Open Access Article
Open Access ArticleEffect of different forms of N fertilizers on the hyperaccumulator Solanum nigrum L. and maize in intercropping mode under Cd stress
Wenmin Huo ab,
Rong Zoua,
Li Wanga,
Wei Guoa,
Dujun Zhanga and
Hongli Fan*a
ab,
Rong Zoua,
Li Wanga,
Wei Guoa,
Dujun Zhanga and
Hongli Fan*a
aInstitute of Agricultural Resources and Regional Planning, Chinese Academy and Agricultural Sciences, Beijing 100081, China
bSchool of Land Science and Technology, China University of Geosciences, Beijing 100083, China. E-mail: fanhongli@caas.cn
First published on 30th November 2018
Abstract
In the present study, we investigated the effects of different forms of nitrogen fertilizers on the hyperaccumulator Solanum nigrum L. and maize in intercropping mode under cadmium (Cd) stress and explored the physiological response mechanism. This research lays the foundation for the appropriate use of nitrogen (N) fertilizer, reduced costs of ecological restoration, and phytoremediation of environmental pollution by using this intercropping system. The main greenhouse pot experiment was conducted using 1.92 mg kg−1 Cd-contaminated soil. NH4+–N fertilizer and NO3−–N fertilizer treatments were performed along with no nitrogen fertilizer treatment as the control. The results indicate that intercropping could decrease the Cd uptake of maize compared with maize monocropping, but the biomass of maize would decrease under the intercropping mode. The application of N fertilizer to the maize–S. nigrum intercropping system could increase the total biomass of maize and S. nigrum. Compared with the NO3−–N fertilizer treatment, the Cd content of stem, leaf and grain tissues of S. nigrum significantly increased by 9.43%, 22.2%, and 8.33%, respectively, under the NH4+–N fertilizer treatment. The bioconcentration and translocation factors of S. nigrum significantly increased by 11.1% and 15.3%. Moreover, the Cd content of stem, leaf, and grain tissues of maize decreased by 26.5%, 21.2%, and 21.4%, respectively. The bioconcentration and translocation factors of maize significantly decreased by 38.8% and 46.7%. The application of N fertilizers promoted the accumulation of Cd in maize roots, while Cd content decreased in maize shoots. Compared with NO3−–N fertilizer, NH4+–N fertilizer can improve Cd accumulation in various S. nigrum tissues under intercropping, which could reduce Cd accumulation in maize under intercropping. Therefore, the application of NH4+–N fertilizer is recommended for satisfactory bioremediation when using the Cd-hyperaccumulator S. nigrum and for supporting the safe production of maize in Cd-contaminated soil, thus enabling the goal of simultaneous agricultural production and remediation.
1. Introduction
Soil contamination by heavy metals is one of the most serious environmental problems worldwide.1 Cadmium (Cd) is a dangerous toxin in the food chain,2 and according to the United Nations Environment program, Cd accumulation in humans results from contaminated food crops.3 According to the 2014 National Survey of the Soil Pollution Bulletin, nationwide, China has a total point excess rate that reaches 16.1%. Among these findings, the point excess of inorganic pollutants represented by heavy metals accounted for 82.8% of total point excess contamination. The rate of soil Cd pollutants exceeded 7.0%; thus, Cd was confirmed to be the primary pollutant in contaminated soil in China.4 Cd in soil was originally released from a variety of anthropogenic activities and other environmental causes,5 including application of phosphate fertilizers, sewage sludge, and recycled wastes such as manure and compost. Therefore, the best remediation approach is to remove heavy metals from the soil without secondary pollution. Phytoremediation is emerging as a potentially cost-efficient and environmentally friendly solution for the remediation of contaminated soils.6 The heavy metals in soils can be extracted by plant roots and eliminated by transport to the shoots of the plant. Current phytoremediation systems usually use hyperaccumulators to eliminate heavy metals from contaminated soil.7,8Hyperaccumulators are plants that can accumulate excess heavy metals. The main factors that should be considered in selecting a hyperaccumulator species include the following: the biomass yield of the plant, bioconcentration, and translocation. Solanum nigrum L., a hyperaccumulator that has been newly identified through pot experiments and small-scale field experiments,9 has the ability to resist Cd poisoning under Cd stress.
Intercropping is a multiple cropping system with two or more crops grown simultaneously in the same field.10 Intercropping has a long history in agricultural production in China, and this technology is common in many developing countries because it enables the efficient use of various natural resources.11 In China, more than 28 million hm2 of annually sown areas are cultivated using intercropping.12 Intercropping promotes plant growth, enhances fertilizer use efficiency, and adjusts the microbe community structure.13
Fertilization is an important agronomic measure for improving the soil fertility of farmland and increasing crop yields. It is also an important auxiliary measure for improving the efficiency of hyperaccumulators in remediating contaminated soils.14 Nitrogen (N) is one of the most important essential nutrients for plant growth and development and is also the primary limiting nutrient for plant growth in the field.15 Accordingly, the application of N fertilizers is important for agricultural production. Relevant studies have shown that the application of nitrogen fertilizers can promote the growth of the heavy metal hyperaccumulator Noccaea caerulescens and its accumulation of heavy metals, including zinc and Cd.16 Research has shown that N fertilizers can improve phytoremediation of heavy metals and promote plant growth. Sarwar et al. suggested that this approach helps to produce proteins that form complexes with metals to improve translocation and/or storage or proteins that are antioxidative enzymes that reduce the level of oxidation caused by heavy metals.17 N fertilizers, which include NH4+–N and NO3−–N forms, have been applied to enhance plant biomass and the phytoextraction efficiency of heavy metals from soil.18 A previous pot experiment investigated the effects of different forms of nitrogen fertilizer (NO3−–N, NH4+–N) on Cd stress in Carpobrotus rossii, a newly identified Cd hyperaccumulator in Australia.19 It was found that the stimulated uptake of nitrogenous nutrient elements was responsible for the stimulation of plant growth.
Under Cd stress, different forms of nitrogen had different effects on plant growth and heavy metal absorption capacity. Research has shown that (NH4)2SO4 promoted the absorption of Cd by spinach. Under hydroponic conditions, the enrichment of Cd by lettuce under a NH4+–N treatment was stronger than that under NO3−–N treatment.20 However, most studies have been conducted on the impact of different forms of N fertilizer on hyperaccumulator plants. The effect of the different N fertilizer forms applied on the biomass and the Cd uptake and transportation under intercropping have not been studied in depth. The agronomic measures for optimizing the efficiency of intercropping and their underlying mechanisms, including regulatory mechanisms, have not been reported yet.
In this study, we observed the hyperaccumulator S. nigrum and maize in an intercropping system to investigate the effects of different nitrogen fertilizer forms (i.e., ammonium–nitrogen fertilizer and nitrate–nitrogen fertilizer), and then investigated Cd absorption and transportation mechanisms in the species.
2. Materials and methods
2.1 Materials for experiment
The soil used in this study was collected from Henan Province, an area that has a temperate continental monsoon climate, annual average temperature of 14 °C, annual sunshine duration of about 2400 h, and annual average rainfall of 656 mm.Soil samples were air-dried and ground to pass through a 2 mm-sieve. The basic physical and chemical properties of the tested soil are shown in Table 1. The Cd content of the soil was 1.92 mg kg−1.
| Total Cadmium (mg kg−1) | Available Cd (mg kg−1) | Available nitrogen (mg kg−1) | Available phosphorus (mg kg−1) | Rapidly available potassium (mg kg−1) | Cation exchange capacity (cmol kg−1) | Organic substance (%) | pH | |
|---|---|---|---|---|---|---|---|---|
| 1.92 | 0.925 | 92.8 | 87.2 | 238 | 20.0 | 2.25 | 8.30 |
The maize used in this study (maize cultivar ‘Zhengdan 958’) was provided by the Henan Academy of Agricultural Sciences. Maize seeds were disinfected with 10% H2O2 solution for 20 minutes, and then rinsed several times with deionized water. Seeds were soaked in deionized water for 15 hours in Petri dishes and sowed into the soil after germination.
The hyperaccumulator selected for this study was wild Solanum nigrum L. Seeds were soaked in warm water for 12 hours, and then wrapped in wet towels maintained at 25 °C until the seedlings grew two true leaves. Seedlings exhibiting uniform growth were then planted in pots, with three seedlings per pot.
2.2 Experimental design
The pot experiment was conducted in a greenhouse with natural light at the Chinese Academy of Agricultural Sciences in June 2017 through September 2017. Each pot was filled with 10 kg of air-dried contaminated soil, and maize seeds and S. nigrum seedlings were transplanted into the pots at the same time.21,22 First, we set two treatments, namely, maize monocropping (T1) and maize–S. nigrum intercropping (T2), with four replicates for each treatment. All the treatments were conducted without the addition of fertilizers. Under maize intercropped with S. nigrum, the following treatments were employed: non-nitrogen fertilizer (the control treatment), ammonium–nitrogen fertilizer, and nitrate–nitrogen fertilizer. Each treatment was replicated three times. The ammonium–nitrogen fertilizer selected was (NH4)2SO4, and the nitrate nitrogen fertilizer selected was Ca(NO3)2. The application rates of fertilizers were equivalent to 0.1 N kg−1. For the pot sets three treatments were conducted: the non-nitrogen fertilizer treatment, the application of Ca(NO3)2, and the application of (NH4)2SO4, with four replicates for each treatment. The soil water content was maintained at around 70% field capacity by watering daily for the experiment's duration. The plants were harvested after 90 days.2.3 Sample collection and index determination
At harvest, soil samples and plant samples were collected after plant maturation. Each pot was sampled with a small stainless steel corer randomly at five points to collect mixed soil samples. The soil samples were air dried and ground through 2 mm- and 0.25 mm-sieves respectively. The plant samples were divided into four parts: roots, stems, leaves, and grains. After washing with deionized water and oven drying at 105 °C for 30 minutes followed by drying at 80 °C, biomass was measured and the tissues were ground through a 0.25 mm-sieve.23The Cd content of the plant material was determined from 0.25 g plant samples that were passed through a 0.25 mm-sieve, followed by HNO3 + HC1O4 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) digestion. Inductively coupled plasma mass spectrometry (ICP-MS) using an Agilent 7700× (Agilent Technologies, Santa Clara, CA, USA) was then used to measure the Cd content. The Cd content of soil was determined from 0.20 g soil samples that were passed through a 0.25 mm-sieve followed by HNO3 + HCl + H2O2 digestion. ICP-MS was performed to measure Cd content. The Cd accumulation was calculated by multiplying Cd content with biomass.
1) digestion. Inductively coupled plasma mass spectrometry (ICP-MS) using an Agilent 7700× (Agilent Technologies, Santa Clara, CA, USA) was then used to measure the Cd content. The Cd content of soil was determined from 0.20 g soil samples that were passed through a 0.25 mm-sieve followed by HNO3 + HCl + H2O2 digestion. ICP-MS was performed to measure Cd content. The Cd accumulation was calculated by multiplying Cd content with biomass.
The Cd content in soil was determined by weighing 5.0 g soil samples that were passed through a 2 mm-sieve into 100 mL triangle bottles, to which 25.0 mL of DTPA extractant was added. Samples were then placed in a horizontal reciprocating shaker at room temperature under reciprocated oscillation at 180 times per minute, and then removed after 2 hours. Samples were then filtered through a filter paper, with the initial filtrate (i.e., 2–3 mL) discarded and the remaining filtrate measured via ICP-MS.
The quality control sample reference material used was GBW10020 (GSB-11), which has a standard Cd value of 0.17 ± 0.02 mg kg−1; the measured value was 0.18 mg kg−1, which is within expected the standard range. ICP-MS was used to determine the Cd content with 72 Ge as an internal standard under He mode. The working conditions were as follows: RF power, 1550 W; carrier gas flow, 0.8 L min−1; plasma gas flow, 15 L min−1; auxiliary gas flow, 0.8 L min−1; atomizer chamber temperature, 2 °C; sample height, 10 m; scanning times, 3; helium flow, 4.3 mL min−1.
2.4 Bioconcentration and translocation factors
The following formulas were used to determine the bioconcentration factor (BCF) and translocation factor (TF):| BCF = Cd content in shoots of plant/Cd content of soil |
| TF = Cd content in shoots of plant/Cd content of roots |
2.5 Statistical analysis
The data were statistically analyzed using Excel 2010 (Microsoft Corp., Redmond, WA, USA) and SPSS 19.0 software (IBM Corp., Armonk, NY, USA) and expressed as means ± standard deviations. All the data were analyzed by one-way analysis of variance (ANOVA). The means of the treatments were compared using Duncan's multiple range tests at P = 0.05.3. Results
3.1 Biomass of maize under monocropping and intercropping
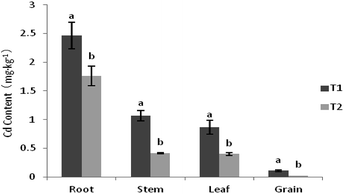
According to the data in Table 2, under intercropping with S. nigrum, the biomass of various organs in maize decreased significantly. The biomass of the root, stem, leaf and grain of maize decreased by 39.8%, 23.2%, 23.1% and 36.2%, respectively. In conclusion, intercropping could inhibit the growth of maize, so the application of fertilizers is necessary to ensure the growth of maize under intercropping with S. nigrum (Fig. 1).| Treatment | Root (g per plant) | Stem (g per plant) | Leaf (g per plant) | Grain (g per plant) |
|---|---|---|---|---|
| a Data are presented as means ± standard errors. Different letters within a column represent significant Duncan's multiple range tests at P = 0.05. | ||||
| T1 | 33.71 ± 1.22a | 35.42 ± 0.35a | 21.15 ± 0.22a | 10.61 ± 0.35a |
| T2 | 20.33 ± 0.21b | 27.17 ± 0.85b | 16.32 ± 1.82b | 6.76 ± 0.30b |
| Treatment | Root (g per plant) | Stem (g per plant) | Leaf (g per plant) | Grain (g per plant) |
|---|---|---|---|---|
| a Data are presented as means ± standard errors. Different letters within a column represent significant Duncan's multiple range tests at P = 0.05. | ||||
| CK | 3.18 ± 0.06b | 10.0 ± 0.33b | 6.79 ± 0.13a | 10.8 ± 0.19b |
| (NH4)2SO4 | 3.38 ± 0.08b | 10.4 ± 0.02ab | 6.94 ± 0.41a | 11.1 ± 0.08a |
| Ca(NO3)2 | 4.42 ± 0.35a | 10.7 ± 0.32a | 7.17 ± 0.08a | 11.7 ± 0.44a |
3.2 The Cd content of maize under monocropping and intercropping
After intercropping with S. nigrum, the Cd content of maize significant decreased, particularly in the grain of maize. Under maize–S. nigrum intercropping mode, the Cd content of root, stem, leaf and grain in maize decreased by 28.6%, 61.4%, 53.8% and 78.5%, respectively. In intercropping mode, the Cd content in various organs of maize decreased significantly. Among them, the Cd content in grain decreased the most. In maize monocropping, the Cd content of grain was 0.103 mg kg−1, which is higher than the limit of Cd content according to the National Food Safety Standard (0.1 mg kg−1). In intercropping, the Cd content of grain was just 0.023 mg kg−1.3.3 Biomass of S. nigrum and maize intercropping
The alternative types of N fertilizer that were applied were associated with distinct effects on the biomass of S. nigrum in the maize–S. nigrum intercropping system (Table 2), with Ca(NO3)2 yielding the highest biomass, followed by (NH4)2SO4, and lastly the control (CK). Compared with no N fertilizer (i.e., CK), under the NH4+–N treatment, the biomass of root, stem, leaf, and grain tissues of S. nigrum increased by 4.97%, 4.01%, 2.21%, and 2.78%, respectively, and the differences in root tissue were significant. Compared with the CK treatment, under the NO3−–N treatment, the biomass of root, stem, leaf, and grain tissues of S. nigrum increased by 37.3%, 7.01%, 5.60%, and 8.33%, respectively, with significant effects for the root and stem tissues. Compared with the NH4+–N treatment, under the NO3−–N treatment, the biomass of root, stem, leaf, and grain tissues in S. nigrum increased by 30.8%, 2.88%, 3.31%, and 5.41%, respectively, and the effect in root tissue was significant. Therefore, under treatments with different N types of fertilizer, there were no significant differences in the shoot biomass for S. nigrum intercropping, while there was a significant difference in root tissue under the NO3−–N treatment.Table 4 shows the effects of no fertilizer, NH4+–N fertilizer, and NO3−–N fertilizer treatments on the biomass of various maize tissues under the maize–S. nigrum intercropping system. N fertilizer application increased the biomass of various maize tissues under the intercropping system, with the Ca(NO3)2 fertilizer having the strongest effect, followed by the (NH4)2SO4 fertilizer and the control (CK). Compared with the CK treatment, under the NH4+–N fertilizer treatment, the biomass of maize root, stem, leaf, and grain tissues increased by 5.05%, 10.3%, 22.0%, and 117%, respectively, with a significant effect observed in the grain. Compared with the CK treatment, under the NO3−–N fertilizer treatment, the biomass of root, stem, leaf, and grain tissues of maize increased by 16.2%, 22.2%, 32.7%, and 120%, respectively, with each tissue exhibiting a significant effect except for the stem tissue. Compared with the NH4+–N fertilizer treatment, under the NO3−–N fertilizer treatment, the root, stem, leaf and grain of maize plants increased by 10.6%, 8.66%, 8.74%, and 1.51%, respectively, though no effects were significant. Thus, N fertilizer application can promote maize growth under this intercropping system, with grain biomass significantly increasing. On applying the different N fertilizer forms, there was no significant difference in the shoot biomass of maize under intercropping.
| Treatment | Root (g per plant) | Stem (g per plant) | Leaf (g per plant) | Grain (g per plant) |
|---|---|---|---|---|
| a Data are presented as means ± standard errors. Different letters within a column represent significant Duncan's multiple range tests at P = 0.05. | ||||
| CK | 19.8 ± 0.92b | 18.5 ± 0.57c | 15.0 ± 0.41b | 3.97 ± 0.25b |
| (NH4)2SO4 | 20.8 ± 0.71ab | 20.4 ± 0.19b | 18.3 ± 0.59b | 7.60 ± 0.23a |
| Ca(NO3)2 | 23.0 ± 0.88a | 27.6 ± 0.47a | 19.9 ± 0.73a | 8.11 ± 0.17a |
3.4 Cd content of S. nigrum and maize under intercropping
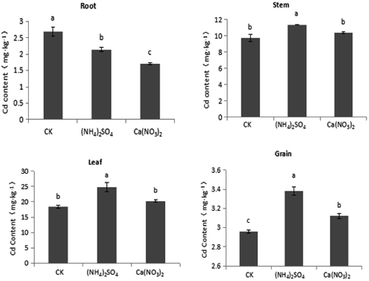
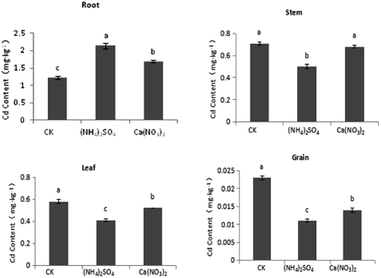
The Cd content of S. nigrum shoot tissue was the highest in the CK treatment, followed by the Ca(NO3)2 and (NH4)2SO4 treatments, with a significant increase in Cd content of shoots under the NH4+–N fertilizer treatment. Further analysis showed that compared with the CK treatment, the Cd content of the stem, leaf, and grain tissues of S. nigrum increased by 6.56%, 9.92%, and 5.41%, respectively, under the NO3−–N treatment, with significant difference observed only for grain. Compared with the CK treatment, the Cd content of S. nigrum stem, leaf, and grain tissues significantly increased by 16.6%, 34.3%, and 14.2%, respectively, under the NH4+–N fertilizer treatment. Compared with the NO3−–N fertilizer treatment, the Cd content of the stem, leaf, and grain tissues of S. nigrum increased by 9.43%, 22.2%, and 8.33%, respectively, under the NH4+–N fertilizer treatment, and each difference was significant (P < 0.05). Therefore, the NH4+–N fertilizer application can promote Cd absorption and translocation in S. nigrum under intercropping. The application of NH4+–N fertilizer can inhibit Cd uptake and transportation from roots to shoots in maize under intercropping (Fig. 2).As shown in Fig. 3, different forms of the nitrogen fertilizer were applied to Cd-contaminated soil, with the (NH4)2SO4 treatment having the greatest effect, followed by the Ca(NO3)2 and CK treatments. The Cd content of shoots was highest in the CK treatment, followed by the Ca(NO3)2 and (NH4)2SO4 treatments. Further analysis showed that compared with the CK treatment, under the NO3−–N fertilizer treatment, the Cd content of maize stem, leaf, and grain tissues decreased by 4.25%, 10.3%, and 43.5%, respectively, with the difference in grain and leaf being significant. Compared with the CK treatment, under the NH4+–N fertilizer treatment, the Cd content of maize stem, leaf, and grain tissues decreased by 29.6%, 29.3%, and 52.2%, respectively, with the difference being significant in all cases. Compared with the NO3−–N fertilizer, under the NH4+–N fertilizer treatment, the Cd content of the stem, leaf, and grain tissues of maize significantly decreased by 26.5%, 21.2%, and 21.4%, respectively. The application of N fertilizers promoted the uptake of Cd in maize roots, but the Cd content in maize shoots significantly decreased. Among the observed differences, the decrement of Cd content in maize grain was the highest under the NH4+–N fertilizer treatment (P < 0.05).
3.5 Cd accumulation of S. nigrum and maize under intercropping
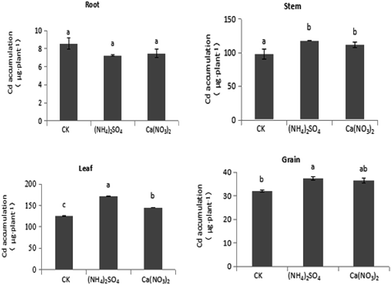
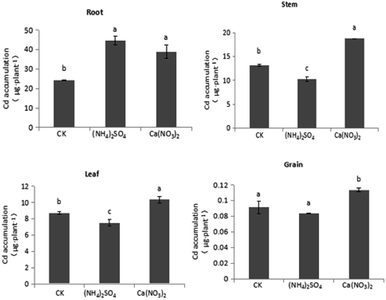
The comparison shown in Fig. 4 reveals that different N fertilizer treatments have various effects on Cd accumulation across S. nigrum tissues under intercropping. Compared with the CK treatment, under the NO3−–N fertilizer treatment, the Cd accumulation of stem, leaf, and grain tissues of S. nigrum increased by 13.9%, 16.1%, and 14.1%, respectively, with the difference in stem and leaf being significant. Compared with the CK treatment, under the NH4+–N fertilizer treatment, the Cd accumulation of stem, leaf, and grain tissues in S. nigrum increased by 20.4%, 37.2%, and 17.0%, respectively. Compared with the NO3−–N fertilizer treatment, under the NH4+–N fertilizer treatment, the Cd accumulation of stem, leaf, and grain tissues in S. nigrum increased by 5.76%, 18.2%, and 2.50%, respectively, and the difference in leaf tissue was significant. Thus, NH4+–N fertilizer increased the Cd accumulation of shoots and promoted Cd transport from root to shoots in S. nigrum under intercropping.As shown in Fig. 5, different N fertilizer forms had significant influences on Cd accumulation in maize under intercropping. Compared with the CK treatment, under the NH4+–N fertilizer treatment, the Cd accumulation of maize shoots significantly decreased, with Cd accumulation of stem, leaf, and grain tissues decreasing by 28.7%, 13.7%, and 8.44%, respectively. Under the NO3−–N fertilizer treatment, the accumulation of various organs on maize significantly increased because the biomass increased more than the decrease in Cd content. Compared with the CK treatment, the NH4+–N fertilizer treatment significantly increased Cd accumulation in the hyperaccumulator S. nigrum while inhibiting Cd absorption of maize under intercropping.
3.6 Bioconcentration and translocation factors in S. nigrum and maize under intercropping
Table 5 shows the BCF and TF values of S. nigrum under intercropping. Compared with the CK treatment, under the NO3−–N fertilizer treatment, the BCF and TF of S. nigrum significantly increased by 15.0% and 31.5%, respectively. Compared with the CK treatment, under the NH4+–N fertilizer treatment, the BCF and TF of S. nigrum increased significantly by 27.8% and 51.7%, respectively. Compared with the NO3−–N fertilizer treatment, under the NH4+–N treatment, the BCF and TF of S. nigrum increased significantly by 11.1% and 15.3%, respectively.| Treatment | BCF | TF |
|---|---|---|
| a Data are presented as means ± standard errors. Different letters within a column represent significant Duncan's multiple range tests at P = 0.05. | ||
| CK | 13.3 ± 0.45c | 29.8 ± 1.79c |
| (NH4)2SO4 | 17.0 ± 0.06a | 45.2 ± 0.62a |
| Ca(NO3)2 | 15.3 ± 0.32b | 39.2 ± 1.56b |
Under the NH4+–N fertilizer treatment, the hyperaccumulator S. nigrum had the highest BCF and TF values, 17.0 and 45.2, respectively. The high TF values suggest that the hyperaccumulator S. nigrum can uptake Cd from soils and store Cd in the shoots with greater efficiency. N fertilizer application appears to promote S. nigrum absorption and transportation of Cd, particularly under the NH4+–N fertilizer treatment.
As shown in Table 6, compared with the CK treatment, under the NO3−–N fertilizer treatment, the TF of maize decreased significantly by 16.7%. Compared with the CK treatment, under the NH4+–N fertilizer treatment, the BCF and TF of maize significantly decreased by 18.4% and 55.6%, respectively. Compared with the NO3−–N fertilizer treatment, under the NH4+–N fertilizer treatment, the BCF and TF of maize significantly decreased by 38.8% and 46.7%, respectively. These results indicate that NH4+–N fertilizer can promote S. nigrum absorption and transportation of Cd while inhibiting the absorption and transportation of Cd in intercropped maize.
| Treatment | BCF | TF |
|---|---|---|
| a Data are presented as means ± standard errors. Different letters within a column represent significant Duncan's multiple range tests at P = 0.05. | ||
| CK | 1.14 ± 0.04b | 0.90 ± 0.13a |
| (NH4)2SO4 | 0.93 ± 0.05c | 0.40 ± 0.03c |
| Ca(NO3)2 | 1.52 ± 0.02a | 0.75 ± 0.02b |
3.7 Total soil Cd content and available soil Cd content
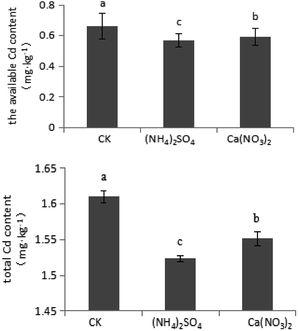
As shown in Fig. 6, compared with the CK treatment, under the NH4+–N and NO3−–N fertilizer treatments, the total Cd content significantly decreased by 5.59% and 3.73%, respectively. Compared with the CK treatment, under the NO3−–N and NH4+–N fertilizer treatments, the available Cd content significantly decreased by 10.6% and 13.6%, respectively. Compared with the NO3−–N fertilizer treatment, under the NH4+–N fertilizer treatment, the available Cd content significantly decreased by 3.39%. Therefore, application of N fertilizer, particularly NH4+–N fertilizer, could enhance remediation effectiveness.4. Discussion
Previous research has shown that Cd inhibits the growth of plants, thus decreasing biomass and even causing plant death owing to the interactions of Cd with photosynthesis, respiration, and nitrogen assimilation in plants.24,25 In the maize–S. nigrum intercropping system, N fertilizer application significantly increased the biomass of various maize and S. nigrum tissues (Tables 3 and 4). These results suggest that N fertilizer promotes the growth of intercropped maize and S. nigrum. Under intercropping, the growth of maize and S. nigrum was promoted by NO3−–N compared with that by NH4+–N, particularly for root biomass, which differed significantly. Similarly, previous research has shown that the NO3−–N fertilizer increases N. caerulescens biomass more than the NH4+–N fertilizer.26–28The application of N fertilizers appeared to increase the Cd content of various S. nigrum tissues. Moreover, Cd content significantly decreased in intercropped maize. NH4+–N-treated S. nigrum accumulated more Cd and maize accumulated less Cd compared with that observed in the NO3−–N-treated plants. This finding confirms previous research findings conducted worldwide. Cheng et al. used a hydroponic experiment to confirm that NH4+–N application increased the Cd uptake and accumulation in the hyperaccumulators C. rossii and S. nigrum.29 N fertilizer forms have a significant effect on Cd translocation from roots to shoots in intercropped S. nigrum and maize. Under intercropping of maize and S. nigrum, the application of N fertilizer promotes the absorption of Cd by the hyperaccumulator S. nigrum, thus reducing the accumulation of Cd in intercropped maize. After nitrogen fertilizer application in soil, NH4+ is formed rapidly by hydrolysis. As the plants absorb NH4+ and the root exudes H+, the rhizosphere is acidified, the solubility of Cd in soil is increased, the amount of adsorbed Cd in soil is decreased, and the biological activity of Cd is increased significantly, thus increasing the Cd absorption in roots of maize and S. nigrum. However, the Cd content in maize shoots significantly decreased, showing that the translocation from roots to shoots was inhibited. The S. nigrum translocation factor significantly increased under the NH4+–N treatment relative to the NO3−–N treatment, similar to previous findings. Wang et al. used a pot experiment to demonstrate that the Cd translocation from roots to shoots in mustard was higher under NH4+–N treatment than under NO3−–N treatment.30 Furthermore, the decreased Cd content in maize shoots after NH4+–N fertilizer application was more significant than that after NO3−–N fertilizer application. These results may clarify the mechanism through which the rhizosphere environment is changed. Relevant research has shown that changes in soil pH can affect the form of heavy metals in soil and the solubility of heavy metals in soil solution. Furthermore, pH changes the distribution of forms of heavy metal in the soil by changing the absorption sites of heavy metals on the soil surface, the stability of the adsorption interface, and coordination forces.31,32 The application of NH4+–N fertilizer can decrease soil pH and increase Cd activity in rhizosphere soil.15 Under intercropping, owing to interspecies competition, maize and S. nigrum can secrete more root exudate to activate soil nutrients in order to compete for nutrients.33 Low molecular organic acids are the main components of root exudates. Organic acids can form chelates with Cd, thus decreasing the pH of the rhizosphere and improving the bioavailability of Cd.34 The Cd2+ amount in the rhizosphere soil is determined prior to its absorption on the root cell surface of a hyperaccumulator.35 The hyperaccumulator S. nigrum can transport Cd accumulated in its roots to its shoots; hence, the Cd content of intercropped maize significantly decreases.
Willaert et al. confirmed that NH4+ application could decrease soil pH and promote Cd uptake of crops.20 Wu et al. examined the effects of different fertilizer forms on Cd uptake by crops; the application of (NH4)2SO4 significantly decreased the soil pH, with significantly more Cd absorbed by cabbage than that observed under the urea treatment.36 Zhang et al. used solution cultures to examine the effect of NH4+–N on Cd, finding that (i) NH4+ decreased the rhizosphere pH and promoted Cd uptake, accumulation, and transportation to shoots in rice, (ii) the Cd concentration in root and shoots was 2.4 times that under the NO3−–N treatment, and (iii) total Cd content was double that of the NO3−–N treatment19. Eriksson studied the effect of ammonium nitrogen and nitrate nitrogen on available Cd, finding that NH4+–N could decrease soil pH and the Cd content absorbed by winter rape (Brassica napus L. var. oleifera Metzgerr) significantly increased.37 Thus, NH4+ application can reduce soil pH values, as NH4+–N fertilizer application rapidly increases NH4+ content, and NH4+ through nitrification in the soil releases H+, which can significantly decrease pH values.38 After crop uptake of NH4+, the pH value of the rhizosphere of crop could also be reduced. A significant body of research has suggested that the pH of soil has an important influence on adsorption and desorption of Cd. The adsorption of Cd by soil decreases as pH decreases and causes the available Cd concentration of soil and rhizosphere to increase, which increases Cd absorption.
In this study, the available Cd concentration in soil decreased significantly under treatments with two different forms of N fertilizer under a maize–S. nigrum intercropping system. The available Cd content decreased by 13.6% and 10.6% under NH4+–N and NO3−–N fertilizer treatments, respectively. This confirms the findings of previous studies. The available Cd in the soil is the main source of heavy metal Cd absorbed by plants. The application of different forms of N fertilizer to Cd-polluted soil would affect the pH of soil and further affect the availability of Cd in soil and the uptake and transportation of Cd by plants. The application of NH4+–N fertilizer owing to the absorption of NH4+–N, results in roots exuding H+, which acidifies the rhizosphere, increases the solubility of Cd in soil, reduces the amount of Cd absorbed by soil and significantly increases the bioactivity of Cd.38 Compared with the NO3−–N fertilizer treatment, the content of Cd in soil was lower under the NH4+–N fertilizer treatment. After NO3−–N fertilizer treatment, NO3−–N is absorbed by plants and OH− or HCO− is excreted by the root system.39 As a result, rhizosphere pH increased and rhizosphere alkalization occurred. Therefore, Cd content in soil decreased with the NO3−–N fertilizer application, which confirms the results of Cheng et al.40 and other groups.
Compared with the control, the NH4+–N treatment significantly increased the Cd content of S. nigrum shoots and decreased the Cd content of various maize tissues. As a Cd hyperaccumulator, shoots were the main area of Cd accumulation. The accumulation of Cd in shoots is a key index for improving the recovery efficiency of S. nigrum. Therefore, the application of NH4+–N could effectively improve the efficiency of S. nigrum remediation in Cd-contaminated soils.
5. Conclusions
The results of our study indicate that intercropping could decrease the Cd uptake of maize, compared with maize monocropping, but the biomass of maize would decrease during intercropping. The application of N fertilizer to the maize–S. nigrum intercropping system could increase the biomass of maize and S. nigrum. When the effects of the different types of N fertilizer were compared, the results showed that NH4+–N fertilizer is more beneficial to the Cd uptake and transportation of maize and S. nigrum in an intercropping system.(1) The application of NH4+–N fertilizers promoted the uptake of Cd in maize root, but the Cd content in shoots of maize decreased. This research showed that compared with CK, under NH4+–N treatment, the Cd content of stem, leaf and grain of S. nigrum increased by 16.6%, 34.3% and 14.2%, respectively, and the difference reached a significant level. Moreover, the Cd content of stem, leaf and grain of the intercropping crop maize decreased by 29.6%, 29.3% and 52.2%, respectively, and the difference reached a significant level.
(2) Compared with CK, under NH4+–N treatment, the BCF and TF of S. nigrum increased by 27.8% and 51.7%, respectively, and the difference reached a significant level. Moreover, the BCF and TF of maize decreased by 18.4% and 55.64%, respectively, and the difference reached a significant level. These results indicated that the application of NH4+–N fertilizer and NO3−–N fertilizer could improve the biomass of various organs of maize and S. nigrum in intercropping. Compared with NO3−–N fertilizer, NH4+–N fertilizer could improve the accumulation of Cd content in various organs of S. nigrum in intercropping, which could reduce the accumulation of cadmium in maize under intercropping.
(3) Different forms of N fertilizer increased the biomass of maize and hyperaccumulator S. nigrum under intercropping mode. Research showed that NH4+–N fertilizer could promote the absorption and transportation of Cd to S. nigrum than NO3−–N fertilizer. Moreover, the Cd content of intercropping crop maize decreased significantly, while the content of Cd in the soil decreased significantly. Thus, ammonium nitrogen fertilizer could be beneficial in improving the efficiency of maize–S. nigrum in the remediation of Cd in contaminated soils, and to ensure its safe production. The results showed that the use of maize–S. nigrum intercropping mode in Cd-contaminated farmland restoration and ammonium fertilizer application could promote maize growth and reduce the absorption of heavy metals. This study provided the theoretical basis and technical support for the future restoration of Cd-contaminated farmland and the security of agricultural products.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors sincerely thank Hong-li Fan from the Laboratory of Plant Nutrition and Fertilizer, Chinese Academy of Agricultural Sciences for excellent technical assistance. This study was supported by National Key Research and Development Projects (2016YFD0800806) and the Key Project from the National Natural Science Foundation of China (31372134).Notes and references
- S. R. Zhang, H. C. Lin, L. J. Deng, G. S. Gong, Y. X. Jia, X. X. Xu, T. Li, Y. Li and H. Chen, Ecological Engineering, 2013, 51, 133–139 CrossRef.
- A. Sebastian and M. N. V. Prasad, Agron. Sustainable Dev., 2014, 34, 155–173 CrossRef CAS.
- United Nations Environment Programme (UNEP), 2008.
- Ministry of Environmental, National survey of soil pollution bulletin [EB/OL], Ministry of Land and Resources, 2014 Search PubMed.
- M. Rizwan, S. Ali, T. Abbas, M. Zia-ur-Rehman, F. Hannan, C. Keller, M. I. Al-Wabel and Y. S. Ok, Ecotoxicol. Environ. Saf., 2016, 130, 43–53 CrossRef CAS PubMed.
- S. P. McGrath, E. Lombi, C. W. Gray, N. Caille, S. J. Dunham and F. J. Zhao, Environ. Pollut., 2006, 114, 115–125 CrossRef PubMed.
- A. Hamzah, R. I. Hapsari and E. I. Wisnubroto, International Journal of Environmental and Agriculture Research, 2016, 2, 8–14 Search PubMed.
- H. M. Tauqee, S. Ali, M. Rizwan, Q. Ali, R. Saeed, U. Iftikhar, R. Ahmad, M. Farid and G. H. Abbasi, Ecotoxicol. Environ. Saf., 2016, 126, 138–146 CrossRef PubMed.
- S. H. Wei, Q. X. Zhou and X. Wang, Chin. Sci. Bull., 2005, 50, 133–138 CrossRef.
- Y. K. Zhang, F. J. Chen, L. Li, Y. H. Chen, B. R. Liu, Y. L. Zhou, L. X. Yuan, F. S. Zhang and G. H. Mi, Sci. China: Life Sci., 2012, 55, 993–1001 CrossRef CAS PubMed.
- N. Li, Z. Li, P. Zhuang, B. Zou and M. McBride, Water Air Soil Pollut., 2009, 199, 45–56 CrossRef CAS.
- L. Li, S. M. Li, J. H. Sun, L. L. Zhou, X. G. Bao, H. G. Zhang and F. S. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 11192–11196 CrossRef CAS PubMed.
- E. Betencourt, M. Duputel, B. Colomb, D. Desclaux and P. Hinsinger, Soil Biol. Biochem., 2012, 46, 181–190 CrossRef CAS.
- J. Wang, L. B. Shen, Z. Li, L. H. Wu and Y. M. Luo, J. Agro-Environ. Sci., 2014, 33, 2118–2124 CAS.
- W. X. Liu, C. J. Zhang, P. J. Hu, Y. M. Luo, L. H. Wu, P. Sale and C. X. Tang, Environ. Sci. Pollut. Res., 2016, 23, 1246–1253 CrossRef CAS PubMed.
- M. Z. Rehman, M. Rizwan, S. Ali, Y. S. Ok, W. Ishaque, S. Ullah, M. F. Nawaz, F. Akmal and M. Waqar, Ecotoxicol. Environ. Saf., 2017, 143, 236–248 CrossRef CAS PubMed.
- N. Sarwar, S. Ullah, S. S. Malhi, M. H. Zia, A. Naeem, S. Bili and G. Farid, J. Sci. Food Agric., 2010, 90, 925–937 CAS.
- V. Giansoldati, E. Tassi, E. Morelli, E. Gabellieri, E. Pedron and M. Barbafieri, Chemosphere, 2012, 87, 1119–1125 CrossRef CAS PubMed.
- C. Zhang, P. W. Sale, A. I. Doronila, G. J. Clark, C. Livesay and C. Tang, Environ. Sci. Pollut. Res., 2014, 21, 9843–9851 CrossRef CAS PubMed.
- G. Willaert and M. Verloo, Pedologie, 1992, 43, 83–91 Search PubMed.
- H. Qin, Z. J. He, J. F. Xiong, L. J. Chen and Y. Bi, J. Agro-Environ. Sci., 2010, 31, 1281–1288 Search PubMed.
- Z. X. Li, Z. Chen, G. L. Chen, Y. C. Xiang, J. W. Zhu, Y. F. Dai and B. Deng, J. Hazard. Mater., 2011, 33, 281–288 Search PubMed.
- S. D. Bao, Soil and agricultural chemistry, China Agriculture Press, 2000 Search PubMed.
- L. S. D. Sanita and R. Gabbrielli, Environ. Exp. Bot., 1999, 41, 105–130 CrossRef.
- M. A. Jalloh, J. H. Chen, F. R. Zhen and G. P. Zhang, J. Hazard. Mater., 2009, 162, 1081–1085 CrossRef CAS PubMed.
- C. Schwartz, G. Echevarria and J. L. Morel, Plant Soil, 2003, 249, 27–35 CrossRef CAS.
- A. C. Monsant, P. Kappen, Y. D. Wang, P. J. Pigram, J. M. B. Alan and C. X. Tang, Plant Soil, 2011, 348, 167–183 CrossRef CAS.
- H. L. Xie, R. F. Jiang, F. S. Zhang, S. P. Mcgrath and F. J. Zhao, Plant Soil, 2009, 318, 205–215 CrossRef CAS.
- M. M. Cheng, P. Wang, P. M. Kopittke, A. A. Wang, P. W. G. Sale and C. X. Tang, J. Exp. Bot., 2016, 67, 5041–5050 CrossRef CAS PubMed.
- X. J. Wang, Y. Song, Y. H. Ma, R. Y. Zhuo and L. Jin, Environ. Pollut., 2011, 159, 3627–3633 CrossRef CAS PubMed.
- G. Sehaller and W. R. Fishner, Z. Pflanzenernahr. Bodenkd., 1985, 148, 306–320 CrossRef.
- T. X. Guan, H. B. He, X. D. Zhang, Z. Bai and H. T. Xie, Chin. J. Soil Sci., 2011, 42, 503–512 Search PubMed.
- S. Mahmood, S. A. Malik, A. Tabassum, U. Younis and M. Athar, J. Soil Sci. Plant Nutr., 2014, 14, 546–553 CAS.
- H. L. Lu, C. L. Yan and J. C. Liu, Environ. Exp. Bot., 2017, 61, 159–166 Search PubMed.
- V. Lakshmana, S. L. Kitto, J. L. Caplan, Y. H. Hsueh, D. B. Kearns, Y. S. Wu and H. P. Bais, Plant Physiol., 2012, 160, 1642–1661 CrossRef PubMed.
- W. F. Wu, Z. R. Nan, S. L. Wang, Z. J. Zhao and T. Zhou, Environmental Science, 2012, 33, 3253–3260 Search PubMed.
- J. E. Eriksson, Water Air Soil Pollut., 1989, 48, 317–335 CrossRef CAS.
- J. Q. Wang, S. H. Ru and D. C. Su, J. Agro-Environ. Sci., 2004, 23, 625–629 CAS.
- P. J. Hu, Y. G. Yin, S. Ishikawa, N. Suzui, N. Kawachi, S. Fujimaki, M. Igura, C. Yuan, J. X. Huang, Z. Li, T. Makino, Y. M. Luo, P. Christie and L. H. Wu, Environ. Sci. Pollut. Res., 2013, 20, 6306–6316 CrossRef CAS PubMed.
- M. M. Cheng, P. Wang, P. M. Kopittke, A. A. Wang, P. W. G. Sale and C. X. Tang, J. Exp. Bot., 2016, 66, 5041–5050 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |