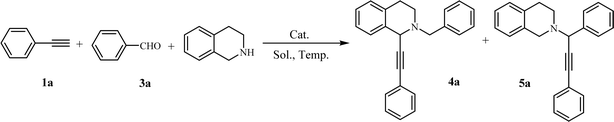
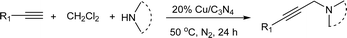Open Access Article
Open Access ArticleRecyclable Cu/C3N4 composite catalyzed AHA/A3 coupling reactions for the synthesis of propargylamines†
Hang Xua,
Jun Wanga,
Peng Wanga,
Xiyu Niua,
Yidan Luob,
Li Zhub and
Xiaoquan Yao *a
*a
aDepartment of Applied Chemistry, School of Material Science and Technology, Nanjing University of Aeronautics and Astronautics, Nanjing 210016, PR China. E-mail: yaoxq@nuaa.edu.cn
bDepartment of Chemistry, School of Pharmacy, Nanjing Medical University, Nanjing 211166, PR China
First published on 24th September 2018
Abstract
The heterogeneous Cu/C3N4 catalyst was found to be efficient for the synthesis of propargylamines using a three-component coupling reaction of alkynes, CH2Cl2 and amines (AHA) without additional base. Moreover, the catalyst also showed highly catalytic activity in the synthesis of C1-alkynylated tetrahydroisoquinolines (THIQs) via an A3 reaction of alkynes, aldehydes and THIQ. The Cu/C3N4-catalyzed multicomponent reactions exhibited good functional group tolerance in most examples. Furthermore, the easily prepared Cu/C3N4 catalyst could be recovered and reused conveniently over 5 times without losing catalytic activities.
Introduction
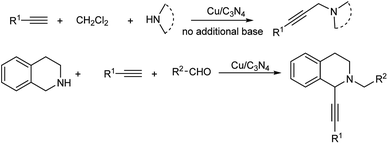
Multicomponent reactions (MCRs) are pretty important and effective in carbon–carbon bond formation because of their considerable economic and ecological merits.1 A lot of organic compounds could be produced via MCRs, for example, propargylamines.Propargylamines are very essential in pharmaceuticals due to their special nitrogen-containing biologically active structure, and have wide application in synthetic chemistry.2 Traditionally, propargylamines were synthesized by stoichiometric nucleophilic attack of metal acetylides on imines3 or through the amination of propargylic halides4 and propargylic triflates.5 In the last decade, a catalytic three-component coupling reaction of alkynes, aldehydes and amines (A3 reaction) was developed as an efficient synthetic methodology for propargylamines, and many metal catalysts were utilized successfully.6 Meanwhile, another multicomponent reaction for the synthesis of propargylamines was also achieved through the coupling of alkynes, dihalomethane and amines (AHA reaction). Remarkable efforts on this method have been made by utilizing effective catalysts such as CuCl,7 nano-In2O3,8 FeCl3,9 AgOAc,10 CoBr2,11 Nipy4Cl2,12 Au NPs13 and K[AuCl4].14 However, in most of the examples above, additional strong bases and additional solvents have to be required. In our previous work, a Cu NPs-catalyzed AHA reaction was accomplished in CH2Cl2 solution.15 Although a highly catalytic activity was observed, additional strong base (Cs2CO3) was also required in the reaction and only low recyclability of the Cu NPs catalyst was observed. On the other hand, a metal-free AHA reaction was also developed, but only moderate yields were obtained.16
Moreover, as a special secondary amine, tetrahydroisoquinolines (THIQs) are found widely existed in natural products and have many biological activities.17 Thus, many novel and valid methods were developed to synthesize THIQ derivatives, and most of them focused on the activation of THIQ's C1 atom.18 Recently, an efficient C1-alkynylation method for THIQs was developed through the A3 coupling reaction of THIQ, aldehydes and alkynes, in which AgOAc,19 CuI,20 and CuBr21 were reported as efficient catalysts. To develop greener approaches for the reaction, some efforts on the catalyst's immobilization were also tried, and polymers22 or magnetic materials23 supported copper species were reported. However, both of them have to suffer lowered catalytic efficiency as well as complicated preparing procedure for immobilized catalysts.
N-doped carbon materials are a kind of novel superior materials and have been wildly used as electrode materials because of its porous structure and amine-containing molecules.24 The materials have also come into the view of organic chemists in recent years due to their superior performance and easy preparation.25 Very recently, we reported a Cu/C3N4 composite-catalyzed homo- & cross-coupling reaction of terminal alkynes,26 in which the easily prepared composite catalyst showed much higher catalytic activity than Cu NPs with excellent recyclability.
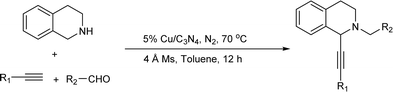
Since the Cu/C3N4 composite shows highly catalytic activity in Glaser–Hay reaction involving the activation of terminal alkynes, herein, we hope to investigate its application in AHA reaction of alkynes, dihaloalkanes and amines, and A3 coupling reaction of THIQ, aldehydes and alkynes (Scheme 1). The Cu/C3N4 catalyst exhibited excellent catalytic activities as well as good functional group tolerance in most examples. Furthermore, excellent recyclability of the catalyst was also achieved.
Experimental
Synthesis of Cu/C3N4 compounds
The Cu/C3N4 catalyst was synthesized following the reported method. Typically, melamine (2 g) was uniformly mixed with copper(II) acetate (625 mg). The resulting mixture was then heated to 550 °C with 2 °C min−1 in a tube furnace under N2 atmosphere and kept for 2 h. After cooling to room temperature, the final solid product (Cu/C3N4) was collected without further purification.Typical procedure for Cu/C3N4-catalyzed AHA reaction of dichloromethane, alkynes and amines
The mixture of p-bromophenylacetylene (0.15 mmol) and piperidine (0.45 mmol), 20% Cu/C3N4 (9.6 mg, 20 mol%) in anhydrous DCM (0.5 mL) was heated at 50 °C for 24 h under nitrogen atmosphere. After completion of the reaction, the mixture was concentrated to yield the crude product, which was further purified by flash chromatography (silica gel, petroleum ether/ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the desired product.
1) to give the desired product.
The detailed characterization data for 2a–2u are provided in the ESI.†
Typical procedure for Cu/C3N4-catalyzed A3 reaction of alkynes, aldehydes and THIQ
The mixture of benzaldehyde (0.2 mmol), phenylacetylene (0.3 mmol), THIQ (0.25 mmol), 5% Cu/C3N4 (6.4 mg, 2.5 mol%) and 4 Å MS (25 mg) in anhydrous toluene (0.5 mL) was heated at 70 °C for 12 h under nitrogen atmosphere. After completion of the reaction, the mixture was concentrated to yield the crude product, which was further purified by flash chromatography (silica gel, petroleum ether/ethyl acetate = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the desired product.
1) to give the desired product.
The detailed characterization data for 4a–4o are provided in the ESI.†
The experiments of catalyst recycle in AHA reaction
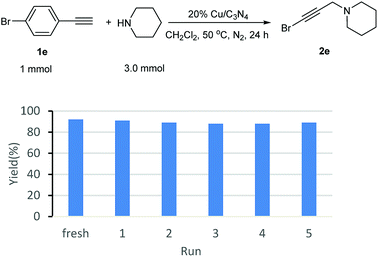
p-Bromophenylacetylene (1.0 mmol), piperidine (3.0 mmol), 20% Cu/C3N4 (64.0 mg), and 2 mL of CH2Cl2 were added into a 30 mL sealed tube under N2. The mixture was stirred at 50 °C for 24 hours. After completion of the reaction, the catalyst was separated by centrifugation and washed by water, ethanol and ether, and then, dried under vacuum at 60 °C. The recovered catalyst was reused for the next cycle with fresh starting materials and solvent.The experiments of catalyst recycle in A3 reaction
Phenylacetylene (2.0 mmol), benzaldehyde (1.0 mmol), THIQ (1.5 mmol), 5% Cu/C3N4 (32.0 mg), 4 Å MS (125 mg), and 2 mL of toluene were added into a 30 mL sealed tube under N2. The mixture was stirred at 70 °C for 12 hours. After completion of the reaction, the catalyst was separated by centrifugation and washed by water, ethanol and ether, and then, dried under vacuum at 60 °C. The recovered catalyst was reused for the next cycle with fresh starting materials and solvent.Results and discussion
With the Cu/C3N4 composite as catalyst, the AHA coupling reaction of p-bromophenylacetylene, dichloromethane and piperidine was selected as the prototype to start our investigation for the optimized reaction conditions, and the results were summarized in Table 1.| Entry | Catalyst | T/°C | Base | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: p-bromophenylacetylene (0.15 mmol), piperidine (0.15 mmol), base (0.3 mmol) and dichloromethane 0.5 mL as solvent under N2 at 50 °C for 24 h.b Isolated yield.c Reaction conditions: p-bromophenylacetylene (0.15 mmol), piperidine (0.45 mmol) and dichloromethane 0.5 mL as solvent under N2 at 50 °C for 24 h.d Reaction conditions: p-bromophenylacetylene (0.15 mmol), piperidine (0.45 mmol) and dichloromethane (0.3 mmol) in 0.5 mL CH3CN under N2 at 50 °C for 24 h.e Reaction conditions: p-bromophenylacetylene (0.15 mmol), piperidine (0.45 mmol) and dichloromethane (0.3 mmol) in 0.5 mL toluene under N2 at 50 °C for 24 h. | ||||
| 1 | 20% Cu/C3N4 (20 mol%) | 50 | Cs2CO3 | 74 |
| 2 | 20% Cu/C3N4 (20 mol%) | 50 | DBU | 87 |
| 3c | 20% Cu/C3N4 (20 mol%) | 50 | None | 92 |
| 4c | 10% Cu/C3N4 (20 mol%) | 50 | None | 77 |
| 5c | 5% Cu/C3N4 (20 mol%) | 50 | None | 57 |
| 6c | 20% Cu/C3N4 (10 mol%) | 50 | None | 85 |
| 7c | 20% Cu/C3N4 (5 mol%) | 50 | None | 75 |
| 8d | 20% Cu/C3N4 (20 mol%) | 50 | None | 61 |
| 9e | 20% Cu/C3N4 (20 mol%) | 50 | None | 69 |
| 10c | 20% Cu/C3N4 (20 mol%) | 30 | None | 35 |
| 11c | 20% Cu/C3N4 (20 mol%) | 60 | None | 92 |
| 12c | Cu NPs (20 mol%) | 50 | None | 30 |
| 13c | None | 50 | None | Trace |
At the beginning, the reaction was carried out with Cu/C3N4 as catalyst and Cs2CO3 as base under N2 atmosphere at 50 °C for 24 hours, and a moderate yield was observed (entry 1, Table 1). When DBU was used as base, 87% of 2e was obtained (entry 2). Noteworthy, an up to 92% of yield was achieved with 3.0 equiv. of piperidine introduced as reactant & base (entry 3). The copper loading amount in catalyst was then evaluated. As can be seen, decreasing the copper loading resulted in worse yields (entries 4 and 5 vs. entry 3). The yields were also decreased with reducing catalyst loading (entries 6 and 7). Other solvents, such as CH3CN and toluene, were ineffective in this catalytic system (entries 8 and 9).
Moreover, the influence of temperature was also investigated. The yield of 2e decreased sharply at 30 °C, while the yield was not changed when the reaction temperature was increased to 60 °C (entries 10 and 11 vs. entry 3).
To understand the effect of C3N4 support on the catalytic activity, a controlled experiment was carried out, in which Cu NPs was used as catalyst. Obviously, without additional strong base,15 much worse result was observed (entry 12). This result might indicate that the C3N4 support increased the catalytic activity of copper species successfully under current conditions. On the other hand, there was no progress of reaction under a catalyst-free condition (entry 13).
Thus, the optimal conditions involved the following parameters: 20 mol% of 20% Cu/C3N4 and 3.0 equiv. of amines in 0.5 mL of dichloromethane at 50 °C under nitrogen for 24 h.
Under the optimized reaction conditions, we then tested the scope of AHA reaction of alkynes, dichloromethane, and amines. As shown in Table 2, aromatic acetylene derivatives underwent the coupling with dichloromethane and piperidine smoothly to afford the respective propargylamines in excellent yields of 85–94% (2a–2g). Less reactive aliphatic alkyne, 1-hexyne, was also suitable for this reaction, and a moderate yield was observed (2h). Moreover, the AHA coupling reaction of alkynes, dichloromethane and pyrrole was also investigated, and good yields were obtained (2i–2m). Delightfully, acyclic secondary amine like diethylamine was also efficiently converted into the corresponding propargylamines in good yields (2n–2s). However, morpholine and diisopropylamine seem not be good substrates in current reaction (2t–2u).
| a Reaction conditions: alkynes (0.15 mmol), amines (0.45 mmol) and dichloromethane 0.5 mL as solvent with 20% Cu/C3N4 (20 mol%) under N2 at 50 °C for 24 h; isolated yield. |
|---|
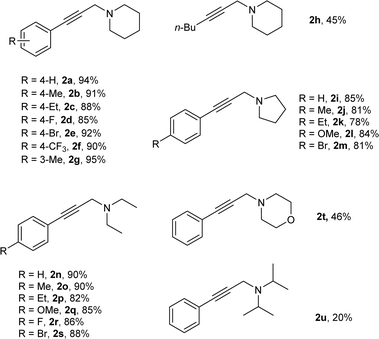 |
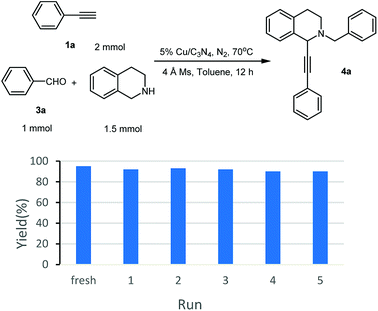
As known to all, one of the advantages of heterogeneous catalysts is their easy separation from the reaction mixture. Without the distribution from additional base, the catalyst could be separated and recovered easily by centrifugation from the reaction mixture, and then, fresh substrates were added to set up a new reaction. Following this procedure, the recyclability of Cu/C3N4 was also investigated. It can be seen from Fig. 1 that the yields were kept in 91–88% in 5 recycles. There was only ca. 1–4% yield decrease compared with the fresh reaction.
As can be seen from above results, the Cu/C3N4 catalyst showed great catalytic activities in AHA coupling reaction as well as good recyclability without additional base. With this catalyst in hand, therefore, we also examined its utilization in A3 coupling reaction of alkynes, aldehydes and THIQ.
At the beginning of the investigation, excess amounts of THIQ and phenylacetylene were used in order to consume all of benzaldehyde, which was inseparable from products 4a and 5a by column chromatography. Firstly, the A3 reaction was carried out in various solvents, with 5% Cu/C3N4 (2.5 mol%) as catalyst, under N2 atmosphere at 70 °C for 24 h. When the reaction was carried in water, a moderate yield of 5a was obtained as the single product (entry 1, Table 3). Both EtOH and isopropanol (IPA) could be processed effectively to afford 4a as the major product (entries 2 and 3). Apparently, an enhanced reaction yield and regioselectivity was obtained in the presence of additional 4 Å MS (entry 4 vs. 3). To our delight, in toluene with 4 Å MS, a regiospecific reaction to 4a was accomplished with excellent yield (entry 5).
| Entry | Catalyst | T/oC | Solvent | Yieldb (%) 4a + 5a | Ratioc 4a/5a |
|---|---|---|---|---|---|
| a Reaction conditions unless otherwise noted: benzaldehyde 3a (0.2 mmol), phenylacetylene 1a (0.3 mmol), THIQ (0.25 mmol), solvent (0.5 mL), reaction time 24 h in cube with N2.b Isolated combined yield of 4a and 5a.c Ratio was determined by NMR prior to purification.d 4 Å MS (25 mg) was added.e 12 h.f 8 h. | |||||
| 1 | 5% Cu/C3N4 (2.5 mol%) | 70 | H2O | 46 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 5% Cu/C3N4 (2.5 mol%) | 70 | EtOH | 54 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 5% Cu/C3N4 (2.5 mol%) | 70 | IPA | 50 | 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4d | 5% Cu/C3N4 (2.5 mol%) | 70 | EtOH | 62 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5d | 5% Cu/C3N4 (2.5 mol%) | 70 | Toluene | 97 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 6d | 20% Cu/C3N4 (2.5 mol%) | 70 | Toluene | 90 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 7d | Cu NPs (2.5 mol%) | 70 | Toluene | 70 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 8d | 5% Cu/C3N4 (1.25 mol%) | 70 | Toluene | 85 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 9d | 5% Cu/C3N4 (5 mol%) | 70 | Toluene | 97 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 10d | 5% Cu/C3N4 (2.5% mol%) | 50 | Toluene | 76 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 11d | 5% Cu/C3N4 (2.5% mol%) | 25 | Toluene | 35 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 12d,e | 5% Cu/C3N4 (2.5 mol%) | 70 | Toluene | 97 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 13d,f | 5% Cu/C3N4 (2.5 mol%) | 70 | Toluene | 78 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
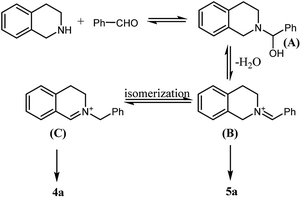
From these results above, water is the key factor affecting the reaction selectivity. Following the mechanism described in Scheme 2,21 iminium B was formed firstly following the dehydrogenation of intermediate A, and then, B was isomerized to give iminium C as the key intermediate to the desired product 4a. Obviously, in the presence of water, the formation of iminium B might be hindered, as a result, the isomerization procedure would be prohibited as well. Therefore, 5a was observed as the single product in water solution.
Other heterogeneous copper catalysts, such as 20% Cu/C3N4 and Cu NPs were also tested, the yields were decreased obviously (entries 6 and 7).
Decreasing the loading of catalyst resulted in worse yield, but the yield was not changed when the loading of catalyst enhanced (entries 8 and 9). Lowering the reaction temperature to 50 °C or 30 °C resulted in decreased yields (entries 10 and 11). When the reaction time was shortened to 12 h, the yield was not changed. However, when the reaction time was further shortened to 8 h, only 78% of 4a was obtained (entries 12 and 13).
Thus, the optimized reaction conditions for A3 coupling reaction were 5% Cu/C3N4 (2.5 mol%) in toluene with 4 Å MS at 70 °C for 12 h under N2 atmosphere.
Under the optimized reaction conditions, a series of terminal alkynes and aldehydes were explored. The results were listed in Table 4. Aromatic aldehydes with electron-donating groups and electron-withdrawing groups worked well to generate the corresponding products in excellent yields (4a–4g). Aliphatic aldehyde like n-caprylic aldehyde could be converted into the desired product with a 72% of yield (4h).
| a Reaction condition: aldehydes (0.2 mmol), alkynes (0.3 mmol), THIQ (0.25 mmol), 4 Å MS (25 mg), toluene (0.5 mL), reaction time 12 h, 70 °C; isolated yield. |
|---|
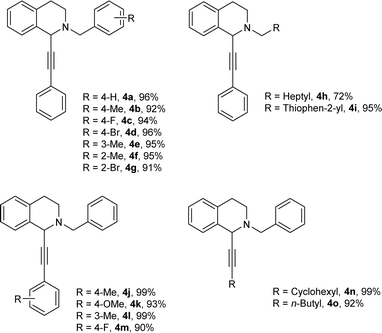 |
Thiophene-2-carbaldehyde was also applicable under the optimized reaction conditions to afford the corresponding product 4i in 95% yield. Various terminal alkynes were then examined for the target reaction. Phenylacetylenes bearing electron-donating groups such as methyl, methoxy at the para or meta positions, underwent the reaction to provide the corresponding products in excellent yields (4j–4l). Aliphatic alkynes, such as ethynylcyclohexane and 1-hexyne, were also suitable for this reaction, and good yields were observed (4n–4o).
Moreover, the recyclability of the Cu/C3N4 catalyst in this reaction was then investigated. Similar to that in AHA reaction, good recyclability was achieved and no significant decrease in yield was observed after 5 recycles (Fig. 2).27
Conclusions
In conclusion, with Cu/C3N4 composite as catalyst, a facile, efficient, and environmental friendly synthetic strategy for propargylic amines was developed by the three-component coupling reaction of alkynes, CH2Cl2 and amines. Good functional group tolerance and high yields were accomplished without additional base required. Moreover, the A3 coupling reaction of alkynes, aldehydes and THIQ for the synthesis of C1-alkynylated tetrahydroisoquinolines (THIQs) could also be achieved by utilizing Cu/C3N4 as catalyst. The Cu/C3N4 catalyst was tolerant of a wide variety of functional groups and good to excellent yields were achieved in most examples. Moreover, the heterogeneous catalyst could be recovered and reused conveniently for several times with satisfactory yields in both of the reactions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the financial support from National Natural Science Foundation of China (21472092 to XY). This is a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.Notes and references
- M. S. Singh and S. Chowdhury, RSC Adv., 2012, 2, 4547 RSC.
- (a) H. Ohno, Y. Ohta, S. Oishi and N. Fujii, Angew. Chem., Int. Ed., 2007, 46, 2295 CrossRef PubMed; (b) B. Yan and Y. Liu, Org. Lett., 2007, 9, 4323 CrossRef PubMed; (c) K. Cao, F. M. Zhang, Y. Q. Tu, X. T. Zhuo and C. A. Fan, Chem.–Eur. J., 2009, 15, 6332 CrossRef PubMed; (d) Y. Ohta, S. Oishi, N. Fujii and H. Ohno, Org. Lett., 2009, 11, 1979 CrossRef PubMed; (e) H. Nakamura, S. Onagi and T. Kamakura, J. Org. Chem., 2005, 70, 2357 CrossRef PubMed; (f) T. Sugiishi, A. Kimura and H. Nakamura, J. Am. Chem. Soc., 2010, 132, 5332 CrossRef PubMed; (g) H. Nakamura, T. Kamakura, M. Ishikura and J. F. Biellmann, J. Am. Chem. Soc., 2004, 126, 5958 CrossRef PubMed.
- S. Czernecki and J. M. Valéry, J. Carbohydr. Chem., 1990, 9, 767 CrossRef.
- I. E. Kopka, Z. A. Fataftah and M. W. Rathke, J. Org. Chem., 1980, 45, 4616 CrossRef.
- T. Murai, Y. Mutoh, Y. Ohta and M. Murakami, J. Am. Chem. Soc., 2004, 126, 5968 CrossRef PubMed.
- (a) C. Wei, J. T. Mague and C.-J. Li, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5749 CrossRef PubMed; (b) C. Wei and C.-J. Li, J. Am. Chem. Soc., 2002, 124, 5638 CrossRef PubMed; (c) L. Shi, Y. Q. Tu, M. Wang, F. M. Zhang and C. A. Fan, Org. Lett., 2004, 6, 1001 CrossRef PubMed; (d) M. Yu, Y. Wang, C.-J. Li and X. Yao, Tetrahedron Lett., 2009, 50, 6791 CrossRef; (e) M. J. Albaladejo, F. Alonso, Y. Moglie and M. Yus, Eur. J. Org. Chem., 2012, 16, 3093 CrossRef; (f) C. Wei, Z. Li and C.-J. Li, Org. Lett., 2003, 5, 4473 CrossRef PubMed; (g) C. Wei and C.-J. Li, J. Am. Chem. Soc., 2003, 125, 9584 CrossRef PubMed; (h) X. Zhang and A. Corma, Angew. Chem., Int. Ed., 2008, 47, 4358 CrossRef PubMed; (i) L. L. Chng, J. Yang, Y. Wei and J. Y. Ying, Adv. Synth. Catal., 2009, 351, 2887 CrossRef; (j) S. Subhasis, G. N. Chandra and M. S. Singh, Tetrahedron Lett., 2010, 51, 5555 CrossRef; (k) W. W. Chen, R. V. Nguyen and C.-J. Li, Tetrahedron Lett., 2009, 50, 2895 CrossRef; (l) T. Q. Zeng, W. W. Chen and C.-J. Li, Green Chem., 2010, 12, 570 RSC; (m) W.-W. Chen, H.-P. Bi and C.-J. Li, Synlett, 2010, 3, 475 Search PubMed; (n) S. Sakaguchi, T. Mizuta, M. Furuwan, T. Kubo and Y. Ishii, Chem. Commun., 2004, 14, 1638 RSC.
- D. Yu and Y. Zhang, Adv. Synth. Catal., 2011, 353, 163 CrossRef.
- M. Rahman, A. K. Bagdi, A. Majee and A. Hajra, Tetrahedron Lett., 2011, 52, 4437 CrossRef.
- J. Gao, Q. W. Song, L. N. He, Z. Z. Yang and X. Y. Dou, Chem. Commun., 2012, 48, 2024 RSC.
- X. Chen, T. Chen, Y. Zhou, C. T. Au, L. B. Han and S. F. Yin, Org. Biomol. Chem., 2014, 12, 247 RSC.
- Y. Tang, T. Xiao and L. Zhou, Tetrahedron Lett., 2012, 53, 6199 CrossRef.
- S. R. Lanke and B. M. Bhanage, Appl. Organomet. Chem., 2013, 27, 729 CrossRef.
- A. Berrichi, R. Bachir, M. Benabdallah and N. Choukchou-Braham, Tetrahedron Lett., 2015, 56, 1302 CrossRef.
- D. Aguilar, M. Contel and E. P. Urriolabeitia, Chem.–Eur. J., 2010, 16, 9287 CrossRef PubMed.
- S. Zeng, S. Xu, Y. Wang, M. Yu, L. Zhu and X. Yao, Chin. J. Org. Chem., 2015, 35, 827 CrossRef.
- V. S. Rawat, T. Bathini, S. Govardan and B. Sreedhar, Org. Biomol. Chem., 2014, 12, 6725 RSC.
- (a) D. Jack and R. Williams, Chem. Rev., 2002, 102, 1669 CrossRef; (b) K. W. Bentley, Nat. Prod. Rep., 2006, 23, 444 RSC; (c) Q. Y. Zhang, G. Z. Tu, Y. Y. Zhao and T. M. Cheng, Tetrahedron, 2002, 58, 6795 CrossRef; (d) A. J. Aladesanmi, C. J. Kelly and J. D. Leary, J. Nat. Prod., 1983, 46, 127 CrossRef; (e) A. Zhang, J. L. Neumeyer and R. J. Baldessarini, Chem. Rev., 2007, 107, 274 CrossRef PubMed; (f) K. Ye, Y. Ke, N. Keshava, J. Shanks, J. A. Kapp, R. R. Tekmal, J. Petros and H. C. Joshi, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 1601 CrossRef PubMed; (g) C. J. Kelleher, L. Cardozo, C. R. Chapple, F. Haab and A. M. Ridder, BJU Int., 2005, 95, 81 CrossRef PubMed.
- (a) K. Alagiri, G. S. R. Kumara and K. R. Prabhu, Chem. Commun., 2011, 47, 11787 RSC; (b) J. Dhineshkumar, M. Lamani, K. Alagiri and K. R. Prabhu, Org. Lett., 2013, 44, 1092 CrossRef PubMed; (c) B. Gröll, P. Schaaf and M. Schnürch, Monatsh. Chem., 2017, 148, 91 CrossRef PubMed; (d) Z. Li and C.-J. Li, J. Am. Chem. Soc., 2005, 127, 3672 CrossRef PubMed.
- G. Shao, Y. He, Y. Xu, J. Chen, H. Yu and R. Cao, Eur. J. Org. Chem., 2015, 4615 CrossRef.
- H. Zheng, W. Meng, G. J. Jiang and Z. X. Yu, Org. Lett., 2013, 15, 5928 CrossRef PubMed.
- W. Lin, T. Cao, W. Fan, Y. Han, J. Kuang, H. Luo, B. Miao, X. Tang, Q. Yu, W. Yuan, J. Zhang, C. Zhu and S. Ma, Angew. Chem., Int. Ed., 2014, 53, 277 CrossRef PubMed.
- (a) H. Zhao, W. He, L. Wei and M. Cai, Catal. Sci. Technol., 2016, 6, 1488 RSC; (b) G. H. Dang, D. T. Le, T. Truong and N. T. Phan, J. Mol. Catal. A: Chem., 2015, 400, 162 CrossRef.
- U. Gulati, S. Rawat, U. C. Rajesh and D. S. Rawat, New J. Chem., 2017, 41, 8341 RSC.
- Some examples of electrode materials: (a) M. Yang, B. Cheng, H. Song and X. Chen, Electrochim. Acta, 2010, 55, 7021 CrossRef; (b) D. H. Jurcakova, M. Kodama, S. Shiraishi, H. Hatori, Z. H. Zhu and G. Q. Lu, Adv. Funct. Mater., 2009, 19, 1800 CrossRef; (c) J. P. Dong, X. M. Qu, L. J. Wang, C. J. Zhao and J. Q. Xu, Electroanalysis, 2008, 20, 1981 CrossRef; (d) K. Jurewicz, R. Pietrzak, P. Nowicki and H. Wachowska, Electrochim. Acta, 2008, 53, 5469 CrossRef; (e) G. Lota, K. Lota and E. Frackowiak, Electrochem. Commun., 2007, 9, 1828 CrossRef; (f) Y. J. Kim, Y. Abe, T. Yanagiura, K. C. Park, M. Shimizu, T. Iwazaki, S. Nakagawa, M. Endo and M. S. Dresselhaus, Carbon, 2007, 45, 2116 CrossRef; (g) W. Li, D. Chen, Z. Li, Y. Shi, Y. Wan, J. Huang, J. Yang, D. Zhao and Z. Jiang, Electrochem. Commun., 2007, 9, 569 CrossRef.
- (a) K. K. R. Datta, B. V. S. Reddy, K. Ariga and A. Vine, Angew. Chem., 2010, 122, 6097 CrossRef; (b) Y. Wang, J. Yao, H. Li, D. Su and M. Antonietti, J. Am. Chem. Soc., 2011, 133, 2362 CrossRef PubMed . Our group also reported two examples:; (c) W. Lv, J. Tian, N. Deng, Y. Wang, X. Zhu and X. Yao, Tetrahedron Lett., 2015, 56, 1312 CrossRef; (d) L. Wang, M. Yu, C. Wu, N. Deng, C. Wang and X. Yao, Adv. Synth. Catal., 2016, 358, 2631 CrossRef.
- H. Xu, K. Wu, J. Tian, L. Zhu and X. Yao, Green Chem., 2018, 20, 793 RSC.
- The slightly decreased catalytic activity might be caused by the loss of copper species. A leaching experiment was carried out with the residue of centrifugal liquid recovered from fresh run as catalyst. ca. 7% of yield to 4a was obtained, which indicated that there was a slight loss of copper species during catalyst recycles. In fact, there are many similar examples reported, also see: (a) Y. Wang, C. Wu, S. Nie, D. Xu, M. Yu and X. Yao, Tetrahedron Lett., 2015, 56, 6827 CrossRef; (b) W. Yan, R. Wang, Z. Xu, J. Xu, L. Lin, Z. Shen and Y. Zhou, J. Mol. Catal. A: Chem., 2006, 255, 81 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra06613b |
| This journal is © The Royal Society of Chemistry 2018 |