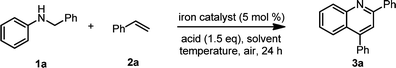
Open Access Article
Open Access ArticleAcid-promoted iron-catalysed dehydrogenative [4 + 2] cycloaddition for the synthesis of quinolines under air†
Jinfei Yang *,
Xiao Meng,
Kai Lu,
Zhihao Lu,
Minliang Huang,
Chengniu Wang and
Fei Sun*
*,
Xiao Meng,
Kai Lu,
Zhihao Lu,
Minliang Huang,
Chengniu Wang and
Fei Sun*
Medical School, Institute of Reproductive Medicine, Nantong University, Nantong 226019, China. E-mail: jfyang@ntu.edu.cn; sunfei@ntu.edu.cn
First published on 10th September 2018
Abstract
An acid-promoted iron-catalysed dehydrogenative [4 + 2] cycloaddition reaction was developed for the synthesis of quinolines using air as a terminal oxidant. Acetic acid was the best cocatalyst for the cycloaddition of N-alkyl anilines with alkenes or alkynes under air. Various quinoline derivatives were obtained in satisfactory-to-excellent yields, and no other byproducts besides water were produced in the reaction. The zebrafish model has become an important vertebrate model for evaluating drug effects. We tested the activity of 3n in zebrafish. The test results showed that 1 μg mL−1 3n treatments resulted in morphological malformation, and 0.01–0.1 μg mL−1 3n treatments led to potent angiogenic defects in zebrafish embryos. The results of this study will be of great significance for promoting drug research in cardiovascular and cerebrovascular diseases.
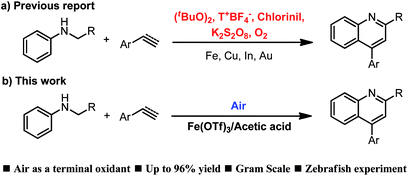
The construction of quinoline motifs has received intensive attention owing to their potential application in photovoltaic devices1 and pharmaceuticals,2 such as anticancer, antiviral, antifungal, antiplatelet aggregation, antimalarial, antibacterial, antileishmanial and anti-inflammatory medicine.3 Because of their importance, many methods have been reported for the synthesis of quinolines.4 Among them, the most attractive strategy for the synthesis of these compounds is the dehydrogenative [4 + 2] cycloaddition through transition-metal catalysis and Lewis/Bronsted acid catalysis. In its most general and classical form, dehydrogenative [4 + 2] cycloaddition catalysed by transition metals such as Fe,5 Cu,6 Pd,7 and others,8 has been used as a potent tool for the synthesis of quinolone derivatives (Scheme 1a). However, these methods require the presence of excess peroxides, chloranil, potassium persulfate or other oxidants to promote the cycloaddition reaction and to obtain good product yields. Furthermore, in these processes, the formation of stoichiometric amounts of acid or tetrachlorohydroquinone waste as byproducts is a substantial problem that has limited their use. To overcome these drawbacks, several methods that utilise oxygen as a terminal oxidant have been reported.9 However, in many cases, the industrial use of these methods is problematic owing to operational difficulty. Therefore, the development of more efficient and economical synthetic methods is still necessary. Undoubtedly, the use of air as a terminal oxidant is the best choice. In addition, in the field of transition-metal catalysis, iron is one of the most commonly used base metals and has been widely applied in various coupling reactions.10 Therefore, it is desirable to develop an iron-catalysed dehydrogenative cycloaddition for the synthesis of quinoline under air.
 | ||
| Scheme 1 Different strategies for [4 + 2] cycloaddition of N-alkyl anilines and alkenes or alkynes by transition-metal catalysis. | ||
Herein, we report the first acid-promoted iron-catalysed dehydrogenative [4 + 2] cycloaddition of N-alkyl anilines with alkenes or alkynes using air as a terminal oxidant (Scheme 1b). Iron-catalysed cycloaddition reaction for the synthesis of quinolines under air has always been a challenge because of metal deactivation after the end of the catalytic cycle. We commenced our studies by treating N-benzylaniline (1a) and styrene (2a) with 5 mol% iron as a catalyst. Initially, we tried to use a variety of iron catalysts to catalyse the cycloaddition of N-alkyl anilines and olefins under air (Table 1, entries 1–6). Trace amounts of the desired product (3a) were obtained with FeCl2, Fe(OTf)2, Fe2(SO4)3 and Fe2O3, as detected by GC analysis, and a 28% or 33% yield was observed when FeCl3 or Fe(OTf)3 was used as a catalyst. To improve the reaction efficiency, different solvents such as ethanol, mesitylene, 1,4-dioxane, nitrobenzene, acetonitrile and toluene were tested (Table 1, entries 6–11).
| Entry | Catalyst (5 mol%) | Acid (0.3 mmol) | Solvent | T (°C) | Yielda (%) |
|---|---|---|---|---|---|
| a Isolated yields.b BNPA = 1,1′-binaphthyl-2,2′-diylhydrogen-phosphate.c AMSA = aminomethanesulfonic acid. | |||||
| 1 | Fe(OTf)2 | No | Toluene | 120 | Trace |
| 2 | FeCl2 | No | Toluene | 120 | Trace |
| 3 | FeCl3 | No | Toluene | 120 | 28 |
| 4 | Fe2O3 | No | Toluene | 120 | Trace |
| 5 | Fe2(SO4)3 | No | Toluene | 120 | Trace |
| 6 | Fe(OTf)3 | No | Toluene | 120 | 33 |
| 7 | Fe(OTf)3 | No | Ethanol | 120 | 0 |
| 8 | Fe(OTf)3 | No | Mysitylene | 120 | 26 |
| 9 | Fe(OTf)3 | No | Dioxane | 120 | 0 |
| 10 | Fe(OTf)3 | No | Nitrobenzene | 120 | 28 |
| 11 | Fe(OTf)3 | No | Acetonitrile | 120 | 23 |
| 12 | Fe(OTf)3 | No | Toluene | 150 | 42 |
| 13 | Fe(OTf)3 | No | Toluene | 140 | 49 |
| 14 | Fe(OTf)3 | No | Toluene | 100 | 16 |
| 15 | Fe(OTf)3 | No | Toluene | 80 | 8 |
| 16 | Fe(OTf)3 | No | Toluene | 60 | Trace |
| 17 | Fe(OTf)3 | No | Toluene | 40 | 0 |
| 18 | Fe(OTf)3 | H2SO4 | Toluene | 140 | 0 |
| 19 | Fe(OTf)3 | TfOH | Toluene | 140 | 0 |
| 20 | Fe(OTf)3 | TFA | Toluene | 140 | 65 |
| 21 | Fe(OTf)3 | PTSA | Toluene | 140 | 61 |
| 22b | Fe(OTf)3 | BNPA | Toluene | 140 | 57 |
| 23 | Fe(OTf)3 | HCOOH | Toluene | 140 | 42 |
| 24 | Fe(OTf)3 | BzOH | Toluene | 140 | 50 |
| 25 | Fe(OTf)3 | AcOH | Toluene | 140 | 82 |
| 26 | Fe(OTf)3 | PhB(OH)2 | Toluene | 140 | 35 |
| 27 | Fe(OTf)3 | B(OH)3 | Toluene | 140 | 15 |
| 28 | Fe(OTf)3 | Phenol | Toluene | 140 | 54 |
| 29c | Fe(OTf)3 | AMSA | Toluene | 140 | 51 |
| 30 | No | AcOH | Toluene | 140 | Trace |
The optimal solvent for the reaction was toluene (Table 1, entry 6). Encouraged by this result, we examined a wide range of reaction temperatures (Table 1, entries 12–17); the best yield was obtained at 140 °C, but it did not meet our expectations.
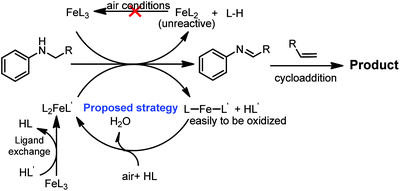
We reasoned that an unreactive catalytic species, FeL2 (Scheme 2), could be formed in the reaction from the interaction of the imine intermediate and FeL3, which could not catalyse the conversion of imines to quinoline. Critically, the FeL2 species was difficult to oxidize to FeL3 under air conditions. Inspired by Birk's work,11 we envisaged that the addition of an acid may promote the oxidation of Fe(II) to Fe(III) under air. Based on this assumption, we proposed that FeL3 can undergo ligand exchange with HL′ to generate the active catalytic species L2FeL′. A subsequent oxidation reaction provided LFeL′, which was easier oxidize to L2FeL′ than FeL2 under air, enabling the next catalytic cycle.
Based on this hypothesis, we investigated some strong acids and moderate acids. Trifluoroacetic acid (TFA) was a cocatalyst that promoted the Fe-catalysed [4 + 2] cycloaddition of N-alkyl anilines and alkenes to deliver 2,4-diphenylquinoline in 65% yield (Table 1, entries 18–20). If 1,1′-binaphthyl-2,2′-diylhydrogen phosphate or p-toluenesulfonic acid (PTSA) were used instead of TFA, the yield was significantly reduced (Scheme 3, entries 21–22). For further improvement of the reaction, other acid such as formic acid (HCOOH), benzoic acid (BzOH), acetic acid (AcOH), phenylboronic acid, boric acid, phenol and carbamic acid were tested (Table 1, entries 23–29). The results showed that the addition of 1.5 equivalents of acetic acid was the best choice, furnishing the corresponding 2,4-diphenylquinoline in 82% yield (Table 1, entry 25). Under the acidic conditions, a strong acid was completely inefficient (Table 1, entry 18), which suggested that the strength of the acid was critical to the reaction. Other acids did not give better results. Therefore, we performed the subsequent reactions between the N-alkyl anilines with alkenes or alkynes in the presence of Fe(OTf)3/AcOH at 140 °C under air conditions for 24 h.
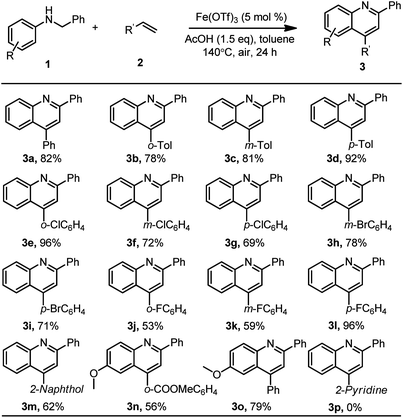 | ||
| Scheme 3 Reaction conditions: substrate 1 (0.2 mmol), aryl olefin (0.4 mmol), Fe(OTf)3 (10 μmol), AcOH (0.3 mmol), toluene (1.0 mL), at 140 °C under air for 24 h, and isolated yields of the products. | ||
With the optimized reaction conditions in hand, a series of aryl ethylenes were investigated for extending the substrate scope (Scheme 3). This acid-promoted iron-catalysed dehydrogenative [4 + 2] cycloaddition reaction displayed good functional group tolerance. Aryl ethylenes with electron-neutral or electron-donating groups on the aryl rings, such as alkyl, phenyl and naphthyl, all gave the corresponding 2,4-diarylquinoline with high selectivity in good yields. Aryls containing an electron-withdrawing group such as fluoro, chloro, bromo and ester were also tolerated and afforded the corresponding 2,4-diarylquinolines 3e–3n in moderate to good yields. Moreover, the reaction of N-benzylaniline 1b containing a substituent (MeO) at the para-position of the aniline ring also produced the corresponding quinoline products 3o in 79% yield. These results indicated that different groups, such as methyl, phenyl, fluoro, chloro, bromo and methoxyl on benzene rings, were tolerated under the optimized reaction conditions. Notably, the retention of the F, Cl and Br atoms in the structures of the products should make the products considerably useful in organic transformations. Unfortunately, the current method could not be applied to olefins containing N heteroatoms, which was likely because of the strong coordination of N atoms with iron.
Next, the scope of arylacetylenes was also investigated, and the results are summarized in Scheme 4. Arylacetylenes could be used instead of arylethylenes for the synthesis of 2,4-diarylquinoline under the optimized reaction conditions. Similar good results were obtained, as shown in Scheme 4. Quinoline derivatives 3a–3g, 3i, 3k–3m and 3o were obtained in satisfactory to good yields (63–96%).
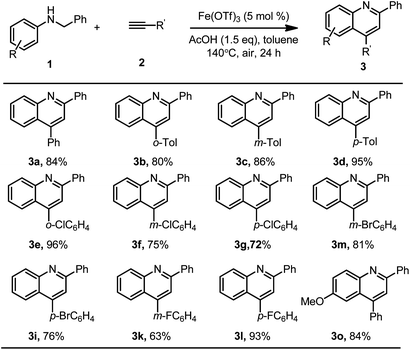 | ||
| Scheme 4 Reaction conditions: substrate 1 (0.2 mmol), aryl alkyne (0.4 mmol), Fe(OTf)3 (10 μmol), AcOH (0.3 mmol), toluene (1.0 mL), at 140 °C under air for 24 h, and isolated yields of the products. | ||
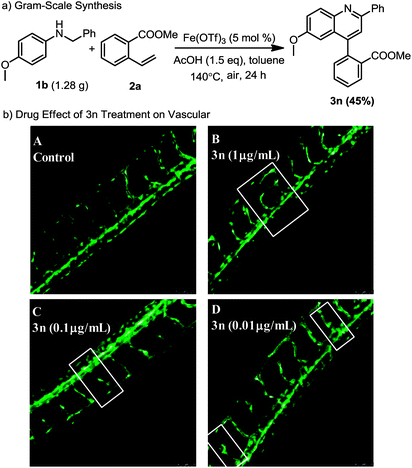
To test the synthetic utility of the current method, a gram scale dehydrogenative [4 + 2] cycloaddition reaction of N-benzyl-4-methoxyaniline with methyl-2-vinylbenzoate was conducted under the optimal conditions, providing the target 3n in 45% yield. To demonstrate the potential of our approach, we conducted molecular docking studies of human phenylethanolamine N-methyltransferase (hPNMT) and the quinoline derivatives. The studies were performed to help visualize possible interactions between hPNMT and the quinoline derivatives. The results showed that methyl-2-(6-methoxy-2-phenylquinolin-4-yl)benzoate 3n may have π–π interactions with ARG 90, and π–cation interactions with TYR 27 in hPNMT. Based on this docking result, 3n is highly likely to be a potent inhibitor of hPNMT. The results of the docked poses of hPNMT and 3n are shown in the ESI.† The zebrafish model has become an important vertebrate model for evaluating drug effects. 12 To demonstrate the drug effect of 3n on the vascular system in the trunk of zebrafish embryos, we tested the activity of 3n in zebrafish. The test results showed treatment of zebrafish embryos with 1 μg mL−1 3n resulted in morphological malformation, and treatment with 0.01–0.1 μg mL−1 3n led to potent angiogenic defects (Scheme 5b). The results of this study will be of great significance for promoting drug research in cardiovascular and cerebrovascular diseases.
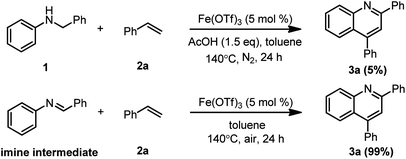
To gain a better understanding of the role of the acid, air and iron in the current cycloaddition reaction, additional experiments were conducted. First, control experiments showed that the absence of any of the three components, air, AcOH and Fe(OTf)3, significantly reduced the reaction yield, implying that each of the components was essential to this reaction. To clarify that the reaction was undergoing the production of an imine intermediate, we employed N-benzylideneaniline as a substrate to test if 2,4-diphenylquinoline could be obtained (Scheme 6). To our great surprise, 3a was obtained in 99% yield. The results showed that a cycloaddition reaction occurred after N-benzylaniline was oxidized to an imine. Based on these results, we proposed the following catalytic cycle: FeL3 first underwent ligand exchange with AcOH to generate an active catalytic species L2FeOAc, leading to subsequent oxidative dehydrogenation to provide the imine intermediate and intermediate LFeOAc while releasing HL. The imine intermediate can then undergo a [4 + 2] cycloaddition with an alkyne or alkene, forming the desired 2,4-diarylquinoline or dihydroquinoline. A subsequent dehydrogenation reaction of dihydroquinoline provided the target product. The intermediate LFeOAc underwent an oxidation reaction in the presence of air to regenerate the catalytic species L2FeOAc.
Conclusions
In summary, we successfully developed a highly efficient acid-promoted iron-catalysed dehydrogenative [4 + 2] cycloaddition for the synthesis of quinoline under air conditions. The new method is compatible with alkenes and alkynes, and we performed molecular docking experiments with 3n, which indicated that this compound might be an inhibitor of hPNMT. Moreover, we used the zebrafish model as an important way to demonstrate the drug effect of 3n treatment on the vascular system in the trunk of zebrafish embryos. The test results showed that 3n had a significant effect on angiogenesis. The results of this study will be of great significance for promoting drug research in cardiovascular and cerebrovascular diseases. Further investigation of cell experiments, animal experiments and other reaction types are under way in our laboratory.Ethics statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Nantong University and Experiments were approved by the Animal Ethics Committee of SYXK(SU) 2007–0021.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (81430027 and 81671510), the National Basic Research Program (973 Program) (2014CB943100) for financial support. This work was also supported by the Fundamental Research Funds of Nantong University (03081105 and 03083002).Notes and references
- For selected reviews, see: (a) A. Danel, E. Gondek and I. Kityk, Opt. Mater., 2009, 32, 267 CrossRef; (b) H. Xu, R. Chen, Q. Sun, W. Lai, Q. Su, W. Huang and X. Liu, Chem. Soc. Rev., 2014, 43, 3259 RSC.
- For selected reviews, see: (a) M. Orhan Puskullu, B. Tekiner and S. Suzen, Mini-Rev. Med. Chem., 2013, 13, 365 Search PubMed; (b) K. Gopaul, S. A. Shintre and N. A. Koorbanally, Anti-Cancer Agents Med. Chem., 2015, 15, 631 CrossRef; (c) O. Afzal, S. Kumar, M. R. Haider, M. R. Ali, R. Kumar, M. Jaggi and S. Bawa, Eur. J. Med. Chem., 2015, 97, 871 CrossRef PubMed.
- For selected reviews, see: (a) M. A. ElSohly and W. Gul, Recent Pat. Anti-Infect. Drug Discovery, 2007, 2, 222 CrossRef; (b) Y. Zhang, T. Han, Q. Ming, L. Wu, K. Rahman and L. Qin, Nat. Prod. Commun., 2012, 7, 963 Search PubMed; (c) P.-Y. Chung, Z.-X. Bian, H.-Y. Pun, D. Chan, A. S.-C. Chan, C.-H. Chui, J. C.-O. Tang and K.-H. Lam, Future Med. Chem., 2015, 7, 947 CrossRef PubMed.
- For related reviews, see: (a) J. Marco-Contelles, E. Pérez-Mayoral, A. Samadi, M. d. C. Carreiras and E. Soriano, Chem. Rev., 2009, 109, 2652 CrossRef PubMed; (b) A. Majumder, R. Gupta and A. Jain, Green Chem. Lett. Rev., 2013, 6, 151 CrossRef; (c) S. M. Prajapati, K. D. Patel, R. H. Vekariya, S. N. Panchal and H. D. Patel, RSC Adv., 2014, 4, 24463 RSC; (d) R. I. Khusnutdinov, A. R. Bayguzina and U. M. Dzhemilev, J. Organomet. Chem., 2014, 768, 75 CrossRef; (e) B. Eftekhari-Sis and M. Zirak, Chem. Rev., 2015, 115, 151 CrossRef PubMed; (f) G. A. Ramann and B. J. Cowen, Molecules, 2016, 21, 986 CrossRef PubMed; (g) V. F. Batista, D. C. G. A. Pinto and A. M. S. Silva, ACS Sustainable Chem. Eng., 2016, 4, 4064 CrossRef; (h) G. Chelucci and A. Porcheddu, Chem. Rec., 2017, 17, 200 CrossRef PubMed.
- (a) H. Richter and O. G. Mancheño, Org. Lett., 2011, 13, 6066 CrossRef PubMed; (b) P. Liu, Z. Wang, J. Lin and X. Hu, Eur. J. Org. Chem., 2012, 1583 CrossRef; (c) P. Liu, Y. Li, H. Wang, Z. Wang and X. Hu, Tetrahedron Lett., 2012, 53, 6654 CrossRef; (d) R. Rohlmann, T. Stopka, H. Richter and O. G. Mancheño, J. Org. Chem., 2013, 78, 6050 CrossRef PubMed.
- (a) H. Huang, H. Jiang, K. Chen and H. Liu, J. Org. Chem., 2009, 74, 5476 CrossRef PubMed; (b) C. Huo, Y. Yuan, F. Chen and Y. Wang, Adv. Synth. Catal., 2015, 357, 3648 CrossRef; (c) X. Q. Yu, J. Wang, Z. W. Xu, Y. Yamamoto and M. Bao, Org. Lett., 2016, 18, 2491 CrossRef PubMed; (d) G. L. Liu, J. R. Qian, J. Hua, F. Cai, X. Li and L. Liu, Org. Biomol. Chem., 2016, 14, 1147 RSC.
- (a) G. C. Senadi, W.-P. Hu, A. M. Gar-khedkar, S. S. K. Boominathan and J.-J. Wang, Chem. Commun., 2015, 51, 13795 RSC; (b) N. Thirupathi, S. Puri, T. J. Reddy, B. Sridhar and M. S. Reddy, Adv. Synth. Catal., 2016, 358, 303 CrossRef.
- (a) X. Yang, L. Li, Y. Li and Y. Zhang, J. Org. Chem., 2016, 81, 12433 CrossRef PubMed; (b) S. Parua, R. Sikari, S. Sinha, S. Das, G. Chakraborty and N. D. Paul, Org. Biomol. Chem., 2018, 16, 274 RSC; (c) S. Das, D. Maiti and S. D. Sarkar, J. Org. Chem., 2018, 83, 2309 CrossRef PubMed.
- (a) X.-D. Jia, F.-F. Peng, C. Qing, C.-D. Huo and X.-C. Wang, Org. Lett., 2012, 14, 4030 CrossRef PubMed; (b) J. Liu, F. Liu, Y.-Z. Zhu, X.-G. Ma and X.-D. Jia, Org. Lett., 2015, 17, 1409 CrossRef PubMed; (c) X. Ma, Y. Zhu, S. Lü, L. Zhang, L. Luo and X. Jia, Tetrahedron Lett., 2016, 57, 1528 CrossRef; (d) M. Ni, Y. Zhang, T. Gong and B. Feng, Adv. Synth. Catal., 2017, 359, 824 CrossRef; (e) W. Ahmed, S. Zhang, X.-Q. Yu, Y. Yamamotoa and M. Bao, Green Chem., 2018, 20, 261 RSC.
- (a) J. Kaschel, T. F. Schneider and D. B. Werz, Angew. Chem., Int. Ed., 2012, 51, 7085 CrossRef PubMed; (b) P. Dupau, M. L. Tran Do, S. Gaillard and J. L. Renaud, Angew. Chem., Int. Ed., 2014, 53, 13004 CrossRef PubMed; (c) F. Jia and Z. Li, Org. Chem. Front., 2014, 1, 194 RSC; (d) W. McNeill and T. Ritter, Acc. Chem. Res., 2015, 48, 2330 CrossRef PubMed; (e) R. B. Bedford, Acc. Chem. Res., 2015, 48, 1485 CrossRef PubMed; (f) C. Cassani, G. Bergonzini and C.-J. Wallentin, ACS Catal., 2016, 6, 1640 CrossRef; (g) R. Shang, L. Ilies and E. Nakamura, Chem. Rev., 2017, 117, 9086 CrossRef PubMed.
- R. Birk, H. Berke, G. Huttner and L. Zsolnaib, Chem. Ber., 1988, 121, 1557 CrossRef.
- Y.-W. Shi, W. Yuan, X. Wang, J. Gong, S.-X. Zhu, L.-L. Chai, J.-L. Qi, Y.-Y. Qin, Y. Gao, Y.-L. Zhou, X.-L. Fan, C.-Y. Ji, J.-Y. Wu, Z.-W. Wang and D. Liu, Sci. Rep., 2016, 6, 30189 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra06826g |
| This journal is © The Royal Society of Chemistry 2018 |