this less active complex for multiple use tests.
Depending on the kind of ionic liquid employed, the product yield changed in subsequent reaction runs. The obtained results clearly show that in the case of the two former ILs (A and B) the hydrosilylation product yield almost did not change and the catalyst maintained its activity even after being used 10 times. Although the activity of the complex [Rh(PPh3)3Cl] immobilized in ionic liquids was somewhat lower in the first run, however, in the case of the liquids A and B the subsequent catalytic runs were characterized by invariably high yield. In contradistinction to the above liquids, in the presence of ionic liquids D and E the catalytic activity gradually decreased and after 7 or 5 reaction runs, respectively, the system became practically inactive. In order to determine the content of rhodium catalyst in the reaction product, inductively coupled plasma-optical emission spectroscopy (ICP-OES) was used. Unfortunately, the initial concentration (1 × 10−5 mole of Rh in the ionic liquid) was close to the limit of detection of the instrument employed. In the case of the samples from the product-containing reaction mixture, the concentration of rhodium was below the detection limit and for this reason we were unable to quantitatively determine the amount of rhodium catalyst present in the product.
We have also conducted another test consisting in adding new portion of substrates to the post-reaction mixture (after the separation of the catalytic system) and subjecting the mixture to heating at 120 °C for 1 hour.
If the catalyst was leached from the catalytic system by the substrates, one could expect that its presence in the post-reaction mixture will result in the continuation of the reaction, but no conversion was detected despite performing several tests with various post-reaction mixtures. Moreover, a homogeneity test has been carried out by adding mercury to the reaction system, but no difference was found in product yields compared to those obtained in reactions conducted in the absence of mercury.
In the further part of the study, measurements were performed of the activity of all catalytic systems, followed by their isolation after each run and reusing many times in octene hydrosilylation. Since TON (turnover number) is a suitable tool for comparing catalytic activities, TON values for all catalytic systems employed in the above reaction were calculated and listed in Table 2.
| Catalyst | Ionic liquid | ||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| a [Mor][HSO4] (A), [Mor][TolSO3] (B), [Mor][CH3SO3] (C), [Mor][NO3] (D), [Mor][NO2] (E). | |||||
| [{Rh(μOSiMe3)(cod)}2] (I) | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
| [{Rh(μ-Cl)(cod)}2] (II) | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
| [RhCl(PPh3)3] (III) | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| [Pt(PPh3)4] (IV) | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
7800 |
| [Pt(PPh3)2Cl2] (V) | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
| PtCl4 (VI) | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
The above results have proved that the kind of ionic liquid influences the catalytic system stability to a considerable extent. Although most of transition metal complexes are characterized by a high activity in the first run (see Table 1), the activity changes during subsequent runs. This can be explained either by different immobilization of the complexes in various ionic liquids or transformation of initial complexes into different catalysts under the influence of ionic liquids. Since all ILs studied contain the same cation, the reason should be sought in the effect of anion. The presence of anions from ionic liquids can cause the exchange with ligands present in the metal coordination sphere. Examples of the exchange of such a type can be found in the literature, particularly for organic reactions catalyzed by Lewis acids (e.g. Friedel–Crafts and Diels–Alder reactions) which in the presence of ionic liquids show large differences in catalytic activity depending on the kind of ionic liquid anion. It has been reported that catalytic activity increased with decreasing nucleophilicity of IL anion.36,37 Ionic liquids employed in our study contain anions originating from acids of different strength. Data listed in Table 3 show acidity constants (pKa) of the studied acids.
| Conjugate acid | pKa |
|---|---|
| H2SO4 | −3.2 |
| CH3C6H4SO3H | −2.8 |
| CH3SO3H | −1.9 |
| HNO3 | −1.4 |
| HNO2 | 3.3 |
As one can notice in Table 3, the lower pKa value the stronger acid, i.e. the weaker is the base conjugated with the acid (or, in other words, the weaker nucleophile). This means that coordinating ability of anions increases from top to bottom of the above table. As a result of ligand exchange, a weakly coordinated ligand appears in the metal coordination sphere, which during the catalytic reaction easily leaves the metal center. When the reaction is completed, coordination of the ligand proceeds again because it occurs in excess (as a component of ionic liquid).
However, the above explanation seems to be only one of possible reasons for changes observed in the catalytic activity of the investigated systems because other factors can be of influence, too. For instance, the results presented in Table 2 indicate that the lowest TON values were obtained in the case of reactions conducted in the liquids D and E. Taking into consideration that the anions NO3− and NO2− are characterized by oxidizing properties, whereas hydrogen siloxanes are reducing agents, a lower product yield can be a result of the above fact. On the other hand, while considering platinum compounds with different oxidation states, one can notice that the immobilization in the morpholinium ILs increased their activity (Table 1). Although the best first-run results were obtained in the presence of such a seemingly simple compound as PtCl4, a better stability was observed in the case of two other platinum complexes. However, a considerably higher activity and stability were shown by rhodium complexes.
Maybe, studies of stoichiometric reactions between metal complexes and ionic liquid, that are underway in our laboratory, will provide more information on the interactions between these compounds and permit to determine the real catalytic system. However, it is important that the catalytic systems developed in this study, particularly those based on rhodium complexes, are characterized by extremely high effectiveness in the process of hydrosilylation and enable multiple use of the catalytic system without loss of catalytic activity.
Conclusions
Studies on the catalytic activity of rhodium and platinum complexes for olefin hydrosilylation in the presence of morpholinium ionic liquids have shown that the introduction of the latter results in the increase in the activity and selectivity of catalytic systems formed, particularly those containing platinum complexes. Stability of the catalytic systems is influenced by the kind of ionic liquid anion: the weaker nucleophile is ionic liquid anion (i.e. the weaker its coordination ability) the more active catalytic system. Rhodium complexes immobilized in morpholinium ionic liquids have higher activity and stability compared to platinum complexes. From among platinum complexes with different oxidation states, PtCl4 has the highest initial activity, however, complexes with lower oxidation states (especially V) are characterized by a higher stability during subsequent catalytic reaction runs.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Financial support from the National Science Center (Poland), grant OPUS UMO-2014/15/B/ST5/04257, is gratefully acknowledged.References
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef PubMed.
- D. J. Adams, P. J. Dyson and S. J. Tavener, Chemistry in Alternative Reaction Media, Wiley, Hoboken, 2004 Search PubMed.
- Ionic Liquids in Synthesis, Ed. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, 2nd edn, 2008 Search PubMed.
- F. M. Kerton and R. Mariott, Alternative Solvents for Green Chemistry, RSC, Cambridge, 2nd edn, 2013 Search PubMed.
- T. Welton, Coord. Chem. Rev., 2004, 248, 2459–2477 CrossRef.
- P. J. Dyson and T. J. Geldbach, Metal Catalysed Reactions in Ionic Liquids, Springer, Dordrecht, 2005 Search PubMed.
- V. I. Parvulescu and C. Hardacre, Chem. Rev., 2007, 107, 2615–2665 CrossRef PubMed.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- H. Olivier-Bourbigou, L. Magna and D. Morvan, Appl. Catal., A, 2010, 373, 1–56 CrossRef.
- J. P. Hallet and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef PubMed.
- Y. Qiao, W. Ma, N. Theyssen, C. Chen and Z. Hou, Chem. Rev., 2017, 117, 6881–6928 CrossRef PubMed.
- R. L. Vekariya, J. Mol. Liq., 2017, 227, 44–60 CrossRef.
- Ionic Liquids (ILs) in Organometallic Catalysis, Ed. J. Dupont and L. Kollar, Springer, Heidelberg, 2015 Search PubMed.
- S. Aubin, F. Le Floch, D. Carrié, J. P. Guegan and M. Vaultier, in Ionic Liquids. Industrial Applications for Green Chemistry, Ed. R. D. Rogers and K. R. Seddon, ACS, Washington, 2002, pp. 334–346 Search PubMed.
- J. Van den Brocke, F. Winter, B. J. Deelman and G. Van Koten, Org. Lett., 2002, 4, 3851–3854 CrossRef.
- B. Weyershausen, K. Hell and U. Hesse, Green Chem., 2005, 7, 283–287 RSC.
- B. Weyershausen, K. Hell and U. Hesse, ACS Symp. Ser., 2005, 902, 133–143 CrossRef.
- T. J. Geldbach, D. Zhao, N. C. Castillo, G. Laurenczy, B. Weyershausen and P. J. Dyson, J. Am. Chem. Soc., 2006, 128, 9773–9780 CrossRef PubMed.
- N. Hofmann, A. Bauer, T. Frey, M. Auer, V. Stanjek, P. S. Schulz, N. Taccardi and P. Wasserscheid, Adv. Synth. Catal., 2008, 350, 2599–2609 CrossRef.
- H. Maciejewski, A. Wawrzyńczak, M. Dutkiewicz and R. Fiedorow, J. Mol. Catal. A, 2006, 257, 141–148 CrossRef.
- B. Marciniec, H. Maciejewski, K. Szubert and M. Kurdykowska, Monatshefte für Chemie, 2006, 137, 605–611 CrossRef.
- H. Maciejewski, K. Szubert, B. Marciniec and J. Pernak, Green Chem., 2009, 11, 1045–1051 RSC.
- J. Pernak, A. Świerczyńska, M. Kot, F. Walkiewicz and H. Maciejewski, Tetrahedron Lett., 2011, 52, 4342–4345 CrossRef.
- N. Taccardi, M. Fekete, M. Berger, V. Stanjek, P. S. Schulz and P. Wasserscheid, Appl. Catal., A, 2011, 399, 69–74 CrossRef.
- J. Pernak, N. Borucka, F. Walkiewicz, B. Markiewicz, P. Fochtman, S. Stolte, S. Steudte and P. Stepnowski, Green Chem., 2011, 13, 2901–2910 RSC.
- H. Maciejewski, K. Szubert and B. Marciniec, Catal. Commun., 2012, 24, 1–4 CrossRef.
- S. Rogalski, P. Żak, M. Miętkiewski, M. Dutkiewicz, R. Fiedorow, H. Maciejewski, C. Pietraszuk, M. Śmiglak and T. J. S. Schubert, Appl. Catal., A, 2012, 445–446, 261–268 CrossRef.
- H. Maciejewski, K. Szubert, R. Fiedorow, R. Giszter, M. Niemczak, J. Pernak and W. Klimas, Appl. Catal., A, 2013, 451, 168–175 CrossRef.
- Y. Xu, Y. Bai, J. Peng, J. Li, X. Xiao and G. Lai, J. Organomet. Chem., 2014, 765, 59–63 CrossRef.
- Y. Bai, F. Zhang, J. Li, Y. Xu, J. Peng and W. Xiao, J. Organomet. Chem., 2015, 794, 65–69 CrossRef.
- R. Kukawka, A. Pawlowska-Zygorowicz, M. Dutkiewicz, H. Maciejewski and M. Smiglak, RSC Adv., 2016, 6, 61860–61868 RSC.
- J. Peng, J. Li, Y. Bai, W. Gao, H. Qiu, H. Wu, Y. Deng and G. Lai, J. Mol. Catal. A, 2007, 278, 97–101 CrossRef.
- J. Peng, J. Li, Y. Bai, H. Qiu, K. Jiang, J. Jiang and G. Lai, Catal. Commun., 2008, 9, 2236–2238 CrossRef.
- D. L. Wang, J. Y. Li, J. J. Peng, Y. Bai and G. Q. Lai, Phosphorus, Sulfur Silicon Relat. Elem., 2011, 186, 2258–2266 CrossRef.
- B. Marciniec and P. Krzyżanowski, J. Organomet. Chem., 1995, 493, 261–266 CrossRef.
- J. H. Kim, J. W. Lee, U. S. Shin, J. Y. Lee, S.-G. Lee and C. E. Song, Chem. Commun., 2007, 4683–4685 RSC.
- J. W. Lee, J. Y. Shin, Y. S. Chun, H. B. Jang, C. E. Song and S.-G. Lee, Acc. Chem. Res., 2010, 43, 985–994 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |