Open Access Article
Open Access ArticleRetracted Article: Titanium dioxide nanoparticles induce mitochondria-associated apoptosis in HepG2 cells
Zhenglin Xia†
 a,
Jingliang He†b,
Bowei Lia,
Ke Hea,
Wenbing Yangc,
Xiaoxun Chend,
Jinqian Zhang
a,
Jingliang He†b,
Bowei Lia,
Ke Hea,
Wenbing Yangc,
Xiaoxun Chend,
Jinqian Zhang *e and
Guoan Xiang*a
*e and
Guoan Xiang*a
aDepartment of General Surgery, Guangdong Second Provincial General Hospital, Southern Medical University, Guangzhou 510515, People's Republic of China. E-mail: guoan_66@163.com
bShunde Hospital of Guangzhou University of Chinese Medicine, Foshan 528300, People's Republic of China
cDepartment of General Surgery, The Second Affiliated Hospital of Xi'an Jiao Tong University, Xi'an 710004, People's Republic of China
dDepartment of Gastrointestinal Surgery, The Guigang City People's Hospital, Guigang, Guangxi 537100, People's Republic of China
eDepartment of Laboratory Medicine, Guangdong Second Provincial General Hospital, Southern Medical University, Guangzhou 510515, People's Republic of China. E-mail: jingwanghou@163.com
First published on 12th September 2018
Abstract
Widespread applications of nanosized materials over the past decade have prompted investigations of desirable properties and potential hazards to humans and the environment. Titanium dioxide (TiO2) nanoparticles are one of the most widely used nanoparticles. To investigate the effect of biological functions induced by TiO2 nanoparticles (10 nm: TiO2 NPs) on human liver cell lines, normal liver cell line L02 and hepatoma cell line HepG2 were co-cultured with exogenous TiO2 NPs. Cell growth and proliferation, cell cycle, and the apoptosis rate were analyzed. The effects of TiO2 NPs on the expression levels of apoptosis-associated protein caspase-3 and the membrane channel protein αENaC and caspase-3/7 activity were determined. Moreover, the influence of TiO2 NPs on the expression levels of the mitochondria-related proteins SIRT3, VDAC1, and ACSS1, the mitochondrial membrane potential and the ADP/ATP ratio were also examined. Our results revealed that TiO2 NPs inhibited the growth and proliferation of HepG2 cells, suppressed the S phase of cell cycling, and induced apoptosis of HepG2 cells. Following an increase in concentration, the inhibitory effect induced by TiO2 NPs on proliferation and cell cycle was more evident, and the apoptosis rate increased in a significant concentration-dependent manner, whereas there was no significant effect on the growth, proliferation, apoptosis, and cell cycle of L02 cells. In addition, the results of western blot showed that in HepG2 cells, TiO2 NPs upregulated the expressions of the apoptosis-related protein caspase-3 and the membrane channel protein αENaC in a concentration-dependent manner. However, in L02 cells, there was no significant difference in the expression levels of caspase-3 or αENaC. Furthermore, TiO2 NPs induced depolarization of the mitochondrial membrane, upregulated the expression levels of the mitochondria-related proteins SIRT3 and VDAC1, and downregulated the expression level of the key respiratory chain protein ACSS1 in HepG2 cells. However, in L02 cells, the expressions of SIRT3, VDAC1, and ACSS1 exhibited no clear change. The apoptosis of HepG2 cells induced by TiO2 NPs may be achieved by regulating intracellular osmotic pressure; moreover, upregulating the expression of the channel protein αENaC or the mitochondrial porin VDAC1 and depolarizing the mitochondrial membrane of HepG2 cells resulted in the loss of Cyt-c and ATP and further activated caspase-3. To further confirm the above results, a nude mouse xenograft model was employed. After a certain period of treatment with TiO2 NPs, the nude mice were sacrificed, tumors were removed, and the expression of related proteins was detected. Immunohistochemistry and western blot results showed that the expressions of the proteins VDAC1 and SIRT3 were clearly upregulated in tissues treated to TiO2 NPs, whereas the expression of ACSS1 was downregulated. The results were consistent with the above in vitro results. All the above results confirmed that TiO2 NPs can act as a safe antitumor agent.
Introduction
Hepatocellular carcinoma (HCC) is a common malignant tumor with high degree of malignancy and rapid development. However, due to the lack of early and accurate diagnosis and effective targeted therapy, its treatment is difficult, which results in high mortality rate.1 The pathogenesis of HCC, which is always one of the most important problems in the prevention and treatment of liver cancer, is still not completely clear.2 The clarification of its mechanism, especially the pathogenesis of HBV-related HCC, and the discovery of effective medicines are most urgent.Nanoparticles are a kind of nanoscale ultrafine material (10−9 m). The outer surface of a nanoparticle has a unique structure, due to which it can not only absorb various molecules via non-covalent forces, but also combine with various chemical groups when the interior space is loaded with ions and small molecules.3,4 Moreover, nanoparticles can cross the cell membrane. They may be used for drug delivery and gene therapy in biomedicine as a result of these beneficial properties. In general, nanoparticles (such as titanium dioxide (TiO2)) have great prospects in terms of applications, especially in the treatment of carcinoma.5–8
Recent studies have shown that long-term exposure to TiO2 NPs can lead to the accumulation of titanium, which can result in DNA damage, changes in gene expressions, inflammatory responses, and liver dysfunction in vivo and in vitro.9–11 TiO2 NPs are also involved in the mitotic index, cell apoptosis, oxidative stress, metabolic processes, cell cycle, signal transduction and cell proliferation, and most of these effects might be caused by a genotoxic mechanism. However, studies involving the relationship between TiO2 NPs and liver cancer are very rare. For the first time, we have attempted to investigate the biological function induced by TiO2 NPs in hepatocellular carcinoma by introducing different concentrations of TiO2 NPs into liver cancer cells and a mouse tumor xenograft model.
Materials and methods
Characterization and analysis of TiO2 NPs
TiO2 NPs are spherical aggregates with anatase-like structures, and the diameter of the aggregates is about 10 nm. The elemental contents of TiO2 NPs were determined using Elementar Analysensysteme GmbH (element analyzer), and the other elemental contents were detected by an S4 Explorer X-ray fluorescence spectrometer.Using a charge-coupled device (CCD) diffractometer (Mercury 3 Versatile CCD Detector, Rigaku Corporation, Tokyo, Japan) employing Ni-filtered Cu Kα radiation, the X-ray diffraction (XRD) patterns of TiO2 NPs were acquired at room temperature.
According to the Brunauer–Emmett–Teller (BET) theory, an Autosorb iQ Station 2 surface area analyzer was used to determine the mesopore size and surface area of TiO2 NPs.
This theory was used to serve as the basis for a crucial analysis technique for the measurement of the specific surface area of TiO2 NPs and to explain the physical adsorption of gas molecules on a solid surface. The particle size of TiO2 NPs (10 μg mL−1 aqueous suspension) was examined by dynamic light scattering (DLS) at room temperature using Zetasizer Nano ZS (Malvern Instrument Ltd).12
The samples were placed under a JEOL JSM-7401 (JEOL Ltd., Tokyo, Japan) scanning electron microscope (SEM) to analyze their morphology. SEM was operated at 100 and 10 kV. Thermogravimetric analysis was carried out with a Q50TGA thermogravimetric analyzer (Thermal Analysis, Inc., New Castle, DE, USA) from room temperature to 1173 K at a rate of 10 K min−1 under an air flow of 30 mL min−1.13
Preparation of plates coated with TiO2 NPs
Solutions of TiO2 NPs with different concentrations of 2.5 μg mL−1, 5 μg mL−1, 7.5 μg mL−1, and 10 μg mL−1 were prepared with alcohol. The solutions of dispersed TiO2 NPs were added to 6-well plates (400 μL per well) or 96-well plates (10 μL per well) and then dried at 100 °C for 2 h in air; subsequently, they were sterilized by ultraviolet irradiation for half an hour.Cell culture and treatment
The human normal hepatic cell line L02 and hepatoma cell line HepG2 obtained from the American Type Culture Collection (Manassas, VA, USA) were seeded into 6-well plates coated with TiO2 NPs and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Gibco, Life Technologies, NY, USA), 100 units per mL penicillin G, and 100 μg mL−1 streptomycin (SW30010, BD, CA, USA) at 37 °C with 5% CO2.Cell counting
L02 (3 × 105) and HepG2 cells (3 × 105) were added to 6-well plates treated by the above methods with 2 mL medium and then, the cells were observed according to the general protocol by an optical microscope. The total cellular scores were counted using a cell counter plate after 48 h.Determination of cellular proliferation and viability
A Cell Counting Kit-8 (CCK-8) (Dojindo, Japan) was used to determine cell viability. Cells were pretreated as described above and then incubated in 96-well plates. After 12, 24 or 48 h of incubation, 10 μL CCK-8 solution was added to each well. Then, the cells were incubated at 37 °C for 4 h, and absorbance values were measured with a plate reader at 450 nm. Each group included five repeated wells. The experiment was based on five independent tests, and the proliferation of cells was observed every time at a certain time point.Cell cycle analysis
Cells (3 × 105) were cultured in uncoated and TiO2 NP-coated dishes for 48 h. After that, the cells were fixed and stained with 7-AAD. The cell cycle was determined by a flow cytometer (BD, USA).Determination of apoptosis
After floating, the detached cells were collected and then treated with an Annexin V-FITC/7-AAD apoptosis detection kit (640906/420404, BioLegend, CA, USA) according to the manufacturer's protocol. Cells were analyzed by flow cytometry (Epics Altra II, Beckman Coulter, CA, USA). The apoptosis rate of cells was measured by Beckman Coulter Epics Altra with the Expo32 software (Beckman Coulter, CA, USA).Caspase-3/7 activity assay
For the caspase-3/7 assay, L02 and HepG2 cells were inoculated into 96-well plates treated by the above methods for 48 h. Then, the culture media were removed, and the Caspase-Glo® 3/7 assay kit (G8092, Promega, CA, USA) was used for determination according to the manufacturer's protocol. Fluorescence was measured with excitation/emission at 485/530 nm using a plate reader (Thermo, USA).Mitochondrial membrane polarization measurement
JC-10 (Enzo Life Sciences, NY) was used to measure the effect of TiO2 NPs on the mitochondrial membrane potential in cells. JC-10 is a cationic fluorophore that is rapidly absorbed by cells and mitochondria due to its negative charge in cells. When the mitochondrial membrane is hyperpolarized, the polymeric form of JC-10 is a J-aggregate that emits fluorescence at 590 nm (red fluorescence). When the mitochondrial membrane is depolarized, the remaining JC-10 in the cytosol retains its monomeric form and emits fluorescence at 525 nm (green fluorescence). After treatment with TiO2 NPs, L02 and HepG2 cells were washed twice and then incubated with 500 μL of 1× JC-10 dye loading solution for 30 min. Then, the cells were analyzed by a flow cytometer (BD Biosciences, San Diego, CA, USA) in the FL1 channel (signal of green fluorescent monomers) and FL2 channel (signal of orange fluorescent aggregates). The intensity ratio of FL1 to FL2 was used to monitor the change in the mitochondrial membrane potential.ADP/ATP ratio assay
L02 and HepG2 cells were inoculated into 96-well plates treated by the above methods and incubated for 24 and 48 h, respectively. After incubation, the culture media were removed and then, an ADP/ATP ratio assay kit (ab65313, Abcam, Cambridge, MA, USA) was used for examination as described in the manufacturer's protocol. Fluorescence was determined in terms of luminescence using a plate reader (Thermo, USA).Western blotting and antibodies
Cells were lysed in a lysis buffer containing a protease inhibitor cocktail (5872s, CST, MA, USA). The protein concentration was determined by the Pierce BCA assay (23225, Thermo Scientific, PA, USA). Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a PVDF membrane (ISEQ00010, Millipore, MA, USA) by electroblotting. After blocking with 5% nonfat dried milk (2321000, BD, MD, USA), the membranes were reacted with primary antibodies such as anti-caspase-3 (sc-7148, Santa Cruz), anti-αENaC (sc-21012, Santa Cruz), anti-SIRT3 (2627, CST), anti-VDAC1/porin antibody-mitochondrial loading control (ab15895, Abcam), anti-ACSS1 (ab69270, Abcam), and anti-GAPDH (5174, CST) in Tris-buffered saline containing 0.1% Tween-20 supplemented with 5% nonfat dried milk. Protein bands were visualized using an enhanced chemiluminescence (ECL) detection kit (Promega, CA, USA).Preparation of animal tissue and volume measurement
Nude mice were purchased from Laboratory Animal Sciences (Southern Medical University). The experiment was initiated with 6 weeks-old mice weighing 15–20 g. HepG2 cell lines were resuspended at 1 × 107 cells per mL, and 0.1 mL of the cell suspension was subcutaneously injected into the right flanks of nude mice (n = 5/group). When tumors in the mice reached 4–10 mm in size, the experiment was initiated, and this was regarded as the 1st day. TiO2 was formulated at a concentration of 0.5 mg mL−1 in PBS. The nude mice were randomly divided into 2 groups and were injected with either TiO2 (50 μL) or vehicle once every two days for 4 times. We measured the volumes of the tumors once every two days before the injections until the 21st day. The mice were sacrificed on the 21st day. Moreover, tumors were obtained and a tumor mass was dissected from each mouse. Then, the tumors were cut in half and stored until the next experiment. The experimental mice were treated according to the standards supported by the Animal Protection Committee of Southern Medical University.All animal procedures were performed in accordance with the Guidelines for Care and Use of the Animal Protection Committee of Southern Medical University, and experiments were approved by the Animal Ethical and Welfare Committee of Southern Medical University (reference no. IACUC-2017-0922).
Immunohistochemical staining
Immunohistochemical reactions were carried out using the labeled streptavidin-biotin method. Tumors dissected from mouse xenograft models were fixed in 10% formalin solution overnight at 4 °C, and sections of 6 μm were cut and embedded in paraffin. Deparaffinized sections were heated for 5 min at 100 °C in a pressure cooker to reactivate the antigens and blocked with hydrogen peroxide. After being washed three times, the samples were incubated with antibodies at 4 °C overnight. The primary antibodies included anti-ACSS1 (Affinity, DF3727, 1:200), anti-VDAC1 (Affinity, DF6140, 1:200), and anti-SIRT3 (Affinity, AF5135, 1:200). After incubation with a secondary antibody at room temperature for 1 h, the samples were washed, and staining was developed by incubation with a DAB substrate kit (Pierce). Immunostained sections were assessed and photographed under a microscope at magnification of 200× (IX71, Olympus, Japan).Tissue protein extraction
Tumor tissues were crushed by a high-speed dispersion cutter on ice and then lysed in a lysis buffer containing a protease inhibitor cocktail (5872s, CST, MA, USA). We carried out lysis on ice for 30 min with oscillation every 5 min. The lysates were centrifuged by a freeze centrifuge at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min. The liquid supernatants were used in the next experiment. The protein concentration was determined by the Pierce BCA assay (23225, Thermo Scientific, PA, USA).
000 rpm for 20 min. The liquid supernatants were used in the next experiment. The protein concentration was determined by the Pierce BCA assay (23225, Thermo Scientific, PA, USA).
Statistics
All data were presented as mean ± SD from at least three averaged replicates of independent experiments. A statistical comparison of quantitative data in the groups was performed by one-way ANOVA or Student's t-test. To determine differences between groups that were not normally distributed, medians were compared using Kruskal–Wallis analysis of variance. The SPSS 19.0 software (SPSS, Inc., Chicago, USA) was used for data analysis. P < 0.05 was regarded as statistically significant.Results and discussion
Characterization of TiO2 NPs
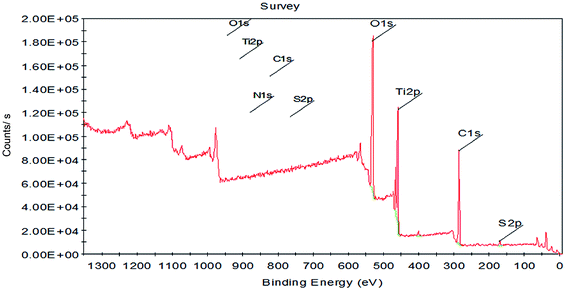
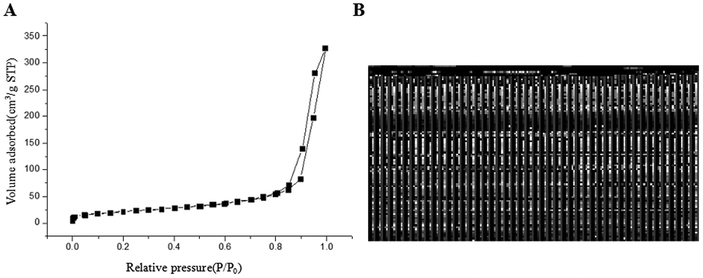
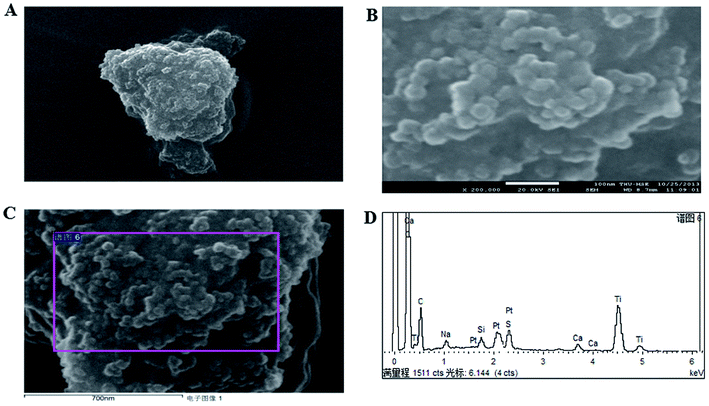
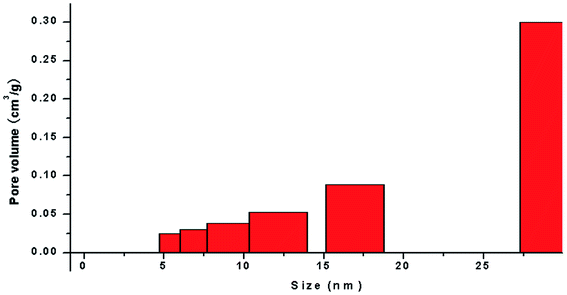
The application of nanotechnology is currently becoming increasingly prevalent, and many innovative high-quality products are used in biomedical applications. However, the hazards of nanomaterials to human health and environment are also increasing.8,9 With the development of nanoscale science and technology, we have constantly understood and assessed the risk of accidental or occupational exposure of humans to nanoparticles (NPS). The elemental composition of TiO2 NPs used in this study is shown in Table 1. The percentage of total metal content was only 2.9%. Fig. 1 shows that the material contained 96.59% TiO2 NPs. The content of transition metals in each sample was less than 2%. The BJH pore size distribution of TiO2 NPs and a plot of the adsorption isotherm are shown in Fig. 2A. Fig. 2B reveals that the BET surface area was 79.299 m2 g−1. The total pore volume with a diameter of less than 343.88 nm at P/P0 = 0.9944002 was 0.5062 cm3 g−1. The average pore diameter was 25.54 nm (Fig. 2B). The particle size distribution of TiO2 NPs is shown in Fig. 3A, and individual anatase-like spherical aggregates of TiO2 nanoparticles with a diameter of 10–30 nm were observed. Hence, our studies revealed that the particles suspended in 75% alcohol were secondary aggregates of primary spherical TiO2 NP aggregates, and the atom diameter was 0.276 nm. SEM images (Fig. 4A, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×; Fig. 4B, 2
000×; Fig. 4B, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×) showed that TiO2 NPs with diameter of about 10 nm were individual spherical particles on the PS surface. When compared with the diameter of the TiO2 NP aggregates, this result indicated that on the PS surface, the structure of secondary aggregates of TiO2 NPs in the volatile alcohol suspension fragmented into individual primary aggregates. According to our speculation, the fact that the stacking interactions between the benzene rings on the PS surface and TiO2 NPs were stronger than those between the TiO2 NP aggregates was the reason for the disintegration of the secondary aggregates of TiO2 NPs. Due to the unstable structure of the aggregates of TiO2 NPs on the PS surface, a fraction of TiO2 NPs could diffuse into the alcohol suspension. The elements were determined from the power spectrum (Fig. 4C and D). Many researchers have shown that NPs not only accumulate in exposed areas, but also penetrate through the vascular epithelium into the blood circulation of animals.10 For this reason, a further assessment of the potential toxicity of NPs is extremely urgent. On the other hand, since NPs can travel in the body, they may contribute to the treatment of diseases. More importantly, nanoscale materials have many unique biological functions such as absorbing various molecules via non-covalent forces, trapping ions or small molecules, and crossing the cell membrane. Thus, TiO2 NPs, as a type of nanoparticles, have great prospects in terms of applications in biomedicine, including drug delivery, gene therapy and molecular imaging.11 It is well-known that the treatment of HCC remains challenging, and the performance of sorafenib as the most efficacious systemic targeted therapy and the only FDA-approved treatment for unresectable HCC has been disappointing. Although many other targeted therapeutic drugs have been found, such as cetuximab, brivanib and regorafenib, none of them can exhibit a satisfactory effect on HCC treatment.12 Perhaps it is time to change the focus of research and try to discover a potential drug carrier that can carry drugs to therapeutic targets and release them steadily. The unique features of TiO2 NPs have attracted the attention of researchers.
000×) showed that TiO2 NPs with diameter of about 10 nm were individual spherical particles on the PS surface. When compared with the diameter of the TiO2 NP aggregates, this result indicated that on the PS surface, the structure of secondary aggregates of TiO2 NPs in the volatile alcohol suspension fragmented into individual primary aggregates. According to our speculation, the fact that the stacking interactions between the benzene rings on the PS surface and TiO2 NPs were stronger than those between the TiO2 NP aggregates was the reason for the disintegration of the secondary aggregates of TiO2 NPs. Due to the unstable structure of the aggregates of TiO2 NPs on the PS surface, a fraction of TiO2 NPs could diffuse into the alcohol suspension. The elements were determined from the power spectrum (Fig. 4C and D). Many researchers have shown that NPs not only accumulate in exposed areas, but also penetrate through the vascular epithelium into the blood circulation of animals.10 For this reason, a further assessment of the potential toxicity of NPs is extremely urgent. On the other hand, since NPs can travel in the body, they may contribute to the treatment of diseases. More importantly, nanoscale materials have many unique biological functions such as absorbing various molecules via non-covalent forces, trapping ions or small molecules, and crossing the cell membrane. Thus, TiO2 NPs, as a type of nanoparticles, have great prospects in terms of applications in biomedicine, including drug delivery, gene therapy and molecular imaging.11 It is well-known that the treatment of HCC remains challenging, and the performance of sorafenib as the most efficacious systemic targeted therapy and the only FDA-approved treatment for unresectable HCC has been disappointing. Although many other targeted therapeutic drugs have been found, such as cetuximab, brivanib and regorafenib, none of them can exhibit a satisfactory effect on HCC treatment.12 Perhaps it is time to change the focus of research and try to discover a potential drug carrier that can carry drugs to therapeutic targets and release them steadily. The unique features of TiO2 NPs have attracted the attention of researchers.
| Element | Weight content (%) | Atom content (%) |
|---|---|---|
| C | 64.19 | 75.57 |
| O | 23.78 | 21.02 |
| Na | 0.66 | 0.41 |
| Si | 0.50 | 0.25 |
| S | 1.17 | 0.52 |
| Ca | 0.52 | 0.18 |
| Ti | 6.26 | 1.85 |
| Pt | 2.92 | 0.21 |
| Total | 100.00 |
 | ||
| Fig. 3 Particle size distribution of TiO2 NPs on dishes. Dishes (cut to a size of 1 × 1 cm) coated with TiO2 NPs were placed under a JEOL JSM-7401 (JEOL Ltd., Tokyo, Japan) scanning electron microscope (SEM) to analyze the morphology of NPs. The SEM was operated at 100 and 10 kV. Thermogravimetric analysis was carried out with a Q50TGA thermogravimetric analyzer (Thermal Analysis, Inc., New Castle, DE, USA) from room temperature to 1173 K at a rate of 10 K min−1 under an air flow of 30 mL min−1.13 Individual anatase-like spherical aggregates of TiO2 nanoparticles with a diameter of 10–30 nm were observed. | ||
Effects of TiO2 NPs on cellular biological function
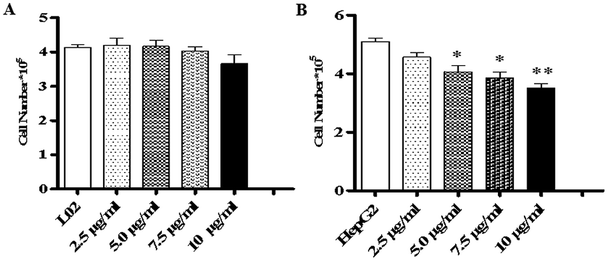
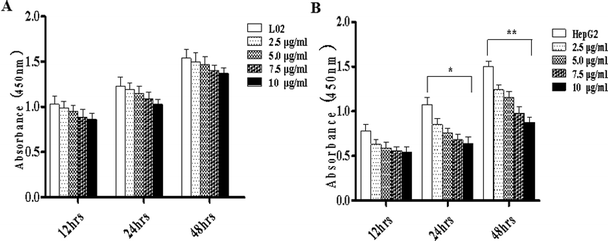
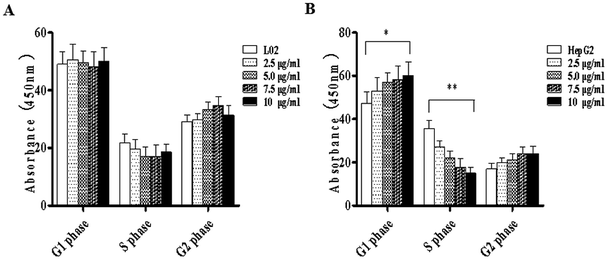
Literature has indicated that TiO2 NPs (30–70 nm) can induce significant oxidative DNA damage in HepG2 cells, as measured by an Fpg-Comet assay even at 1 μg mL.13 In different cell lines, different effects are induced by TiO2 NPs. This might be related to the characteristics of the test materials or the quantities introduced into the cells. Considering the difference in metabolism between tumor cells and normal cells, there may be differences in the quantities introduced into the cells.To study the effects of TiO2 NPs on normal cells L02 and tumor cells HepG2, the effects of TiO2 NPs on cell growth, cell proliferation, cell cycle and cell apoptosis were first analyzed. By a cell counting method, the growth state of cells could be understood quickly. In addition, the cell inoculation concentration was used to determine the quantity, cell survival rate and proliferation state. After collection, the cells were counted using a blood cell counting plate and then, a suitable concentration of the cell suspension was prepared and co-cultured with TiO2 NPs for 24 h. Then, the cells were harvested and counted again. The results indicated that after exposure to TiO2 NPs, the growth state of L02 cells underwent no significant changes (Fig. 5A), whereas the growth of HepG2 cells was significantly inhibited (Fig. 5B) and exhibited a certain concentration dependence with a statistically significant difference (*p < 0.05, **p < 0.01). For further experiments with a certain guiding significance, the effect of TiO2 NPs on the proliferation of L02 and HepG2 cells was analyzed using a CCK-8 cell proliferation assay kit. The cells were co-cultured with TiO2 NPs for 12 h, 24 h, and 48 h and then, cell proliferation was determined. The results showed that for L02 cells, at 12 h, 24 h and 48 h time points, compared with the results of the control group, the cell proliferation ability of the experimental group was slightly inhibited, but there were no statistically significant differences (Fig. 6A). For HepG2 cells, all four concentrations in the experimental groups caused a significant decrease in cell proliferation ability with clear concentration dependence. The OD values displayed no statistically significant difference after culturing for 12 h, but the OD values at 24 h decreased clearly. Furthermore, they decreased more clearly at 48 h, and there were statistically significant differences compared to that of other groups (Fig. 6B) (*p < 0.05, **p < 0.01). After co-culturing with TiO2 NPs for 48 h, staining with 7-AAD and detection by flow cytometry were performed to identify the changes induced by TiO2 NPs in the cell cycles of L02 and HepG2 cells in the G1, S, and G2 phases. The results showed that TiO2 NPs had no significant effects on the G1, S, and G2 phases in L02 cells (Fig. 7A). In HepG2 cells, with an increase in the concentration of TiO2 NPs, the number of cells in the G1 phase of the cell cycle increased (Fig. 7B) with significant difference compared with that of the control group (*p < 0.05). Moreover, following an increase in the concentration of TiO2 NPs, the percentage of cells in the S phase decreased significantly with significant difference compared with that of the control group (**p < 0.01). These results revealed that TiO2 NPs could significantly inhibit cell growth and cell proliferation, arrest the cell cycle in the S phase, and induce cell apoptosis with certain concentration and time dependence in HepG2 cells. The drug concentrations that were used did not induce corresponding phenomena in L02 cells. The difference in biological behaviors between normal cells and tumor cells indicated that TiO2 NPs might be a safe antitumor drug.
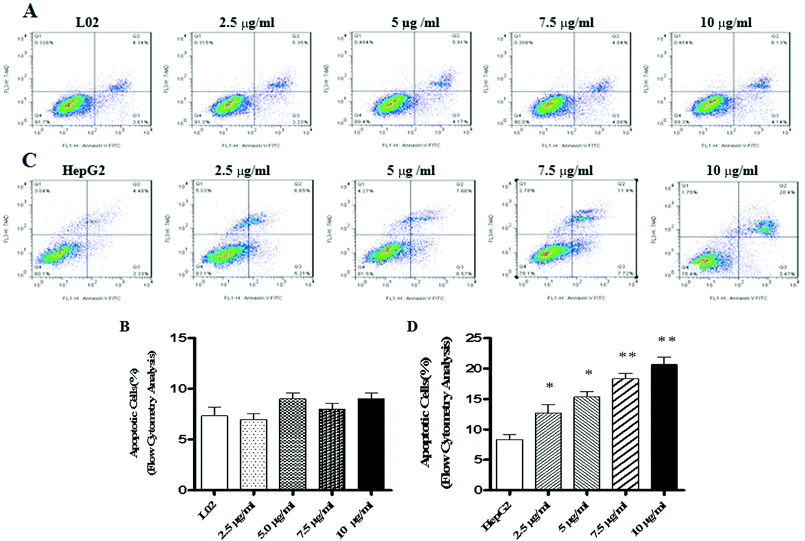
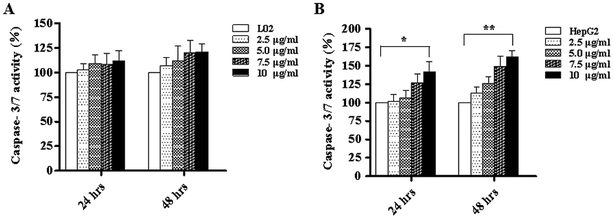
We know that apoptosis is a programmed cell death process regulated by a wide variety of genes, and it plays a crucial role in maintaining the internal environment of an organism.12 It is usually regulated by endogenous cellular genes, enzymes and exogenous signal transduction pathways. However, excessive apoptosis can lead to functional disorder in the body.14,15 At present, it has been found16 that the occurrence of tumors results from an imbalance between cell apoptosis and proliferation, and the inhibition of apoptosis is an important mechanism of tumorigenesis. The disorders of apoptosis pathways such as the overexpression of anti-apoptosis factors and apoptosis-inhibiting factors and the suppression of apoptosis gene expression can lead to the development of tumors. In other words, the promotion of apoptosis is a common strategy for cancer treatment. There are two common apoptotic pathways in humans, namely, external (death receptor-mediated) and endogenous (mitochondria-mediated) pathways.17 Both of these pathways are eventually initiated via activation of caspase and other apoptosis-related molecules.18,19 The results of western blot demonstrated that TiO2 NPs can significantly upregulate the expression of the apoptosis-related protein caspase-3 in HepG2 cells without affecting the expression of caspase-3 in L02 cells. Caspase-3 can cleave poly(ADP-ribose) polymerase (PARP) into 31 kDa and 85 kDa fragments, increase the activity of Ca/Mg-dependent endonucleases, and cleave DNA between nucleosomes, which leads to apoptosis.20 To determine the effect of TiO2 NPs on apoptosis, Annexin V/7-AAD double staining flow cytometry was used to detect the apoptosis of HepG2 cells and L02 cells co-cultured with TiO2 nanoparticles for 48 h. The results showed that no clear apoptotic cells appeared after 48 h in L02 cells (Fig. 8A and B). In HepG2 cells, with an increase in the concentration, the apoptosis rate increased and a significant difference was observed in comparison with that of the control group (Fig. 8C and D) (*p < 0.05, **p < 0.01). The influence of TiO2 NPs on cell apoptosis was further elucidated by measuring the activity of caspase-3/7. The activity of caspase-3/7 in HepG2 cells induced by TiO2 NPs increased significantly after 48 h (Fig. 9B) (*p < 0.05, **p < 0.01). However, there was no clear effect on L02 cells (Fig. 9A). These findings further demonstrated that TiO2 NPs can reduce the activity of HepG2 cells, promote cell apoptosis, and increase the activity of caspase-3/7. Clearly, TiO2 NPs could safely and stably activate caspase-3 in HepG2 cells to promote apoptosis. The analysis of caspase-3/7 activity also indicated that TiO2 NPs significantly increased the activation of caspase-3/7 in HepG2 cells, which further confirmed this observation.
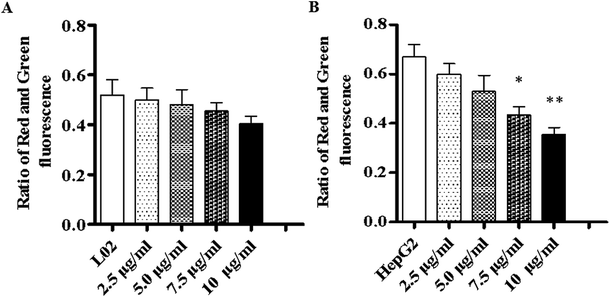
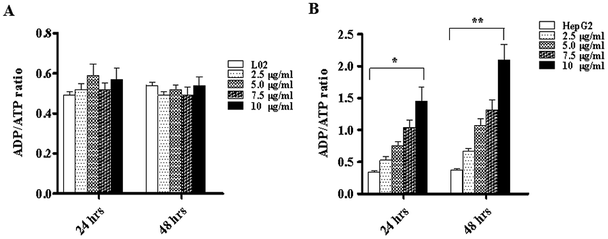
To investigate the way in which TiO2 NPs activate caspase-3, a JC-10 detection kit was used to detect changes in the mitochondrial membrane potentials of L02 and HepG2 cells. The results showed that after co-culturing with TiO2 NPs for 48 h, the mitochondrial membrane potential of L02 cells underwent no clear change (Fig. 10A). In HepG2 cells (Fig. 10B), depolarization of the mitochondrial membrane exhibited a gradual trend in a concentration-dependent manner, and the difference was statistically significant (*p < 0.05, **p < 0.01). The mitochondrial membrane potential maintained the preconditions for the oxidative phosphorylation of mitochondria and the production of adenosine triphosphate (ATP), which helped maintain the normal physiological function of the cells.21 TiO2 NPs enabled the downregulation of the expression of the mitochondria-associated protein ACSS1 in HepG2 cells, which was possibly related to the inhibition of the respiratory chain induced by TiO2 NPs in mitochondria; this behavior could lead to disorders in the generation of ATP and cause accumulation of ADP in mitochondria. In addition, the polarization of the mitochondrial membrane in HepG2 cells induced by TiO2 NPs may cause further loss of ATP. The changes in the ADP/ATP ratio could be discovered with an ADP/ATP detection reagent kit. After co-culturing with TiO2 NPs for 48 h, the ADP/ATP ratio in L02 cells exhibited no clear change (Fig. 11A). In HepG2 cells, the ADP/ATP ratio gradually increased in a time- and dose-dependent manner, and the difference was statistically significant (Fig. 11B) (*p < 0.05, **p < 0.01). These results suggested that TiO2 NPs were likely to promote apoptosis by inducing mitochondrial dysfunction. Mitochondrial energy metabolism was inhibited, which resulted in decline in the rate of cell proliferation, depolarization of the mitochondrial membrane, disturbances of energy metabolism and inhibition of the cell cycle.22,23 The increase in the ADP/ATP ratio after HepG2 cells were exposed to TiO2 NPs further proved the above conclusion. Hence, TiO2 NPs can depolarize the mitochondrial membrane of HepG2 cells, disrupt mitochondrial function, and reduce ATP production. It can be concluded that TiO2 NPs can possibly activate caspase-3 via a mitochondria-mediated pathway and promote apoptosis.
TiO2 NPs induced HepG2 cell apoptosis through a mitochondrial pathway, which may be related to αENaC
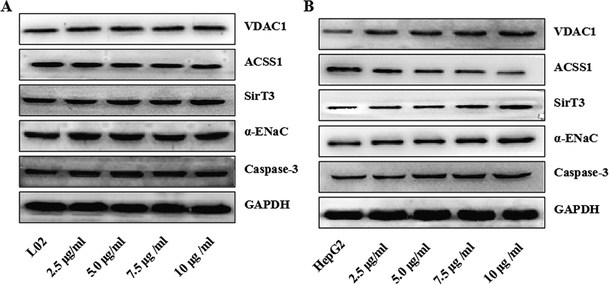
To confirm that the apoptosis of HepG2 cells induced by TiO2 NPs was related to the endogenous mitochondrial pathway, the expressions of the mitochondria-related proteins VDAC1, ACSS1 and cytochrome c (Cyt c) were determined in vitro and in vivo. After co-culturing with TiO2 NPs for 48 h or regular injection of TiO2 NPs to treat transplanted tumors in nude mice, the expression level of VDAC1 increased, and the expression level of ACSS1 was downregulated; both occurred in a concentration-dependent manner in vitro. VDAC1 encodes a voltage-dependent anion channel protein, which is an important component of the outer mitochondrial membrane. The encoded protein adjusts the exchange of metabolites and ions in the outer mitochondrial membrane and regulates the function of mitochondria. The release of ATP within mitochondria can also be controlled by VDAC1.24 Upregulation of the expression of VDAC1 is closely related to apoptosis and leads to depolarization of the mitochondrial membrane, increase in membrane permeability, and release of Cyt c, further activating caspase-3.25 Mitochondrial acetyl-CoA synthetase, which is encoded by the ACSS1 gene, is the key enzyme in the mitochondrial respiratory chain, providing vital energy required for ketogenic conditions via the conversion of acetic acid to acetyl-CoA; thus, it can be used to produce ATP by three oxidation steps in the tricarboxylic acid cycle. This may be the reason why TiO2 NPs can induce increase in the ADP/ATP ratio in HepG2 cells. This result proved that TiO2 NPs can alter the expression of mitochondrial proteins, depolarize the mitochondrial membrane, inhibit mitochondrial energy metabolism, inhibit cell growth and proliferation, release Cyt c, activate caspase-3 (ref. 26 and 27) and induce apoptosis in HepG2 cells.The interactions between physical and chemical properties of NPs play a key role in their transmembrane transport. However, for a long time, we have been unable to discover how NPs are taken up by the cells. Cell transmembrane transport occurs via the following five routes: (1) simple diffusion involving transmembrane transport of fat-soluble substances such as O2, CO2, and NH3; (2) facilitated diffusion, which is divided into two types: one involves transport by a carrier such as that of glucose into the blood by red blood cells, and the other is diffusion mediated or facilitated by a channel protein such as K+, Na+, or Ca2+ concentration gradient across the membrane; (3) primary active transport such as transmembrane transport of K+, Na+, or Ca2+ ions against a concentration gradient or potential gradient; (4) secondary active transport, such as the absorption and reabsorption of glucose by the small intestinal mucosal membrane and renal tubular epithelial cells; and (5) exocytosis and endocytosis, such as the processes of leukocyte phagocytosis of bacteria and foreign bodies for endocytosis and the release of neurotransmitters by secretory cells for exocytosis. The findings of Vranic et al.28 have shown that the uptake of NCI-H292 by SiO2-NP cells was dose- and time-dependent, and the saturation state of the cells appeared. In addition, by confocal microscopy, the internalization pathway of the cells was shown to be an energy-dependent process, but a fraction of the NPs could also enter the cells by passive diffusion. TiO2 NPs can be observed inside the cells by transmission electron microscopy.29 The metabolism of tumor cells is also more robust than that of normal cells. We suspected that TiO2 NPs might exert these differences by influencing the cellular energy metabolism. To investigate whether TiO2 NPs could enter cells via channel proteins and to determine the molecular mechanism of the apoptosis of HepG2 cells induced by TiO2 NPs, the cells were incubated with different concentrations of TiO2 NPs for 48 h. The western blotting method was used to detect the expressions of apoptosis-related factors such as caspase-3, αENaC, SIRT3, ACSS1, and VDAC1. In L02 cells, as shown in Fig. 12A, there were no significant changes in the expressions of caspase-3, αENaC, SIRT3, ACSS1 and VDAC1 compared with that of the control group. In HepG2 cells, as shown in Fig. 12B, TiO2 NPs significantly upregulated the expressions of caspase-3, αENaC, SIRT3 and VDAC1 and significantly downregulated the expression of ACSS1 in a concentration-dependent manner. The results showed that in HepG2 cells, TiO2 NPs enabled the upregulation of the expression of αENaC, which is a functional element of the osmotic pressure-induced cation channel.30 Moreover, the expression of SIRT3 protein was detected. The coding of this protein was dependent on the silent information regulator (Sir2) family, which depends on NAD+, and displayed changes in intracellular osmotic pressure of cells.31 With an increase in concentration, the expression gradually increased. The internal and external osmotic pressure together maintained the cell volume in a steady state.32 When TiO2 NPs entered the cells, they caused a change in intracellular osmotic pressure, but we have not investigated whether TiO2 NPs are directly transported by αENaC, by changes in osmotic pressure that indirectly upregulate αENaC expression, or both factors together. This requires us to carry out further study.
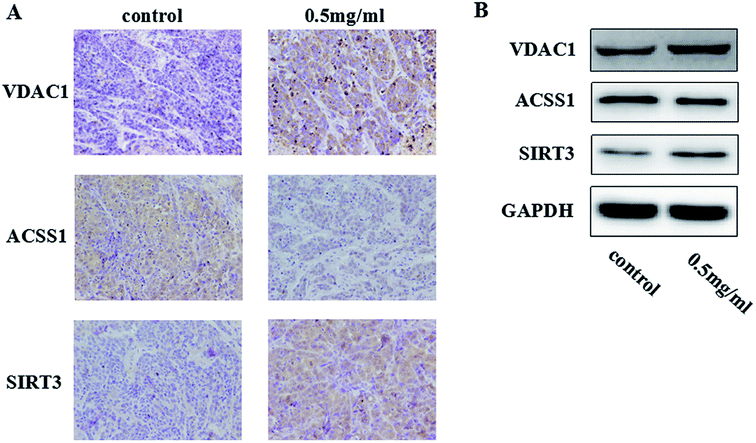
TiO2 NPs suppressed tumor growth and induced differential expressions of mitochondria-related proteins in xenografts of nude mice
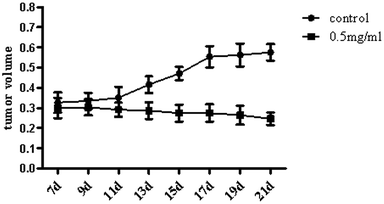
We researched the effects of TiO2 NPs on cellular biological functions in vitro; to further study the influence of TiO2 NPs on liver cancer cells in vivo, we constructed animal models of liver cancer. Tumor volumes decreased after treatment with TiO2 NPs compared with that of the vehicle group (Fig. 13). This showed that TiO2 NPs can also have good effect on tumors in vivo. The results of immunohistochemistry and western blot showed that the expressions of VDAC1 and SIRT3 proteins were clearly upregulated in tissues of the xenografts treated with TiO2 NPs, whereas the expression of ACSS1 was downregulated. These results were consistent with the above in vitro results (Fig. 14A and B). In vivo, tumor growth may have been inhibited by the same mechanism of action, and apoptosis of tumor cells was induced. With the development of research on nanoscale science and technology, TiO2 NPs may have great prospects in terms of applications in biomedicine.33Conclusions
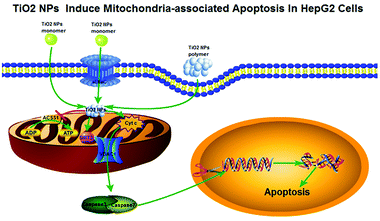
In summary, all the above phenomena can be summed up together as follows: TiO2 NPs might cross the cell membrane and accumulate in mitochondria (Fig. 15), resulting in depolarization of the mitochondrial membrane, decrease in ATP production via downregulation of the expression of ACSS1, inhibition of cell growth and proliferation, and arrest of the cell cycle. In addition, TiO2 NPs could upregulate the expression of VDAC1, and a large number of pro-apoptotic substances such as Cyt c produced as a result of mitochondrial dysfunction could be released from the mitochondria via this channel into the cytoplasm and irreversibly activated caspase-3, which caused substantial degradation of intracellular DNA and induced apoptosis of HepG2 cells. Moreover, due to the strong carrying capacity and negligible effect on L02 cells, TiO2 NPs are expected to become an effective and safe tool for the struggle against human tumors.Ethics approval
All animal procedures were performed in accordance with the Guidelines for Care and Use of the Animal Protection Committee of Southern Medical University, and experiments were approved by the Animal Ethical and Welfare Committee of Southern Medical University (reference no. IACUC-2017-0922).Conflicts of interest
The authors declare that there is no conflict of interest.Acknowledgements
This work was funded by grants from the Natural Science Foundation of Guangdong Province (No. 2017A030313510), the Science and Technology Planning Project of Guangdong Province (No. 201707010305), and the Introduction of Talent Fund of Guangdong Second Provincial General Hospital (No. YY2016-006).References
- R. Siegel, J. Ma and Z. Zou, et al., Cancer statistics, Ca-Cancer J. Clin., 2014, 64(1), 9–29 CrossRef PubMed.
- A. Gerbes, F. Zoulim and H. Tilg, et al., Gut round table meeting paper: selected recent advances in hepatocellular carcinoma, Gut, 2018, 67(2), 380–388 CrossRef PubMed.
- A. Ruggiero, C. H. Villa and E. Bander, et al., Paradoxical glomerular filtration of carbon nanotubes, Proc. Natl. Acad. Sci. U. S. A., 2010, 107(27), 12369–12374 CrossRef PubMed.
- K. Kostarelos, A. Bianco and M. Prato, Promises, facts and challenges for carbon nanotubes in imaging and therapeutics, Nat. Nanotechnol., 2009, 4(10), 627–633 CrossRef PubMed.
- J. Zhang, Q. Sun, J. Bo, R. Huang, M. Zhang, Z. Xia, L. Ju and G. Xiang, Single-walled carbon nanohorn (SWNH) aggregates inhibited proliferation of human liver cell lines and promoted apoptosis, especially for hepatoma cell lines, Int. J. Nanomed., 2014, 9, 759–773 CrossRef PubMed.
- R. B. Baggs, J. Ferin and G. Oberdorster, Regression of pulmonary lesions produced by inhaled titanium dioxide in rats, Vet. Pathol., 1997, 34(6), 592–597 CrossRef PubMed.
- F. Afaq, P. Abidi, R. Matin and Q. Rahman, Cytotoxicity, pro-oxidant effects and antioxidant depletion in rat lung alveolar macrophages exposed to ultrafine titanium dioxide, J. Appl. Toxicol., 1998, 18(5), 307–312 CrossRef PubMed.
- A. Nemmar, H. Vanbilloen, M. F. Hoylaerts, P. H. Hoet, A. Verbruggen and B. Nemery, Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster, Am. J. Respir. Crit. Care Med., 2001, 164(9), 1665–1668 CrossRef PubMed.
- M. Siegrist, A. Wiek, A. Helland and H. Kastenholz, Risks and nanotechnology: the public is more concerned than experts and industry, Nat. Nanotechnol., 2007, 2(2), 67 CrossRef PubMed.
- J. Petkovic, B. Zegura and M. Stevanovic, et al., DNA damage and alterations in expression of DNA damage responsive genes induced by TiO2 nanoparticles in human hepatoma HepG2 cells, Nanotoxicology, 2011, 5(3), 341–353 CrossRef PubMed.
- Y. Cui, X. Gong and Y. Duan, et al., Hepatocyte apoptosis and its molecular mechanisms in mice caused by titanium dioxide nanoparticles, J. Hazard. Mater., 2010, 183(1–3), 874–880 CrossRef PubMed.
- M. Bupathi, A. Kaseb and F. Meric-Bernstam, et al., Hepatocellular carcinoma: where there is unmet need, Mol. Oncol., 2015, 9(8), 1501–1509 CrossRef PubMed.
- T. Chen, J. Yan and Y. Li, Genotoxicity of titanium dioxide nanoparticles, J. Food Drug Anal., 2014, 22(1), 95–104 CrossRef PubMed.
- L. Li, J. Zhang and Y. Yang, et al., Single-wall carbon nanohorns inhibited activation of microglia induced by lipopolysaccharide through blocking of Sirt3, Nanoscale Res. Lett., 2013, 8(1), 100 CrossRef PubMed.
- A. Behzadnia, M. Montazer, A. Rashidi and M. M. Rad, Sonosynthesis of nano TiO2 on wool using titanium isopropoxide or butoxide in acidic media producing multifunctional fabric, Ultrason. Sonochem., 2014, 21(5), 1815–1826 CrossRef PubMed.
- M. Pacurari, V. Castranova and V. Vallyathan, Single- and multi-wall carbon nanotubes versus asbestos: are the carbon nanotubes a new health risk to humans, J. Toxicol. Environ. Health, Part A, 2010, 73(5), 378–395 CrossRef PubMed.
- E. Belade, L. Armand and L. Martinon, et al., A comparative transmission electron microscopy study of titanium dioxide and carbon black nanoparticles uptake in human lung epithelial and fibroblast cell lines, Toxicol. In Vitro, 2012, 26(1), 57–66 CrossRef PubMed.
- M. Geiser and W. G. Kreyling, Deposition and biokinetics of inhaled nanoparticles, Part. Fibre Toxicol., 2010, 7, 2 CrossRef PubMed.
- S. Vranic, N. Boggetto and V. Contremoulins, et al., Deciphering the mechanisms of cellular uptake of engineered nanoparticles by accurate evaluation of internalization using imaging flow cytometry, Part. Fibre Toxicol., 2013, 10, 2 CrossRef PubMed.
- G. S. Choudhary, S. Al-Harbi and A. Almasan, Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis, Methods Mol. Biol., 2015, 1219, 1–9 CrossRef PubMed.
- B. G. Heerdt, M. A. Houston and A. J. Wilson, et al., The intrinsic mitochondrial membrane potential (Deltapsim) is associated with steady-state mitochondrial activity and the extent to which colonic epithelial cells undergo butyrate-mediated growth arrest and apoptosis, Cancer Res., 2003, 63(19), 6311–6319 Search PubMed.
- T. S. Hauck, A. A. Ghazani and W. C. Chan, Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells, Small, 2008, 4(1), 153–159 CrossRef PubMed.
- E. C. Cho, L. Au, Q. Zhang and Y. Xia, The effects of size, shape, and surface functional group of gold nanostructures on their adsorption and internalization by cells, Small, 2010, 6(4), 517–522 CrossRef PubMed.
- Y. Okada, E. Maeno and S. Mori, Anion channel involved in induction of apoptosis and necrosis, Adv. Exp. Med. Biol., 2004, 559, 205–209 CrossRef PubMed.
- J. C. Rathmell and C. B. Thompson, Pathways of apoptosis in lymphocyte development, homeostasis, and disease, Cell, 2002, 109, S97–S107 CrossRef PubMed.
- R. A. Frye, Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity, Biochem. Biophys. Res. Commun., 1999, 260(1), 273–279 CrossRef PubMed.
- M. P. Mattson, C. Culmsee and Z. F. Yu, Apoptotic and antiapoptotic mechanisms in stroke, Cell Tissue Res., 2000, 301(1), 173–187 CrossRef PubMed.
- S. Vranic, N. Boggetto and V. Contremoulins, et al., Deciphering the mechanisms of cellular uptake of engineered nanoparticles by accurate evaluation of internalization using imaging flow cytometry, Part. Fibre Toxicol., 2013, 10(2) Search PubMed.
- E. Belade, L. Armand and L. Martinon, et al., A comparative transmission electron microscopy study of titanium dioxide and carbon black nanoparticles uptake in human lung epithelial and fibroblast cell lines, Toxicol. In Vitro, 2012, 26(1), 57–66 CrossRef PubMed.
- M. Bondarava, T. Li, E. Endl and F. Wehner, Alpha-ENaC is a functional element of the hypertonicity-induced cation channel in HepG2 cells and it mediates proliferation, Pflügers Arch., 2009, 458(4), 675–687 CrossRef PubMed.
- H. Y. Liu, H. H. Qian and X. F. Zhang, et al., Improved method increases sensitivity for circulating hepatocellular carcinoma cells, World J. Gastroenterol., 2015, 21(10), 2918–2925 CrossRef PubMed.
- W. Hu and J. J. Kavanagh, Anticancer therapy targeting the apoptotic pathway, Lancet Oncol., 2003, 4(12), 721–729 CrossRef PubMed.
- X. X. Chen, B. Cheng and Y. X. Yang, et al., Characterization and preliminary toxicity assay of nano-titanium dioxide additive in sugar-coated chewing gum, Small, 2013, 9(9–10), 1765–1774 CrossRef PubMed.
Footnote |
| † Contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |