Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of free ammonia inhibition on NOB activity in high nitrifying performance of sludge
Fan Zhanga,
Hong Yang *a,
Jiawei Wanga,
Ziqi Liua and
Qingkun Guanb
*a,
Jiawei Wanga,
Ziqi Liua and
Qingkun Guanb
aKey Laboratory of Beijing for Water Quality Science and Water Environment Recovery Engineering, Beijing University of Technology, Beijing 100124, China. E-mail: yhong@bjut.edu.cn
bBeijing General Municipal Engineering Design & Research Institute Co. Ltd., China
First published on 13th September 2018
Abstract
The inhibition of free ammonia (FA) on nitrite-oxidizing bacteria (NOB) was investigated using an enriched NOB community with high nitrifying performance. A continuous-flow reactor was operated for the enrichment of the bacterial community. High-throughput sequencing analysis showed that Nitrospira (NOB) using in batch experiments was extended from 4.78% to 12.08% during the under continuous-flow operation for 27 days. For each batch experiments, an ammonia injection at the start-up resulted in the desired initial FA concentration (at pH = 8.1–8.2, T = 25 °C), and a continuous ammonia feeding stream allowed for a relatively stabilized FA levels as much as the initial one. Results indicated that FA inhibition on NOB was not instantaneous but occurred gradually at a certain reaction time. Low concentrations of FA (18.08–24.95 mg L−1) had a limited inhibition on NOB with increasingly high nitrate production rates, whereas high FA levels (36.06–50.66 mg L−1) exerted a significant negative impact on the NOB. Also, strong adaptation happened in these high levels of FA inhibition on NOB, which resulted in an overall low NOB activity during the whole aerobic operation.
1. Introduction
Traditional nitrification is a two-step process commonly utilized in wastewater treatment plant (WWTP). These steps are performed by two different autotrophic bacterial population. First, ammonia is oxidized to nitrite by the ammonia-oxidizing bacteria (AOB) and subsequently, the nitrite is converted to nitrate by nitrite-oxidizing bacteria (NOB). Recently, partial nitrification (ammonia oxidation to nitrite) has drawn much attention as it could reduce the oxygen and organic compound demand by 25% and 40%, respectively, compared with nitrification and denitrification.1 The success of the process depends on the elimination of NOB, thereby resulting in nitrite as the primary final product.2 Therefore, attempts have been made to devise a microbiological method for AOB enrichment and NOB washout to enable optimal partial nitrification.3,4Some previous works have proved that temperature, pH and dissolved oxygen (DO) are responsible for nitrite build-up.5–7 Nevertheless, the direct utilization of temperature (30–35 °C) changes is not feasible in municipal WWTP due to high energy consumption and capital investment. Especially in winter, when the temperature outside is almost close to 0 °C, it is hard to maintain the chamber temperature at 30–35 °C for effective partial nitrification. It has been suggested that high DO concentration improves the ammonia oxidation rate.8 Therefore, maintaining a low DO concentration (<0.5 mg L−1) for partial nitrification fails to work in highly efficient nitrogen removal systems. Means to maintain high pH and ammonia levels for partial nitrification in WWTP consume huge material resources, thus incurring high operation costs. Therefore, a better solution is proposed using a method for entire NOB washout by bacteria screening from biological system, resulting in the sole existence of AOB.9,10 Such sludge used in WWTP treating municipal and high-strength wastewater, achieves saving of operation and infrastructure costs. It is unnecessary to deliberately control low-level of DO, which enables to improve the efficiency of partial nitrification in wastewater treatment.
Washing out the entire NOB population by bacteria screening depends on inhibitors, free ammonia (FA)11,12 and free nitrous acid (FNA).13,14 Traditionally, FA is the first choice for nitrite build-up and NOB washout. In recent years, some researchers have proposed a new strategy to increase bioenergy recovery from wastewater or sludge treatment using FA.15–18 They demonstrate that pre-treatment of the activated sludge with FA for 24–40 h could reduce sludge yields by 20%. At the same time, the NOB activity could be decreased to some extent without any adverse impact on the reactor performance.19–21 Consequently, it is imperative that the characteristics and regulations of FA inhibition are discussed.
It is known that the inhibitory effect of FA exerts severe pressure on NOB activity.22 Although FA is known to slow down AOB activity, NOB is considered to be much more sensitive to FA than AOB.23 Anthonisen et al.13 suggested that inhibition of NOB begins at a FA concentration of 0.1–1 mg L−1, whereas the inhibition threshold for AOB was 10–150 mg L−1. FA concentrations of 4 mg L−1 and 6 mg L−1 have been reported to inhibit the NOB in two different studies.24,25 In addition, Abeling et al.26 reported that NOB was inhibited when FA reached 1–5 mg L−1.
The data mentioned above clearly shows that the threshold FA concentration for NOB inhibition varies. However, these FA values were calculated from the initial ammonia concentrations without considering the nitrification rate and ammonia consumption. Hence, the variation in FA concentration is because of the consumption of ammonia under different nitrification rates. Thus, the initial FA concentration should not be considered the real inhibitory value. Very few reports focus on exploring relatively stabilized FA inhibition of NOB with high nitrifying performance in a certain time.
In this paper, a series of batch experiments were designed to establish the relationship between relatively stable FA inhibitory concentrations and NOB response. An enriched NOB culture with high nitrate production rate was used, taken from the continuous-flow reactor with 99% conversion ratio of ammonia to nitrate. The NOB response was assessed systematically by monitoring NOB activity and nitrate production.
2. Materials and methods
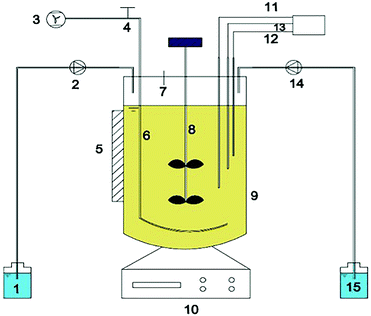
2.1. Continuous-flow operation to enrich NOB
A continuous-flow reactor with a working volume of 95 L was employed to selectively grow NOB for 62 days. Nitrifying sludge from the Gao Bei Dian Wastewater Treatment Plant (WWTP) in Beijing, China was used as the inoculum. This reactor was fed with stock solutions of NH4Cl (nitrogen source), KH2PO4 (phosphorus source) and Na2CO3 (pH buffer, alkalinity source) as well as a mineral stock solution detailed in Table 1. The 1 mL trace element stock solution contained 0.5 g L−1 ZnSO4·7H2O, 0.5 g L−1 MnCl2·4H2O, 0.4 g L−1 CoCl2·6H2O, 0.4 g L−1 CuSO4·5H2O, 0.2 g L−1 NiCl2·6H2O and 0.05 g L−1 Na2MoO4·2H2O. The hydraulic retention time (HRT) was maintained at 5 h. Temperature and pH inside the reactor were set at 25 ± 1 °C and 7.0 ± 0.1, respectively, whereas DO was controlled in the range of 1.0–1.5 mg L−1. Throughout the operation period, 99% of the incoming ammonia was oxidized to nitrates. Mixed liquor suspended solids (MLSS) was calculated as 2438 mg L−1 on the first day of the continuous-flow culture, which increased up to 4675 mg L−1 on day 62.| Compound | Concentration (mg L−1) |
|---|---|
| NH4Cl | 65–812 |
| KH2PO4 | 13–163 |
| Na2CO3 | 2000 |
| FeSO4·7H2O | 30 |
| CaCl2 | 15 |
| MgSO4·7H2O | 30 |
| Trace element stock | 1 ml L−1 |
2.2. Determination of NOB inhibition by FA
| Test | NPR0 (mg (L−1 h)) | Initial FA (mg L−1) | Initial NH4+–N (mg L−1) | MLSS (mg L−1) |
|---|---|---|---|---|
| 1 | 72.04 | 18.13 | 190.32 | 2867 |
| 2 | 101.57 | 20.86 | 244.07 | 3033 |
| 3 | 115.78 | 22.13 | 280.78 | 3566 |
| 4 | 128.05 | 24.93 | 307.61 | 3896 |
| 5 | 133.65 | 36.58 | 451.36 | 4273 |
| 6 | 142.64 | 40.75 | 502.83 | 4412 |
| 7 | 143.35 | 44.04 | 543.41 | 4415 |
| 8 | 154.06 | 50.66 | 625.03 | 4675 |
(i) Before each experiment, two sludge samples with same characteristics were collected from the continuous-flow reactor simultaneously. Their MLSS was consistent with that in continuous-flow reactor. All samples were washed thrice with deionized water so that NH4+–N, NO2−–N and NO3−–N were not detected. One sample was injected with a certain concentration of NH4+–N solution (e.g., 80 mg L−1 for Test 1) followed by 1 h operation to attain the actual NPR0 (Blank I). The other sample was used to determine the inhibitory effects of FA depending on the actual NPR0.
(ii) At the beginning of the test, a certain volume of NH4+–N stock solution was added into the reactor, resulting in different initial NH4+–N concentrations: 190.32, 244.07, 280.78, 307.61, 451.36, 502.83, 543.41 and 625.03 mg L−1, which were corresponding to the initial FA concentrations: 18.13, 20.86, 22.13, 24.93, 36.58, 40.75, 44.04 and 50.66 mg L−1 at pH 8.1 ± 0.1, respectively (Table 2). At the same time, the heater, stirrer and aeration devices were turned on to initiate the reaction.
(iii) Ammonia was fed to the sample during the whole 3 h aerobic reaction. The flow rate was controlled at 83.3 mL h−1 in order to ensure nitrification in 3 h. Concentration of the NH4+–N feed stream was three times the actual NPR measured before the experiments, which were 216.13, 304.71, 347.34, 384.15, 400.95, 427.92, 430.05 and 462.18 mg L−1, respectively. The ammonia feed stream could consistently supply NH4+–N and NO2−–N to complete the nitrification process. Thus, the NH4+–N concentrations in the reactor were almost maintained at the same level with the initial NH4+–N concentrations. The desired FA levels could be stabilized in the original values. For each FA inhibition test, liquor samples were collected at different times (0, 0.5, 1, 1.5, 2, 2.5 and 3 h) to assess the effect of FA on the sludge with different NPR values. During the aerobic reaction, the NO2−–N concentration in the reactor increased gradually due to FA inhibition. However, the calculated FNA concentration was below 0.008 mg L−1 (T = 25 ± 1 °C, pH = 8.1 ± 0.1),14 which is less than the inhibitory concentration of 0.02 mg L−1. Therefore, free nitrous acid (FNA) had negligible influence on the reaction.
(iv) On reaching 3 h, the heater, stirrer, aeration devices, pH controller and NH4Cl feeding pump were turned off in each batch reaction. The mixed liquor was discarded from the reactor. As the continuous-flow reaction progressed, the NPR0 of the sludge improved gradually. When the NPR0 reached a high value, fresh sludge was withdrawn from the continuous-flow reactor to investigate the inhibitory effect of high FA levels.
2.3. Analytical methods
The influent and effluent samples were withdrawn from the continuous-flow reactor on a daily basis and analyzed immediately for NH4+–N, NO2−–N and NO3−–N. Mixed liquor suspended solids (MLSS) was also measured inside the continuous-flow reactor on a daily basis. All analyses were performed according to standard methods.27 In the batch experiments, NH4+–N, NO2−–N and NO3−–N were measured for the calculation of FA, FNA and nitrate production rate. DO and pH were monitored in the continuous-flow reactor using programmable logic controller, whereas an installed Mettler-Toledo GmbH DO Probe, (Mettler-Toledo, Switzerland) and pH meter (Mettler-Toledo CH-8902, Switzerland) were used in the batch tests, respectively.In the batch experiments, concentration of FA and FNA were calculated using eqn (1) and (2) proposed by Anthonisen et al. (1976)13:
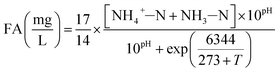 | (1) |
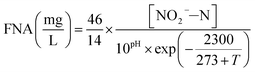 | (2) |
The nitrate production rate (NPR0) in the batch experiments was estimated according to eqn (3)
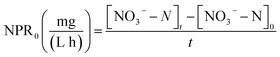 | (3) |
FA has a strong negative effect on the biosynthesis process of NOB resulting in the reduction of NOB activity. Hence, the formulae for the calculation of NOB activity in batch experiments were established as follows:
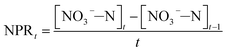 | (4) |
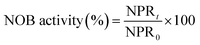 | (5) |
2.4. High-throughput 16S rRNA gene sequencing
To test the presence of AOB and NOB during the continuous-flow phase, three samples were collected and analysed using high-throughput sequencing (SinoGenoMaxCorp., China). The first sample was taken from the seed sludge, whereas the second and third were withdrawn from the continuous-flow reactor on days 35 and 62. Samples were prepared for DNA extraction. Then, the extracted DNA was supplied for PCR amplification of 16S rRNA in V3–V4 region. The PCR products were verified by electrophoresis to generate sequencing libraries. The libraries were then sequenced and assessed on an Illumina HiSeq2500 PE250 platform. Then, the reads obtained were classified into operational taxonomic units (OTUs), which were subsequently used for microbial diversity analysis.3. Results and discussion
3.1. Continuous-flow performance
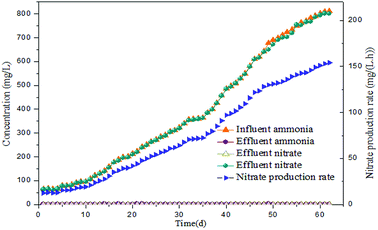
Long-term monitoring of the reactor performance revealed that complete nitrification was achieved. As illustrated in Fig. 2, influent ammonia was increased in steps from an initial concentration 65 to 812 mg L−1. After reaction time of 5 h, the concentration of ammonia was 2.01 mg L−1, while that of nitrite was a negligible 0.47 mg L−1 in the effluent. Thus, the ammonia was almost completely oxidized into nitrates (over 99%) and the calculated nitrate production rate (the nitrate production rate in this reactor was assessed using the difference of nitrate in the influent and effluent per hour) was only 12.51 mg (L h)−1. Low nitrate production rate in the initial phase indicated that the activity of NOB was less and inappropriate for FA inhibition experiments. However, when the influent ammonia was continually improved, it was almost completely transformed into nitrate (99% of ammonia into nitrate transformation ratio during the operation phase), thus reflecting the enhancement of NOB activity. Besides, the output of nitrite was maintained 0.24–0.56 mg L−1 through the 62 days. When the nitrate production rate reached 72.04 mg (L h)−1 on day 35, the mixed liquor was collected for FA inhibition experiments. With the increase of nitrate production rate in the following days, the sludge was periodically removed for use as the bacterial source for FA inhibition experiments. Additionally, MLSS stepped up from the initial 2438 mg L−1 to a final value of 4675 mg L−1 implying enrichment of the NOB population. High-throughput sequencing analysis indicated that the microbial culture of the enriched NOB was Nitrospira, which increased by approximately 5.6 times from 2.14% (day 1) to 12.08% (day 62). A significant increase of the NOB fraction (4.78%) was detected on day 35, when the first batch experiment was initiated. As the continuous-flow proceeded, the NOB activity and ratio increased progressively to reach 154.06 mg (L h)−1 and 12.08%, respectively, when the final batch experiment was conducted on day 62. Thus, the enriched NOB taken from day 35 to 62 triggered a more evident consequence of the NOB response upon FA.3.2. Effect of low FA concentration on NOB activity
Several tests were performed on the samples with varied NPR0 under different FA levels (details in Table 2) in order to investigate the biocidal effect of FA on the activated sludge. Fig. 3 reflects the variation of NOB activity in samples with different NPR0 values after being exposed to different FA concentrations. FA levels were maintained in a relatively stable range (approximately 18.08–18.83, 20.13–20.86, 22.13–22.81, 24.14–24.93 mg L−1) through a continuous mode of ammonia feeding and an ammonia injection at the beginning. The continuous ammonia feeding mode was to ensure the ammonia to nitrate transformation so that the initial ammonia was estimated for FA concentration instead of participating in the transformation of ammonia into nitrate. Therefore, the ammonia concentration was maintained stable during the whole aerobic period resulting in relatively level-off profiles of the calculated FA concentration. The subtle fluctuation in the FA profiles was because the ammonia (assessed for FA levels) was oxidized into nitrate more or less. Since the oxidation of ammonia into nitrate was based on NOB activity, the nitrate production profiles clearly indicated the inhibitory impact of FA on NOB. It should be noted that NOB activity was 100% at 0 h as there was no influence of FA.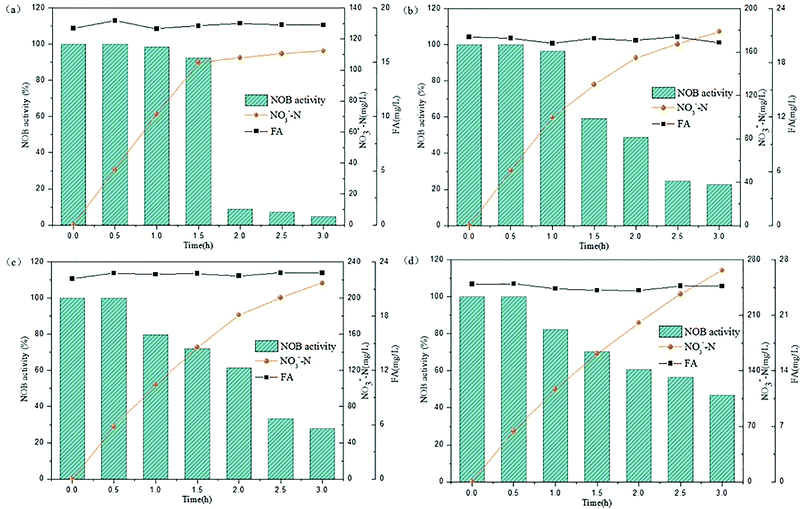 | ||
| Fig. 3 Inhibitory effect of FA on different NPR0 values: (a) NPR0 = 72.04 mg (L h)−1; (b) NPR0 = 101.57 mg (L h)−1; (c) NPR0 = 115.78 mg (L h)−1; (d) NPR0 = 128.05 mg (L h)−1. | ||
As shown in Fig. 3(a), the FA range of 18.08–18.83 mg L−1 (Test 1) was selected to investigate the effect on NOB with NPR0 of 72.04 mg (L h)−1. The NO3−–N production profile ascended sharply to 104.86 mg L−1 upto 1.5 h following by a sudden transition to a slow increase to 112.28 mg L−1. Similarly, NOB activity decreased gradually from 100% at 0.5 h to 92.45%, while an unexpected drop happened from 8.85% to 4.61%. This observation correlated well with previous studies,28–30 which reported that FA had a negative impact on NOB activity. It could be hypothesized that NOB suppression was not instantaneous but occurred gradually after 1.5 hours. Surprisingly, the percentage of NOB activity decreased intensely reaching 4.61% at the end of the reaction. The results indicated that the FA concentration ranging from 18.08 to 18.83 mg L−1 appeared to be the threshold for the sludge at 72.04 mg (L h)−1. When the NPR0 value reached 101.57 mg (L h)−1 (Test 1) as shown in Fig. 3(b), more FA (20.13–20.86 mg L−1) was introduced resulting in the rapid improvement of NO3−–N production before 1 h accompanied by a relatively gradual increase in the subsequent hours. This trend could be due to the fall of FA to the bottom range before 1 h. However, a slight increase of FA level after 1 h allowed for considerable suppression of NOB activity. Therefore, NOB activity was reduced only to 96.48% in 1 h, while an abrupt drop in NOB activity from 96.48% to 22.74% was observed in 1–3 h. In comparison, the NOB activity at NPR0 of 115.78 and 128.05 mg (L h)−1 corresponding to FA levels of 22.13–22.81 (Test3) and 24.14–24.93 (Test 4) mg L−1, respectively, showcased a similar behaviour as well. The NOB activity was not reduced instantly by FA but occurred gradually. Notably, the NO3−–N production at the end of the aerobic reaction period showed an upward trend from Tests 1 to 4 (112.29, 178.78, 216.79 to 266.74 mg L−1). These clearly indicated that the oxidization of nitrite to nitrate was increasingly unrestricted with the increase of NPR0, even though FA concentrations were enhanced as well. Moreover, NOB activity also presented the same trend with values 4.61%, 22.74%, 27.87% and 46.83%, respectively, at the end of each test. It could be concluded that FA concentrations up to 24.14–24.93 mg L−1 inhibited the NOB population (with high nitrifying performance) to a limited extent. The NOB activity was 100% at 0.5 h in all the above conditions. Subsequently, the NOB activity started to decline sharply in the following hours. It is, therefore, hypothesized that the NOB response for FA inhibition did not occur in a momentary time but in a long term. With the enhancement of NPR0, NO3−–N production increased (corresponding to 112.28, 178.78, 216.79 to 266.74 mg L−1 at the end of the four tests) regardless of FA. This clearly implied that low FA concentrations had a limited inhibitory effect on NOB. Furthermore, tests 1 to 4 were vaccinated separately to the mixed sludge from continuous-flow reactor at days 35, 42, 45 and 48, respectively.
3.3. Effect of high FA concentration on NOB activity
The above experiments illustrated that the varying trends in NOB activity was observed in a lower FA concentration range below 25 mg L−1. Rather, the FA values would increase stepwise in practice because the NPR0 values inclined gradually. At this stage, the NPR0 was further enhanced to values 133.65, 142.64, 143.35 and 154.06 mg (L h)−1, respectively. Thus, NOB inhibition was further investigated at higher NPR0 under higher FA concentrations. The inoculum from continuous-flow reactor in Tests 5 to 8 were withdrawn at days 52, 56, 57 and 62, respectively.As can be seen in Fig. 4(a), NO3−–N accumulated gradually reaching a concentration of 185.41 mg L−1 when inhibition by FA became apparent in the range of 36.06–36.73 mg L−1 (Test 5). Reduction NOB activity to 78.43% occurred at 0.5 h, which was likely due to the strong inhibitory effect of FA at high-levels. In the following hour, the NOB activity began to decline rapidly from 0.5 to 1 h, followed by a slower fall later. Similar result was obtained for the inhibition of NOB by FA at 40.06–40.81 mg L−1 concentration, which is depicted in Fig. 4(b) (Test 6). However, NO3−–N production reached only up to 128.05 mg L−1 at the end of the reaction. Furthermore, the NOB activity was estimated as 37.36% at 0.5 h and reduced to 15.93% to a limited extent, which was mainly because of the intense inhibition by FA present at high levels of 40.06–40.81 mg L−1. In addition, when the mixed liquor with an initial NPR0 of 143.35 mg L−1 was collected from the continuous-flow culture on day 57 (Test 7), NO3−–N accumulation was increased to 63.05 mg L−1 in the final reaction phase. Fig. 4(c) demonstrates the NOB performance under a higher FA level of 44.03–44.96 mg L−1. NOB activity declined significantly to 24.17% after 0.5 h. Subsequently, a slow descent in the NOB activity led to 8.37%, which represented a suppressed and inactive NOB response due extreme inhibition by such high levels of FA. Interestingly, a further increase of FA concentration to 50.04–50.66 mg L−1 (Test 8) did show further inhibitory effect on NOB activity. As shown in Fig. 4(d), the sludge with the highest NPR0 of 154.06 mg (L h)−1 produced minimal NO3−–N compared to the other 7 experiments accumulating only 44.35 mg L−1 at the end of the reaction. Moreover, the NOB activity dropped suddenly down to 13.76% at 0.5 h and then gradually to 5.31%. According to these results, NOB was inhibited intensely with incremental FA levels in the higher range despite the NPR0 increasing only on a small scale. Notably, the high FA concentration at the beginning of the reaction had significant inhibitory effect NOB activity at the startup (0.5 h) in all four experiments discussed above. However, the biomass had started to adapt to the strong inhibition by high in initial stage, thus resulting in the sustenance of NOB activity i.e. ammonia to nitrate transformation through the whole aerobic reaction period. This was in agreement with Wong et al.,31 whose results suggested that NOB adapted to FA above 40 mg L−1 and could resist the inhibition.
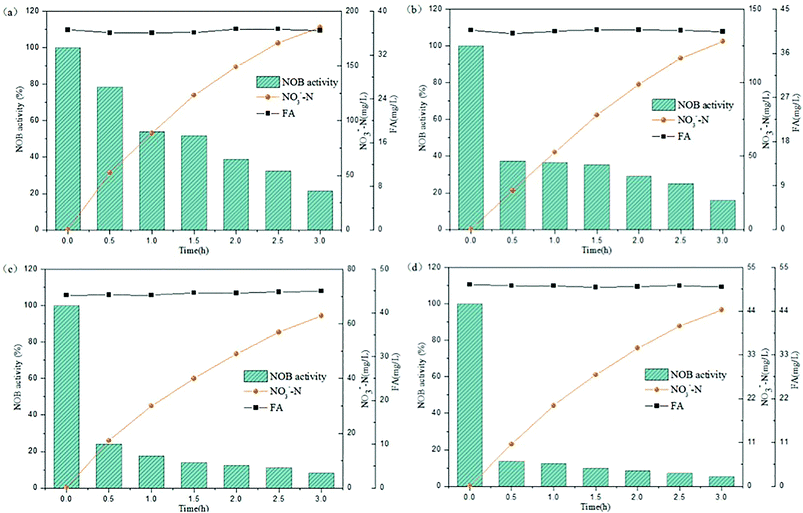 | ||
| Fig. 4 Performance of FA effect on different NPR0: (a) NPR0 = 133.65 mg (L h)−1; (b) NPR0 = 142.64 mg (L h)−1; (c) NPR0 = 143.35 mg (L h)−1; (d) NPR0 = 154.06 mg (L h)−1. | ||
3.4. Microbial diversity analysis
High-throughput sequencing was used to analyze the microbial diversity of the seed sludge and the cultivated sludge for comparison. A coverage 0.96 was achieved in the three samples indicating that the result exclusively represented the microorganisms present in the sludge samples. Abundance estimators Chao1 and ACE were applied to determine the OTUs. Greater richness was reflected by a higher value. Moreover, diversity indices Shannon and Simpson were calculated to understand the diversity of the microbial components. High value for Shannon and low value for Simpson indicated the existence of great diversity in the microbiome. The values of OTUs, Chao1, Shannon and ACE in the seed sludge were 4658, 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 687.51, 8.4 and 69
687.51, 8.4 and 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 893.04, respectively (Table 3). A decrease in these values was noted during the 35 days of cultivation showing values 3547, 22
893.04, respectively (Table 3). A decrease in these values was noted during the 35 days of cultivation showing values 3547, 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 476.63, 4.87 and 52
476.63, 4.87 and 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 663.95, respectively. They further decreased to 2773, 18
663.95, respectively. They further decreased to 2773, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 705.24, 4.21 and 41
705.24, 4.21 and 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 317.76, respectively, after 62 days of enrichment. On the contrary, the value of Simpson exhibited an upward trend increasing from 0.02, 0.03 to 0.05. The most feasible explanation for this phenomenon is that the microbial abundance and diversity declined while enriching the sludge for nitrifying bacteria. Meanwhile, nitrifiers had been predominant among the microbial communities in the sample. In other words, the performance of the system and richness of the specifically functional population were improved with a reduction in the microbial diversity as previously reported.32,33
317.76, respectively, after 62 days of enrichment. On the contrary, the value of Simpson exhibited an upward trend increasing from 0.02, 0.03 to 0.05. The most feasible explanation for this phenomenon is that the microbial abundance and diversity declined while enriching the sludge for nitrifying bacteria. Meanwhile, nitrifiers had been predominant among the microbial communities in the sample. In other words, the performance of the system and richness of the specifically functional population were improved with a reduction in the microbial diversity as previously reported.32,33
| Sample | OTUs | Chao1 | Shannon | ACE | Simpson | Coverage |
|---|---|---|---|---|---|---|
| a Sample 0 represented the seed sludge from WWTP; Sample 1 and 2 were cultivated sludge at days 35 and 62. | ||||||
| 0 | 4658 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 687.51 687.51 |
8.4 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 893.04 893.04 |
0.02 | 0.96 |
| 1 | 3547 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 476.63 476.63 |
4.87 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 663.95 663.95 |
0.03 | 0.96 |
| 2 | 2773 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 705.24 705.24 |
4.21 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 317.76 317.76 |
0.05 | 0.96 |
The structure of bacterial community at the genus level is presented in Fig. 5. The seed sludge showed that the Aridibacter, Ferruginibacter and Thauera were the predominant genera as depicted in Fig. 5(a). Their relative abundances were 5.25%, 2.88% and 2.85%, respectively. They belonged to the phyla Acidobacteria, Bacteroidetes and Proteobacteria, respectively, which were the main components of the activated sludge in the wastewater treatment system.34,35 Nitrosomonas (affiliated to β-Proteobacteria phylum, called ammonia-oxidizing bacteria) and Nitrospira (a distinct phylum, called nitrite-oxidizing bacteria) were detected at 0.43% and 2.14%, respectively, in municipal wastewater treatment.36,37 These implied that the abundance of the specific functional species (Nitrospira) was very low. Hence, the seed sludge was not suitable for investigating the inhibitory effect of FA. With the increase of continuous-flow operation, the Nitrospira improved up to ratios of 4.78% and 12.08%, respectively, at days 35 and 62. Tests 1 to 8 were conducted using increasingly enriched Nitrospira from 4.78% to 12.08%. The results were consistent with this showing excellent biological nitrification performance i.e. high nitrate production rates ranging from 72.04 mg (L h)−1 in Test 1 to 154.06 mg (L h)−1 in Test 8. Thus, the negative impact of FA on Nitrospira (NOB) is distinct and persuasive. As represented in Fig. 5(b) and (c), the ratio of Nitrosomonas (AOB) increased from 0.54% to 24.42% during the operation period of 62 days compared to the seed sludge that contained only 0.43%. The increase in Nitrosomonas was 45 times, while Nitrospira was enriched 5.6 folds compared to the seed sludge. This is in agreement with the previous reports that NOB grew more slowly than AOB.38–40 At the same time, other biological communities were gradually reduced and washed out of the continuous-flow reactor allowing the enrichment of Nitrosomonas (AOB) and Nitrospira (NOB) communities.
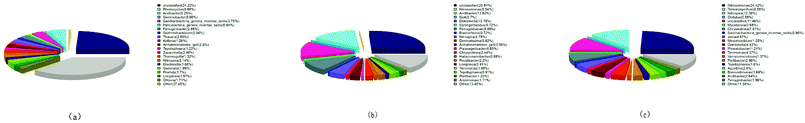 | ||
| Fig. 5 Distributions of microorganisms at the genus level: (a) the seed sludge; (b) cultivated sludge at days 35; (c) cultivated sludge at days 62. | ||
3.5. Proposed role of FA inhibition on NOB washout
The compound (FA) had been demonstrated to be the most effective inhibitor for NOB washout.41 Previous studies clearly showed that AOB had a much higher level of tolerance to FA compared to NOB, which may have contributed significantly to the elimination of NOB from the biological system. However, considering that wastewater treatment should be economic and environment friendly, it is impossible to employ high levels of FA in municipal WWTP. Thus, FA may provide a feasible solution for the washout of NOB in the lab scale.Based on the above results, two main aspects about FA inhibition on NOB could be concluded. Firstly, the reaction time of FA inhibition was of supreme importance, as the FA inhibition on NOB was not instantaneous but occurred gradually. As presented in Fig. 3 and 4, the NOB activity declined gradually as the reaction progressed. In addition, lower and higher levels of FA had significantly varied inhibitory effects on NOB. Lower-level of FA (18.13–24.93 mg L−1) had a limited inhibitory effect, whereas NOB could develop resistance to some extent towards higher levels of FA (36.58–50.66 mg L−1). It has been reported previously that FA concentration up to 200 mg L−1 could suppress NOB activity severely.42,43 Also, an elevated level of FNA could be supplied for further inhibition of NOB growth resulting in the complete elimination of NOB.44 Therefore, combination of high-levels of inhibitors FA and FNA could attain the desired NOB washout. It is imperative to investigate the combined effect of FA and FNA ion the inhibition and NOB washout in subsequent experiments.
It should be mentioned that the NOB culture used in this study coexists with AOB as evident from the high-throughput sequencing, which detected Nitrosomonas (AOB) levels of 0.54% and 24.42% at days 35 and 62 compared to 0.43% in the seed sludge. The existence of AOB in the system was inevitable while studying the effect of FA inhibition on NOB. Thus, the response of AOB has not been captured in the data from this study. However, the ammonia fed into the fermenter could transform into nitrate only because of the cooperative activity of AOB and NOB populations. Consequently, the AOB community seems to have still maintained a relative high activity under these FA concentrations. The results (FA inhibition on NOB) obtained may be valid for achieving NOB washout in the presence of AOB. This FA approach could be used to guide AOB enrichment and complete NOB washout in high nitrifying performance systems for efficient and cost-saving partial nitrification in municipal WWTP.
4. Conclusions
The feasibility of a continuous-flow configuration was demonstrated for the enrichment of NOB with high performance by conducting a series of experiments of FA inhibition. High-throughput sequencing implied that Nitrospira (NOB) used in the batch experiments increased from 4.78% to 12.08% during the continuous-flow operation period corresponding to the high nitrate production rate from 72.04 to 154.06 mg (L h)−1. For each batch test, the initial FA generation was through the ammonia injection at the beginning (at pH = 8.1–8.2, T = 25 °C) of the culture and the maintenance of these FA levels by a continuous ammonia feeding stream throughout the aerobic reaction period. Results showed that the negative impact of FA on NOB was not immediate but occurred progressively. Low levels of FA (18.08–24.95 mg L−1) were found to have limited effect on NOB with increasingly high nitrate production rates. However, much higher levels of FA (36.06–50.66 mg L−1) had a strong inhibition on NOB leading to the drastic reduction of activities. Additionally, the NOB was capable of adaption to FA inhibition and thus, the microorganisms were able to remain active at high concentrations of FA. Notably, 18.08–18.83 mg L−1 FA concentrations is likely to be the threshold value for the sludge at 72.04 mg (L h)−1. It could be suggested that FA inhibitor was effective in NOB suppression and could be applied as an inhibitor for NOB washout where partial nitrification is desired.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by “Beijing Municipal Commission of Education” under the research titled “Research on reinforcement and stability of nitrogen removal performance in wastewater treatment based on the new landmark conditions” (Z161100004516015) owned by municipal government of Beijing.Notes and references
- C. Fux, K. Lange, A. Faessler, P. Huber, B. Grueniger and H. Siegrist, Water Sci. Technol., 2003, 48, 9–18 CrossRef PubMed.
- M. A. Dytczak, K. L. Londry and J. A. Oleszkiewicz, Water Res., 2007, 42, 2320–2328 CrossRef PubMed.
- Z. G. Yuan and L. L. Blackall, Water Res., 2002, 36, 482–490 CrossRef PubMed.
- I. Jubany, J. Lafuente, J. A. Baeza and J. Carrera, Water Res., 2009, 43, 2761–2772 CrossRef PubMed.
- C. W. Randall and D. Buth, J. - Water Pollut. Control Fed., 1984, 56, 1045–1049 Search PubMed.
- C. Hellinga, A. A. J. C. Schellen, J. W. Mulder, M. C. M. van Loosdrecht and J. J. Heijnen, Water Sci. Technol., 1998, 37, 135–142 CrossRef.
- J. P. Li, D. Elliott, M. Nielsen, M. G. Healya and X. M. Zhan, Biochem. Eng. J., 2011, 55, 215–222 CrossRef.
- L. K. Wang, G. M. Zeng and Z. H. Yang, Biochem. Eng. J., 2014, 87, 62–68 CrossRef.
- D. Wang, Q. L. Wang, A. Laloo and Y. Xu, Sci. Rep., 2016, 6, 25547 CrossRef PubMed.
- D. Wang, Q. Fu, Q. Xu, Y. Liu and H. N. Hao, Bioresour. Technol., 2017, 244, 920–928 CrossRef PubMed.
- S. Villaverde, F. Fdz-Polanco and P. A. Garcia, Water Res., 2000, 34, 602–610 CrossRef.
- D. J. Kim, D. L. Lee and J. Keller, Bioresour. Technol., 2006, 97, 459–468 CrossRef PubMed.
- A. C. Anthonisen, R. C. Loher, T. B. S. Prakasam and E. G. Srinath, J. - Water Pollut. Control Fed., 1976, 48, 835–852 Search PubMed.
- V. M. Vadivelu, Z. Yuan, C. Fux and J. Keller, Environ. Sci. Technol., 2006, 40, 4442–4448 CrossRef PubMed.
- D. B. Wang, Y. Y. Duan, Q. Yang and Z. G. Yuan, Water Res., 2018, 133, 272–281 CrossRef PubMed.
- C. Zhang, Y. G. Qin, Q. X. Xu and Q. L. Wang, ACS Sustainable Chem. Eng., 2018, 6, 9120–9129 CrossRef.
- X. Liu, Q. X. Xu, D. B. Wang and L. Q. Yang, Bioresour. Technol., 2018, 268, 230–236 CrossRef PubMed.
- G. J. Yang, Q. X. Xu, D. B. Wang and Q. Yang, Chemosphere, 2018, 205, 484–492 CrossRef PubMed.
- Q. X. Xu, X. Liu, D. B. Wang and Q. L. Wang, Bioresour. Technol., 2018, 267, 141–148 CrossRef PubMed.
- D. B. Wang, K. Shuai, Q. X. Xu and X. Liu, Bioresour. Technol., 2018, 262, 114–123 CrossRef PubMed.
- D. Wang, B. Liu, Q. Xu and Q. Yang, Chemosphere, 2018, 206, 491–501 CrossRef PubMed.
- S. Philips, H. J. Laanbroek and W. Verstraete, Environmental Science and Biotechnology, 2002, 1, 115–141 CrossRef.
- S. Park, W. Bae and B. E. Rittmann, Environ. Sci. Technol., 2009, 44, 335–342 CrossRef PubMed.
- W. Bae, S. Baek and J. Chung, Biodegradation, 2001, 12, 359–366 CrossRef PubMed.
- V. M. Vadivelu, J. Keller and Z. Yuan, Water Sci. Technol., 2007, 56, 89–97 CrossRef PubMed.
- U. Abeling and C. F. Seyfried, Water Sci. Technol., 1992, 26, 1007–1015 CrossRef.
- APHA, Standard Methods for the examination of water and wastewater, American Public Health Association, Washington D.C. US, 20th edn, 1998 Search PubMed.
- K. I. Gil and E. S. Choi, Biotechnol. Lett., 2001, 23, 2021–2026 CrossRef.
- J. Carrera, I. Jubany, L. Carvallo, R. Chamy and J. Lafuente, Process Biochem., 2004, 39, 1159–1165 CrossRef.
- S. Park, W. Bae and B. E. Rittmann, Process Biochem., 2009, 44, 631–640 CrossRef.
- G. M. C. Wong and R. C. Loehr, Water Res., 1978, 12, 605–660 CrossRef.
- J. X. Ma, Z. W. Wang, Y. Yang, X. J. Mei and Z. C. Wu, Water Res., 2013, 47, 859–869 CrossRef PubMed.
- L. W. Shen, Y. Yao and F. G. Meng, J. Membr. Sci., 2014, 462, 139–146 CrossRef.
- X. W. Zhang, J. Zhang and Z. Hu, RSC Adv., 2015, 5, 61345–61353 RSC.
- J. Snaidr, R. Amann and I. Huber, Appl. Environ. Microbiol., 1997, 63, 2884–2896 Search PubMed.
- H. Daims, J. L. Nielsen and P. H. Nielsen, Appl. Environ. Microbiol., 2001, 67, 5273–5284 CrossRef PubMed.
- S. Siripong and B. E. Rittmann, Water Res., 2007, 41, 1110–1120 CrossRef PubMed.
- C. Hellinga, M. C. M. van Loosdrecht and J. J. Heijnen, Math. Comput. Model. Dyn. Syst., 1999, 5, 351–371 CrossRef.
- U. Dongen, M. S. Jetten and M. C. M. van Loosdrecht, Water Sci. Technol., 2001, 44, 153–160 CrossRef PubMed.
- B. Ma, B. Peng, Y. Wei, G. Zhu, Z. Yuan and Y. Peng, Sci. Rep., 2015, 5, 13048 CrossRef PubMed.
- V. M. Vadivelu, J. Keller and Z. G. Yuan, Water Res., 2007, 41, 826–834 CrossRef PubMed.
- Q. L. Wang, H. R. Duan, W. Wei and Z. G. Yuan, Environ. Sci. Technol., 2017, 51, 9800–9807 CrossRef PubMed.
- Q. L. Wang, ACS Sustainable Chem. Eng., 2017, 5, 9630–9633 CrossRef.
- Q. L. Wang, L. Ye, G. M. Jiang, S. H. Hu and Z. G. Yuan, Water Res., 2014, 55, 245–255 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |