Open Access Article
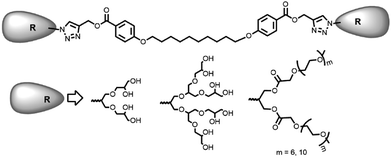
Open Access ArticleSynthesis of non-ionic bolaamphiphiles and study of their self-assembly and transport behaviour for drug delivery applications†
Rashmia,
Abhishek K. Singha,
Katharina Achazib,
Boris Schade c,
Christoph Böttcherc,
Rainer Haagb and
Sunil K. Sharma
c,
Christoph Böttcherc,
Rainer Haagb and
Sunil K. Sharma *a
*a
aDepartment of Chemistry, University of Delhi, Delhi 110 007, India. E-mail: sksharma@chemistry.du.ac.in; Tel: +91-11-27666646
bInstitut für Chemie und Biochemie, Freie Universität Berlin, Takustraße 3, 14195 Berlin, Germany. E-mail: haag@zedat.fu-berlin.de; Fax: +49-30-838-452633; Tel: +49-30-838-52633
cForschungszentrum für Elektronenmikroskopie, Institut für Chemie und Biochemie, Freie Universität Berlin, Fabeckstraße 36a, 14195 Berlin, Germany
First published on 12th September 2018
Abstract
A series of four bolaamphiphiles with different hydrophilic units has been synthesised. All the amphiphiles were well characterised from their physiochemical data. The aggregation tendency of newly synthesised amphiphiles was studied using fluorescence spectroscopy, dynamic light scattering (DLS), and cryogenic electron microscopy (cryo-TEM). Furthermore, their application as nanocarriers for hydrophobic guests was demonstrated by using two established standards, i.e. the dye Nile red and the drug nimodipine. A cytotoxicity and cellular uptake study has been carried out using A549 cells. Due to the presence of an ester linkage in PEG based bolaamphiphiles, a drug release study was performed in the presence of an immobilized enzyme Novozym-435 (a lipase).
Introduction
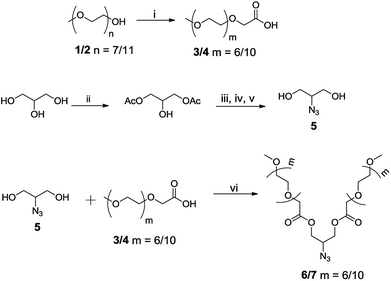
Inspired by nature, the self-assembly of amphiphilic molecules mimics various biological systems depending on their structure, concentration, solution environment, and temperature. The spontaneous organization of sophisticated molecules like lipids, proteins, saccharides and nucleic acids provides vital information to understand the factors responsible for their self-assembly. Various non-covalent interactions like hydrogen bonding, hydrophobic interactions, π–π stacking, etc. provide the momentum for self-aggregation. In recent years, this concept has largely been used for various applications in biomedical and material sciences.1–8 As many drugs suffer from limited aqueous-solubility and shorter half-life in the bloodstream, resulting in their limited bioavailability, the supramolecular nano-carriers have shown promising behaviour in the target specific drug delivery. For the enhanced therapeutic action of such drugs, these are encapsulated in the hydrophobic core of the amphiphiles and the hydrophilic groups surfaced the assembly to keep the guest intact from aqueous medium and solubilize the whole molecule.9–18 For the past decades the amphiphilic architectures have earned considerable attention to such delivery systems. To explore the usefulness of such systems, new amphiphilic architectures have been designed that can be used as nano-carriers for encapsulation and release of drug/dyes in a systematic manner. Among various amphiphilic architectures studied, bolaamphiphiles with two hydrophilic end groups separated by a hydrophobic spacer, exhibit unique hierarchically self-assembled structures from micelles to large cylindrical, vesical and monolayer formation at air–water interface. Furthermore, compared to the conventional surfactants, bolaamphiphiles have higher aqueous solubility and reduced critical aggregation concentration.19,20 Although a number of ionic and non-ionic bolaamphiphiles constituted from carbohydrates and amino acids have been reported for various pharmaceutical applications,21–27 however the bolaamphiphiles based on polyethylene glycol (PEG) and dendritic polyglycerol (dPG) are not very common besides being biocompatible, nontoxic, stable, and aqueous soluble. Furthermore, PEG and PG are known to evade recognition by the reticulo-endothelial system (RES), which in turn qualify them for use in drug delivery applications.28–35Herein we report the synthesis of four different pegylated and dendronized symmetrical bolaamphiphiles by coupling together, a novel hydrophobic aryl–alkyne conjugate with different hydrophilic PEG/dPG azides by following the CuAAC methodologies (Fig. 1). The synthesized bolaamphiphiles were characterized using NMR, IR, HRMS and GPC. The aggregation behaviour of amphiphiles was studied using DLS and cryo-TEM. Their drug encapsulation potential and release profile was studied using UV and fluorescence spectroscopy.
Results and discussion
Synthesis and characterization
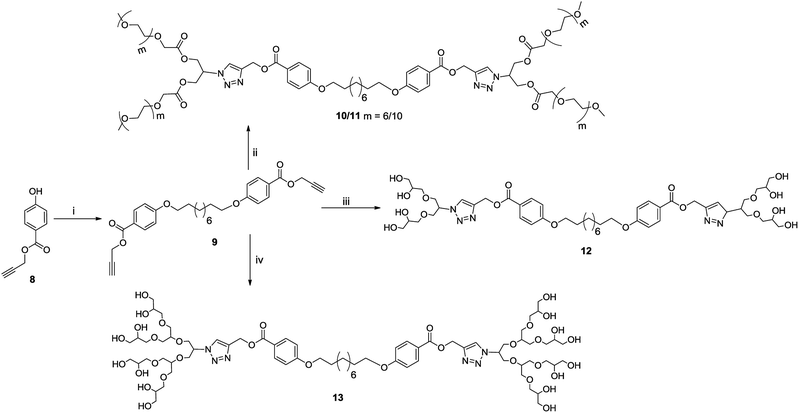
The chemical and physical properties of amphiphilic systems vary with respect to their hydrophobic and hydrophilic content. To systematically explore the influence of hydrophilic groups on aggregation behaviour, i.e. size, morphology, encapsulation and release profile of guests, etc., bolaamphiphiles with different polar head groups were synthesised by coupling together hydrophobic and hydrophilic moiety using CuAAC methodologies. Four symmetrical bolaamphiphiles have been synthesised by utilizing PEG (Mn: 350/550) and dendritic polyglycerol [G1.0/G2.0] as hydrophilic moieties. The synthesis of hydrophilic groups 6/7 involves acylation of azidodiol 5 with mono methoxy PEG acid [(Mn: 350/550) 3/4 ], mPEG acids (3/4) and azidodiol were in turn prepared by following literature procedure (Scheme 1).36 G1.0 and G2.0 dendritic polyglycerol azides (dPGs azides) were also synthesised by following literature procedure.37 For the synthesis of the hydrophobic core, p-hydroxybenzoic acid was first converted quantitatively to its propargyl ester 8 by using propargyl bromide in the presence of a mild base. Compound 8 was then reacted with 1,10-dibromodecane on its both ends to yield the desired diacetylinic product 9. The hydrophobic alkyne and PEG/dPG azides were then subjected to click reaction to obtain the target bolaamphiphiles (10–13) (Scheme 2). All the bolaamphiphiles synthesized were characterized on the basis of their physical and spectral data i.e. 1H and, 13C-NMR spectroscopy, HRMS and gel permeation chromatography (GPC).Critical aggregation concentration (CAC)
The CAC of bolaamphiphiles (10–13) was determined by fluorescence measurement using a hydrophobic dye Nile red. The assembly was initially probed with Nile red by dissolving respective bolaamphiphiles in water. The plot of fluorescence intensity of encapsulated Nile red versus log[amphiphile conc.] gave the CAC value at the point of inflection, and it was observed to be in the range of 1.5 × 10−4 to 9.2 × 10−5 M (Table 1). Employing the Griffin equation38 for calculating HLB (Hydrophilic–Lipophilic Balance) shows that in the pegylated bolaamphiphiles Bola-PEG6 with low HLB exhibit higher CAC value as compared to the Bola-PEG10 having an enhanced hydrophilic character. A similar behaviour was observed with dendronized bolaamphiphiles i.e. Bola-[G2.0] having a higher HLB exhibit lower CAC as compared to Bola-[G1.0] (Fig. S16, see the ESI†).Dynamic light scattering (DLS) and cryo-TEM measurement
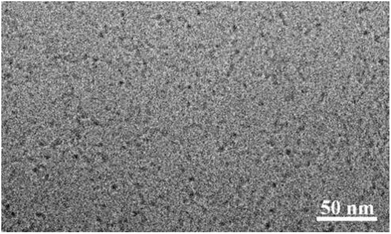
The hydrodynamic size of the particles was determined by dynamic light scattering (DLS) technique at a concentration of 5.0 mg mL−1. The particle size of the nanoparticles in the aqueous solution obtained from DLS was observed to be in the range 4 to 7 nm (Fig. 2) for all the four bolaamphiphiles synthesized. The size distribution was monomodal in volume and number, but bimodal in intensity. In the case of bimodular system, the two peaks were assigned for molecular aggregates assemblies, whereas single peaks in volume and number corresponds to simple molecular assembly in aqueous medium, indicating that mainly small size particles are formed as compared to larger molecular aggregates. Nanoparticles of up to 200 nm size show prolonged delivery of therapeutic agents and exhibit higher drug uptake compared to microparticles.39 The nano sized aggregates formed by the bolaamphiphiles in this study were found to be of appropriate size for studying their biological applications. As the amphiphiles in aqueous medium agglomerate, so the actual size and morphology cannot be evaluated rather a hydrodynamic size is obtained from the DLS study. For the precise measurement of particle size and to visualize the actual morphology in aqueous medium cryo-TEM technique was utilized for one of the representative amphiphile Bola-[G1.0]. The cryo-TEM image for Bola-[G1.0] (Fig. 3) shows small elongated aggregates with an average diameter of ∼3 nm, which is in good agreement with the DLS data by considering the fact that the hydrodynamic size is generally greater than the visible assembly structure.40 Such smaller molecular aggregates are of optimum size to be considered for therapeutic applications.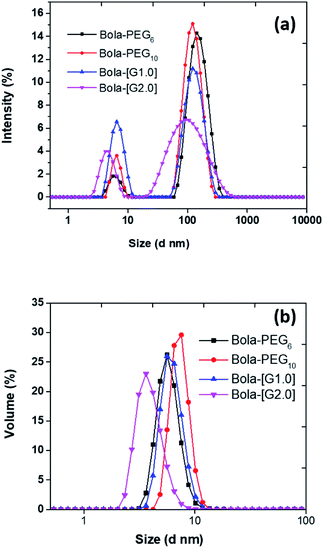 | ||
| Fig. 2 Plot of (a) intensity versus size; (b) volume versus size for calculating hydrodynamic size (nm) of bolaamphiphiles obtained from dynamic light scattering (DLS). | ||
Cytotoxicity study
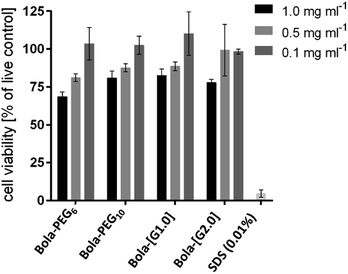
Cytotoxicity of the bolaamphiphiles was assessed by an MTS assay using A549 lung cancer cells. All of the amphiphiles showed a similar trend i.e. low toxicity at the highest test concentration of 1 mg mL−1 (Fig. 4). However, at 0.1 mg mL−1, all the bolaamphiphiles were found to be non-cytotoxic and thus suitable for biological applications.Encapsulation study
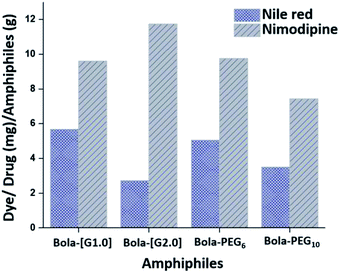
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 at 552 nm for the dye. In accordance with the HLB values, the dendronized bolaamphiphiles constituted from [G1.0] dendron (Bola-[G1.0]) exhibit higher encapsulation of Nile red 5.68 mg g−1 as compared to its analogue Bola-[G2.0]. Similarly, among the pegylated bolaamphiphiles, the one with a higher lipophilic ratio, i.e. Bola-PEG6 encapsulated more amount of Nile red as compared to Bola-PEG10 (Fig. 5).
000 M−1 cm−1 at 552 nm for the dye. In accordance with the HLB values, the dendronized bolaamphiphiles constituted from [G1.0] dendron (Bola-[G1.0]) exhibit higher encapsulation of Nile red 5.68 mg g−1 as compared to its analogue Bola-[G2.0]. Similarly, among the pegylated bolaamphiphiles, the one with a higher lipophilic ratio, i.e. Bola-PEG6 encapsulated more amount of Nile red as compared to Bola-PEG10 (Fig. 5).
Cellular uptake study
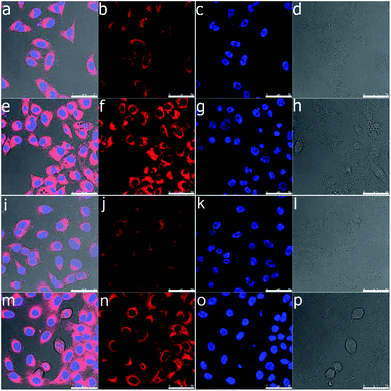
Confocal laser scanning microscopy was used to analyse the cellular uptake behaviour of Nile red loaded bolaamphiphiles Bola-PEG6 and Bola-[G1.0] to simulate drug transport by the amphiphiles. A test concentration of 0.1 mg mL−1 of the bolaamphiphiles was chosen considering the toxicity study results. Both the amphiphiles exhibit a similar uptake pattern of Nile red inside the cell. Cellular uptake in the cell was seen even after the first hour of incubation (Fig. 6). After 24 h, the bolaamphiphiles were observed to be accumulated in the cells' cytosol. However, no Nile red was visible in the nucleus. The results indicate that the amphiphiles are well suited to carry drugs in the cytosol of cells.Enzyme-triggered release of encapsulated guest
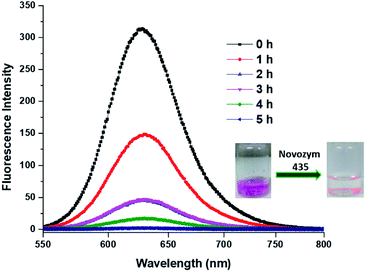
Besides encapsulation, the release of encapsulated therapeutic entity is also significantly important. We studied the enzyme triggered release of the encapsulated guest from the inner hydrophobic core of amphiphilic aggregates. Since the amphiphiles Bola-PEG6 and Bola-PEG10 constitute from ester functionality, we studied the enzyme mediated ester cleavage. Such an act can be triggered in biological systems as a number of hydrolytic enzymes are available over there.We performed the enzyme mediated release study using the same hydrolytic enzyme that was used for the synthesis of bolaamphiphiles i.e. immobilized Candida antarctica lipase (Novozym-435). The release study was performed in dark and at 37 °C. It was observed that within 5 h of enzyme exposure a complete release of encapsulated dye takes place, however the control experiment performed without the enzyme does not exhibit any noticeable release of encapsulated dye (Fig. 7).
Conclusions
A set of four linear and dendronized bolaamphiphiles have been synthesised in a chemo-enzymatic manner by employing the immobilized enzyme Novozym 435 and following a CuAAC methodologies. Furthermore, biocompatible materials like polyethylene glycol (PEG) and polyglycerol dendrons (dPGs) were used as building blocks. The bolaamphiphiles synthesised were well characterized from their spectroscopic and physical data, e.g. 1H-NMR, 13C-NMR, HRMS, and GPC techniques. Fabrication of nano-carrier has been achieved by allowing the amphiphile aggregation in aqueous medium. The size of the aggregates was studied using DLS and cryo-TEM. Nile red as a model dye and nimodipine as a model drug were used for evaluating the encapsulation potential of amphiphiles.Bola-PEG6 and Bola-[G1.0] exhibit a higher encapsulation tendency than their larger pegylated and dendronized analogues i.e. Bola-PEG10 and Bola-[G2.0] respectively. The enzyme mediated release of the encapsulated dye was also studied using Bola-PEG6, which shows an efficient dye release from the hydrophobic core of the amphiphile. Cell viability study shows that all bolaamphiphiles are well tolerated upto a concentration of 0.5 mg mL−1. In conclusion the good biocompatibility, the higher drug loading capacity and fast enzymatic release shows that the synthesised bolaamphiphiles can be used as a starting point to design and develop efficient nanocarriers for drugs and dyes.
Conflicts of interest
There is no conflicts of interest to be declare.Acknowledgements
We gratefully acknowledge the DST, Government of India and DFG, Germany and the core facility BioSupraMol, FU-Berlin for supporting a collaborative research project financial assistance and UGC-CSIR, New Delhi, for providing fellowship to Rashmi.References
- M. Ramanathan, L. K. Shrestha, T. Mori, Q. Ji, J. P. Hill and K. Ariga, Phys. Chem. Chem. Phys., 2013, 15, 10580–10611 RSC.
- A. Sorrenti, O. Illa and R. M. Ortun, Chem. Soc. Rev., 2013, 42, 8200–8219 RSC.
- P. A. Albouy, S. Deville, A. Fulkar, K. Hakouk, M. Imperor-Clerc, M. Klotz, Q. Liu, M. Marcellinib and J. Perezd, Soft Matter, 2017, 13, 1759–1763 RSC.
- P. Calandra, D. Caschera, V. T. Liveri and D. Lombardoc, Colloids Surf., A, 2015, 484, 164–183 CrossRef.
- M. Lee, B. K. Cho and W. C. Zin, Chem. Rev., 2001, 101, 3869–3892 CrossRef PubMed.
- J. A. A. W. Elemans, A. E. Rowan and R. J. M. Nolte, J. Mater. Chem., 2003, 13, 2661–2670 RSC.
- C. Wang, Z. Wang and X. Zang, Acc. Chem. Res., 2012, 45, 608–618 CrossRef PubMed.
- Y. Tu, F. Peng, A. Adawy, Y. Men, L. K. E. A. Abdelmohsen and D. A. Wilson, Chem. Rev., 2016, 116, 2023–2078 CrossRef PubMed.
- R. Lin and H. Cui, Curr. Opin. Chem. Eng., 2015, 7, 75–83 CrossRef.
- R. Haag, Angew. Chem., Int. Ed., 2004, 43, 278–282 CrossRef PubMed.
- H. Chen, C. Khemtong, X. Yang, X. Cjang and J. Gao, Drug Discovery Today, 2011, 16, 354–360 CrossRef PubMed.
- P. Khadka, J. Ro, H. Kim, I. Kim, J. T. Kim, H. Kim, J. M. Cho, G. Yun and J. Lee, Asian J. Pharm. Sci., 2014, 9, 304–316 CrossRef.
- N. A. Kotov, Nanostruct. Mater., 1999, 12, 789–796 CrossRef.
- S. Prasad, K. Achazi, C. Bottcher, R. Haag and S. K. Sharma, RSC Adv., 2017, 7, 22121–22132 RSC.
- X. Hu, R. Liu, D. Zhang, J. Zhang, Z. Li and Y. Luan, ACS Biomater. Sci. Eng., 2018, 4, 973–980 CrossRef.
- W. He, X. Hu, W. Jiang, R. Liu, D. Zhang, J. Zhang, Z. Li and Y. Luan, Adv. Healthcare Mater., 2017, 6, 1700829–1700842 CrossRef PubMed.
- J. Zhang, D. Zhang, X. Hu, R. Liu, Z. Lib and Y. Luan, RSC Adv., 2018, 8, 13103–13111 RSC.
- P. Zhang, H. Zhang, W. He, D. Zhao, A. Song and Y. Luan, Biomacromolecules, 2016, 17, 1621–1632 CrossRef PubMed.
- M. Fariya, A. Jain, V. Dhawan, S. Shah and M. S. Nagarsenker, Adv. Pharm. Bull., 2014, 4, 483–491 Search PubMed.
- N. Nuraje, H. Bai and K. Su, Prog. Polym. Sci., 2013, 38, 302–343 CrossRef.
- S. Drescher, A. Meister, V. M. Garamus, G. Hause, C. J. Garvey, B. Dobner and A. Blume, Eur. J. Lipid Sci. Technol., 2014, 116, 1205–1216 CrossRef.
- M. Popov, S. Grinberg, C. Linder, T. Waner, B. Levi-Hevroni, R. J. Deckelbaum and E. Heldman, J. Controlled Release, 2012, 160, 306–314 CrossRef PubMed.
- Y. Jin, N. Qia, L. Tong and D. Chen, Int. J. Pharm., 2010, 386, 268–274 CrossRef PubMed.
- C. Lee and S. Lee, RSC Adv., 2017, 7, 38989–38997 RSC.
- Z. Khan, S. A. Al-Thabaiti and M. A. Malik, RSC Adv., 2016, 6, 45993–46001 RSC.
- A. M. Reddy, A. Sharma, Q. Maqbool and A. Srivastava, RSC Adv., 2013, 3, 18900–18907 RSC.
- S. Schmid, D. Y. W. Ng, E. Mena-Osteritz, Y. Wu, T. Weil and P. Bäuerle, Chem. Commun., 2016, 52, 3235–3238 RSC.
- Y. Zhang, E. Mintzer and K. E. Uhrich, J. Colloid Interface Sci., 2016, 482, 19–26 CrossRef PubMed.
- K. J. Harrington, G. Rowlinson-Busza, K. N. Syrigos, P. S. Uster, R. G. Vile and J. S. W. Stewar, Clin. Cancer Res., 2000, 6, 2528–2537 Search PubMed.
- T. M. Allen, C. Hansen, F. Martin, C. Redemann and A. Yau-Young, Biochim. Biophys. Acta, 1991, 1066, 29–36 CrossRef.
- B. Obermeie, F. Wurm, C. Mangold and H. Frey, Angew. Chem., Int. Ed., 2011, 50, 7988–7997 CrossRef PubMed.
- S. Gupta, R. Tyagi, V. S. Parmar, S. K. Sharma and R. Haag, Polymer, 2012, 53, 3053–3078 CrossRef.
- B. N. S. Thota, L. H. Urner and R. Haag, Chem. Rev., 2016, 116, 2079–2102 CrossRef PubMed.
- A. K. Singh, B. N. S. Thota, B. Schade, K. Achazi, A. Khan, C. Böttcher, S. K. Sharma and R. Haag, Chem.–Asian J., 2017, 12, 1796–1806 CrossRef PubMed.
- L. H. Urner, B. N. S. Thota, O. Nachtigall, S. Warnke, G. Helden, R. Haag and K. Pagel, Chem. Commun., 2015, 51, 8801–8804 RSC.
- S. Gupta, B. Schade, S. Kumar, C. Böttcher, S. K. Sharma and R. Haag, Small, 2013, 9, 894–904 CrossRef PubMed.
- M. Wyszogrodzka and R. Haag, Chem.–Eur. J., 2008, 14, 9202–9214 CrossRef PubMed.
- W. C. Griffin, J. Soc. Cosmet. Chem., 1949, 1, 311–326 Search PubMed.
- R. Singh and J. W. Lillard Jr, Exp. Mol. Pathol., 2009, 86, 215–223 CrossRef PubMed.
- B. N. S. Thota, H. Berlepsch, C. Böttcher and R. Haag, Chem. Commun., 2015, 51, 8648–8651 RSC.
- M. Kumari, S. Gupta, K. Achazi, C. Böttcher, J. Khandare, S. K. Sharma and R. Haag, Macromol. Rapid Commun., 2015, 36, 254–261 CrossRef PubMed.
- M. Kumari, M. Billamboz, E. Leonard, C. Len, C. Böttcher, A. K. Prasad, R. Haag and S. K. Sharma, RSC Adv., 2015, 5, 48301–48310 RSC.
- G. M. Soliman, R. Sharma, A. O. Choi, S. K. Varshney, F. M. Winnik, A. K. Kakkar and D. Maysinger, Biomaterials, 2010, 31, 8382–8392 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra05921g |
| This journal is © The Royal Society of Chemistry 2018 |