Open Access Article
Open Access ArticleMolecular insights into competitive adsorption of CO2/CH4 mixture in shale nanopores
Wenning Zhou *ab,
Zhe Zhanga,
Haobo Wanga,
Yuying Yanc and
Xunliang Liuab
*ab,
Zhe Zhanga,
Haobo Wanga,
Yuying Yanc and
Xunliang Liuab
aSchool of Energy and Environmental Engineering, University of Science and Technology Beijing, Beijing 100083, China. E-mail: wenningzhou@ustb.edu.cn; Tel: +86 10 62332730
bBeijing Key Laboratory of Energy Saving and Emission Reduction for Metallurgical Industry, University of Science and Technology Beijing, Beijing 100083, China
cFluids & Thermal Engineering Research Group, Faculty of Engineering, University of Nottingham, Nottingham NG7 2RD, UK
First published on 3rd October 2018
Abstract
In the present study, competitive adsorption behaviour of supercritical carbon dioxide and methane binary mixture in shale organic nanopores was investigated by using grand canonical Monte Carlo (GCMC) simulations. The model was firstly validated by comparing with experimental data and a satisfactory agreement was obtained. Then the effects of temperature (298–388 K), pressure (up to 60 MPa), pore size (1–4 nm) and moisture content (0–2.4 wt%) on competitive adsorption behaviour of the binary mixture were examined and discussed in depth. It is found that the adsorption capacity of carbon dioxide in shale organic nanopores is much higher than that of methane under various conditions. The mechanism of competitive adsorption was discussed in detail. In addition, the results show that a lower temperature is favorable to both the adsorption amount and selectivity of CO2/CH4 binary mixture in shale organic nanopores. However, an appropriate CO2 injection pressure should be considered to take into account the CO2 sequestration amount and the exploitation efficiency of shale gas. As for moisture content, different influences on CO2/CH4 adsorption selectivity have been observed at low and high moisture conditions. Therefore, different simulation technologies for shale gas production and CO2 sequestration should be applied depending on the actual moisture conditions of the shale reservoirs. It is expected that the findings in this work could be helpful to estimate and enhance shale gas resource recovery and also evaluate CO2 sequestration efficiency in shale reservoirs.
Introduction
With the increasing global demand for energy, there is a growing interest in unconventional oil and gas resources. Shale gas, as a clean and efficient unconventional gas resource, has become an important alternative to conventional fossil energy. Over the last decade, shale gas has been successfully recovered due to the development of horizontal drilling and massive hydraulic fracturing technology and recently extensively explored in the US, Canada, Australia, Europe, China, etc.1,2 However, hydraulic fracturing has a few drawbacks, including potential surface water shortage due to high-volume hydraulic fracturing, risks of ground and surface water contamination caused by additives in the fracturing fluid, the accumulation of toxic and radioactive elements in soil, risk of induced seismicity by large-scale wastewater disposal, etc.3–5 Therefore, seeking an alternative fracturing fluid has received considerable attention from researchers. Meanwhile, carbon emission has been another hot topic due to the fact of global warming. Carbon capture, utilization and storage (CCUS) has been proposed as the most attractive and promising technology to reduce carbon emission and thereby mitigate global warming.6–8 Among various carbon utilization and storage techniques, the most promising systems are depleted oil reservoirs, particularly those suited to CO2-based Enhanced Oil/Gas Recovery (EOR/EGR), and deep saline formations.9Carbon dioxide enhanced shale gas recovery has recently drawn more and more attention. Shale gas is mainly composed of free gas in fractures and pores, adsorbed gas in organic matter and clay minerals and a small amount of dissolved gas in liquid phase. Of the three forms above, the adsorbed gas takes up 20–85% of the total gas-in-place. The percentage could account for 60–85% in the organic-rich shale.10 Due to the characteristics of supercritical carbon dioxide (Sc-CO2), including its low viscosity, high diffusion capacity near gas and high density near liquid, it has unique advantages for the shale gas extraction.11 Additionally, much research has shown that preferential adsorption of carbon dioxide over methane, which indicates the feasibility of CO2 sequestration with enhanced gas recovery (CS-EGR) in shale reservoirs.12,13 Although the CS-EGR technique in shale has not yet been widely commercialized, extensive investigations have been carried out in this field.14–16 Weniger et al.17 conducted experiments of high-pressure methane and carbon dioxide sorption on shale examples. They reported that the CO2 and CH4 sorption capacities correlated with the organic carbon content (TOC) and CO2/CH4 sorption capacity ratio ranged between 1.9 and 6.9 for carbonaceous shale. Duan et al.18 experimentally examined the adsorption equilibrium isotherms of CH4, CO2 and their mixtures on Sichuan Basin shale, China. Their results clearly indicated that adsorbed CO2 is in a more highly ordered arrangement than CH4 on shale and their selectivity depends upon the composition and pore structure of the shale. In addition, a series of experiments have been carried out to investigate carbon dioxide and methane adsorption capacity on the shale and discuss the influences of pressure, temperature, pore structure and moisture content.19–22 More recently, researchers also performed fracturing experiments on shale samples using Sc-CO2 and water.23,24 Their results demonstrated Sc-CO2 fracturing has absolute advantages over hydraulic fracturing in the fracture initiation pressure and creating more extensive fracture network, thereby increasing the permeability and promoting gas transport. Although much experimental work has been done, it is difficult to reveal the essence of CO2/CH4 competitive adsorption behaviours through laboratory experiments due to the complexity of the shale structure and associated geological conditions.
In recent years, molecular simulation has emerged as an effective approach in addition to experimental and theoretical methods to study the microscopic molecular interaction mechanism.25 Supercritical methane diffusion behaviour in the slit-shaped nanopores in shale was investigated by molecular simulation in the work.26 Their results showed that methane molecules in shale nanopores diffuse more rapidly with the increasing pore size and temperature but diffuse more slowly with an increase in pressure. Liu et al.27 carried out molecular dynamics simulations to study the competitive adsorption and diffusion performances of CO2/CH4 binary mixture within shale organic nanochannels. They found preferential adsorption of CO2 over CH4 on the nanochannel surface and CO2/CH4 selectivity and mobility are affected by temperature. Zhang et al.28 used molecular simulation to explore the adsorption and CO2/CH4 selectivity in functional group rich shale nanopores. They reported the functional group rich organic matter in shale has a significant effect on the selectivity of CO2/CH4 and suggested an optimum depth for CO2 sequestration. Pathak et al.29 employed molecular simulations to investigate CO2/CH4 adsorption performance on kerogen in organic rich shale matrix. The results revealed that the carbon dioxide is more strongly retained than methane in the bulk kerogen matrix. Lin et al.30 and Yu et al.31 examined methane adsorption and diffusion behaviours in slit-shaped shale organic nanopores represented by graphene. The adsorption properties of in quartz clay minerals of shale matrix were also studied by employing molecular simulations.32,33 Moreover, the effects of moisture content on adsorption of methane adsorption and diffusion in kerogen and kaolinite were explored by molecular simulations.26,34,35 Their results indicated that moisture content has a great influence on methane adsorption and diffusion behaviours in shale. Although much work has been carried out on studying shale gas adsorption and diffusion mechanisms, only a few of them focus on CO2/CH4 mixture and the mechanism of competitive adsorption in organic shale nanopore has not been fully understood. In this study, molecular simulation was adopted to investigate CO2/CH4 binary mixture competitive adsorption behaviours in slit nanopores of shale organic matter. Effects of temperature, pressure, pore size and moisture content on CO2/CH4 binary mixture competitive adsorption performances were investigated and discussed in depth. The results are expected to serve as a reference for the enhancement of shale gas exploitation and evaluation of CO2 sequestration capacity in shale matrix.
Models and methods
Model construction
The constituents of shale matrix include organic matter and inorganic substances, with large amount of shale gas mainly absorbing on organic matter. The nanopores in shale gas reservoirs are mainly organic matter nanopores. When the adsorption and desorption properties of shale gas are concerned, graphene slit-shaped nanochannel were widely employed as a representation of organic matter nanopore and showed satisfactory results.25–27,29,30 In this study, a multilayer graphene slit was built up to investigate CO2/CH4 binary mixture competitive adsorption characteristics, as shown in Fig. 1. This periodic, optimized, slit-shaped nanochannel consists of upper and lower three-layer graphene with the interlayer spacing of 0.335 nm.36 The distance between upper and lower grapheme layer, i.e., size of shale organic nanopores, ranges from 1 nm to 4 nm.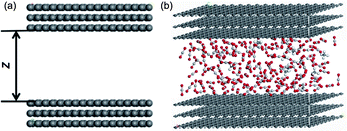 | ||
| Fig. 1 Graphene slit model for the organic nanopore in shale: (a) 2D; (b) 3D vision with adsorbed CO2/CH4 binary mixture. | ||
Molecular simulation
In the present study, the grand canonical Monte Carlo (GCMC) method was employed and all simulations were performed with Accelrys Materials Studio software. The COMPASS force field was adopted in the simulations. Simulation cases were carried out with 1 × 107 Monte Carlo steps.37,38 The first 5 × 106 steps were performed to guarantee the equilibrium state. The latter 5 × 106 steps were used for the ensemble averages to calculate the required physical parameters. Moreover, we used the Ewald method to describe the electrostatic interactions with the accuracy of 10−3 kcal mol−1. The van der Waals (vdW) interactions were calculated within a fine cutoff distance of 15.5 Å by the atom-based method. Interactions between organic matter and gas molecules were described using the Lennard-Jones (LJ) 12-6 potential:
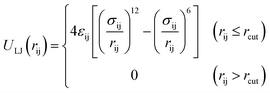 | (1) |
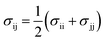 | (2) |
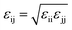 | (3) |
It should be mentioned that in the GCMC simulation, chemical potential, volume and temperature are independent variables. The chemical potential is a function of fugacity rather than pressure. Therefore, the Peng–Robinson (P–R) equation of state was used to calculate the fugacity of CH4 and CO2 mixture in this study.39 The adsorption amount obtained in simulations is the absolute amount. To compare with the adsorption amount in experiments, where excess amount is used, the following expression is applied:
| nex = nabs − ρbVads | (4) |
To examine the competitive adsorption behaviours, the adsorption selectivity of CO2 over CH4 is defined:
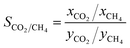 | (5) |
To study the effects of water content on absorption, we used the average water density to quantify the moisture content:
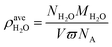 | (6) |
Results and discussion
Model validation
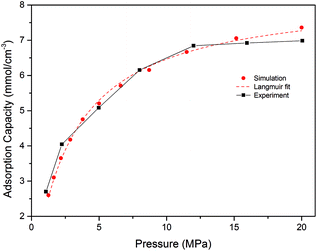
To validate the proposed model, simulation results of CH4 adsorption isotherms were compared with experimental results40 and Langmuir single-molecular layer adsorption theory,41 which is widely applied to describe gas adsorption in shale matrix, as shown in Fig. 2. It should be mentioned that the excess adsorption capacity has been converted to absolute adsorption capacity to compare with the simulation results obtained by the GCMC. The simulation was performed in a pressure range of 1.25–20 MPa at the temperature of 333.15 K, which are the same conditions with those in experimental work. It is found that simulation results fit very well with Langmuir equation and also show satisfactory agreement with experimental data. It should be noted that, besides temperature and pressure, the adsorption capacity of CH4 in actual shale organic matter relates with many other factors such as the total carbon content (TOC), organic matter type and maturity, pore structure, moisture, etc.38,42 The simulation result in the present study was obtained at the pore size of 1.5 nm shale organic nanopore in dry condition. Nevertheless, the agreement in the tendency and data of the comparison has proved the GCMC as an effective and valid tool to explore the adsorption characteristics of the gases at microscopic scale in shale matrix. Therefore, the validity of the model is justified and further investigation on CO2/CH4 binary mixture adsorption can be then carried out.Effects of temperature on competitive adsorption isotherms
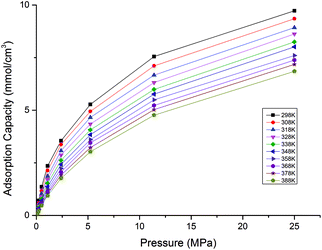
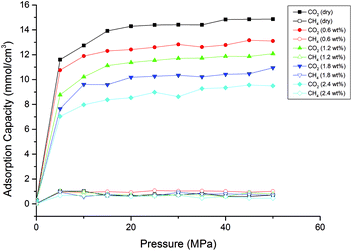
In actual shale formations, the reservoir depth varies from 1000 m to 4000 m. According to the geothermal gradient of ∼3 °C/100 m, the temperatures of shale formations would be different depending on their depths. In this study, a wide temperature range of 298 K to 388 K was examined. The effects of temperature on CH4, CO2 and CO2/CH4 binary mixture adsorption behaviours were investigated. Fig. 3 and 4 display the effects of temperature on adsorption isotherms for CH4 and CO2, respectively.The results show that for both CH4 and CO2, the adsorption capacities decrease with the increasing temperature. It is because that CH4 and CO2 adsorption in shale organic matter is physical adsorption.19,20 The increasing temperature leads to increases of the mean kinetic energy of CH4 and CO2 gas molecules, which would enable them to conquer and escape from the adsorption layer easier. It can be also observed from the figures that CO2 is adsorbed much faster into shale organic matter than CH4 as the pressure increases.
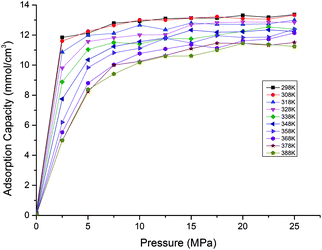
Fig. 5 presents the effects of temperature on the competitive adsorption behaviour of CO2/CH4 binary mixture in shale organic nanopore. It is found that, under the same temperature and pressure condition, the adsorption capacity of CO2 is far greater than that of CH4. The reasons might be as follows. As the adsorption of CO2/CH4 on shale organic matter is physical adsorption, the adsorption strength is mainly determined by adsorbent–adsorbate interaction energy. Compared with CH4 molecules, the large quadrupole moment of CO2 results in strong Coulomb interaction between CO2 and the surfaces of the nanopore.43 So both Coulomb interaction and van der Waals interaction, which is determined by the polarizability, contribute the interactions between CO2 and shale organic matter, with the Coulomb interaction being much stronger. While CH4 is a nonpolar molecule, the van der Waals force is the only interaction between CH4 and shale organic nanopore. The properties of the adsorbates CO2 and CH4 can be seen in Table 1. Besides adsorbent–adsorbate interaction, adsorbate–adsorbate interaction also contributes the adsorption strength.44 On the one hand, the critical temperature for CO2 is 31.1 °C, which is higher than that for CH4 (−82.6 °C). Thus it is relatively easy for CO2 gas to be liquefied. The stronger attraction between CO2 molecules makes them much easier to be adsorbed by shale organic matter. On the other hand, larger dynamic viscosity of CH4 under shale reservoirs conditions results in more intense and irregular thermal motion of CH4 molecules, causing higher desorption ability of CH4 from the shale organic matter. Therefore, the interactions between CO2 and shale organic nanopore are much stronger than that between CH4 and the nanopore. It can be seen from Fig. 5, at the pressure of 20 MPa and temperature of 298 K, the competitive adsorption capacity of CH4 is only around 1 mmol cm−3, while the adsorption capacity of CO2 is as high as 12 mmol cm−3. The adsorption capacity of CH4 single component is around 7.5 mmol cm−3 according to the data in Fig. 3. That is to say, the adsorption capacity of CH4 is largely suppressed under competitive conditions with CO2 in shale organic matter. In another word, CO2 is preferentially adsorbed over CH4 in organic nanopore in shale. Similar results have been reported in previous experimental works.18,20,45
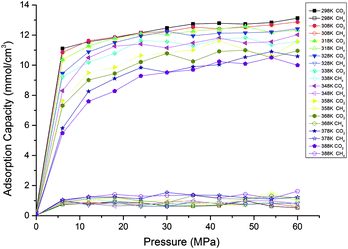 | ||
| Fig. 5 Competitive adsorption isotherms of CO2/CH4 in shale organic nanopore at different temperatures. | ||
| Properties | CO2 | CH4 |
|---|---|---|
| Molecular weight (g mol−1) | 44 | 16 |
| Kinetic diameter (Å) | 3.3 | 3.8 |
| Polarizability × 1025 cm3 | 26.3 | 26.0 |
| Quadrupole moment × 1026 esu cm2 | 4.3 | 0 |
| Dipole moment × 1018 esu cm | 0 | 0 |
Fig. 6 plots the adsorption selectivity of CO2/CH4 binary mixture in shale organic nanopore as a function of temperature at different CO2 injection pressures. It shows that the temperature has great influence on the selectivity of CO2/CH4, i.e., the CO2 sequestration efficiency. A lower temperature is favourable to the enhancement of selectivity. As the temperature goes up, the selectivity decreases significantly. At the same partial pressure of CH4, a lower CO2 injection pressure would be also helpful to the increase of the selectivity.
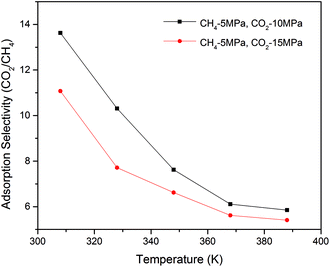 | ||
| Fig. 6 Adsorption selectivity of CO2/CH4 in shale organic nanopore as a function of temperature at different pressures. | ||
Effects of pressure on competitive adsorption behaviours
To reveal the effects of pressure on CO2/CH4 competitive adsorption characteristics, a series of simulation cases were conducted. The pressure conditions as a function of depth can be estimated by the pressure gradient ∼1.0 MPa/100 m. Indeed, the pressure change of shale gas is complicated and exhibits a tendency of decline as the exploitation progresses. Thus a wide range of CH4 partial pressure 5–20 MPa and CO2 injection pressure 7–30 MPa was applied in the simulations. Fig. 7(a) illustrates the concentration profile of CO2/CH4 binary mixture in the nanopore of shale organic matter at the temperature of 308 K with the CO2 injection pressure of 10 MPa and CH4 partial pressure of 5 MPa. It is found that, when together with carbon dioxide, the adsorption amount of CH4 on shale organic matter decreases significantly. The snapshot of the adsorption is shown in Fig. 7(b).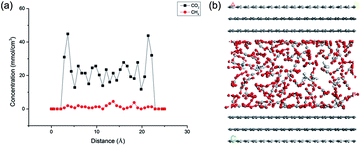 | ||
| Fig. 7 Concentration profile (a) and snapshot (b) of CO2/CH4 binary mixture adsorption in the slit-like nanopore of shale organic matter. | ||
Fig. 8 presents the adsorption amount of CO2/CH4 binary mixture as a function of CO2 injection pressure at different CH4 partial pressures. It is observed that with the increasing CO2 injection pressure, the adsorption amount of CO2 has a gradual rise while the trend has a decline for CH4. However, the increasing CO2 injection pressure also leads to the decrease of CO2/CH4 adsorption selectivity, as shown in Fig. 6. Therefore, an appropriate CO2 injection pressure should be chosen to take into account CO2 sequestration amount and CO2/CH4 adsorption selectivity in CS-EGR project. In addition, it can be seen from the figure as the partial pressure of CH4 goes up, the differences between CO2 and CH4 adsorption capacity become smaller. In another word, the efficiency of CO2 sequestration and enhancement recovery of shale gas would have a gradual decline as the pressure (i.e., the depth of shale formations) increases.
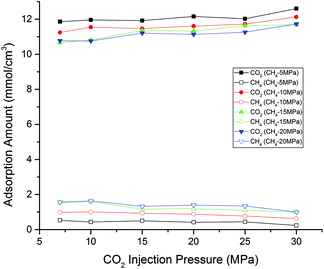 | ||
| Fig. 8 Adsorption amount of CO2/CH4 binary mixture as a function of CO2 injection pressure at different CH4 partial pressures. | ||
Effects of pore size on competitive adsorption behaviours
Due to the fact that a large proportion of pores in shale matrix are nanosized, it is essential to examine the influences of pore size on the competitive adsorption behaviours of CO2/CH4 binary mixture. Fig. 9 shows the snapshots of CO2/CH4 binary mixture adsorption for nanopores with the pore size ranging from 1 nm to 2.5 nm.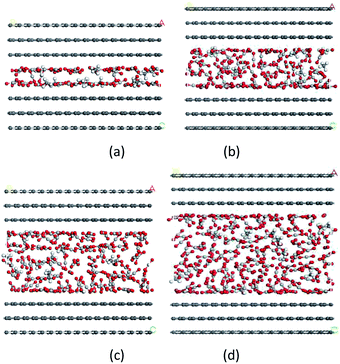 | ||
| Fig. 9 Snapshots of CO2/CH4 binary mixture adsorption for nanopores with different pore sizes: (a) 1 nm; (b) 1.5 nm; (c) 2 nm; (d) 2.5 nm. | ||
Fig. 10 displays the competitive adsorption isotherms of CO2/CH4 in shale nanopores with different pore sizes from 1 nm to 4 nm at the temperature of 318 K. It is found that, under a wide range of pressure, the adsorption amount for CO2 increases significantly when the pore size increase from 1 nm to 2.5 nm. However, when the pore size further increases, the increase of CO2 adsorption amount becomes more gradual.
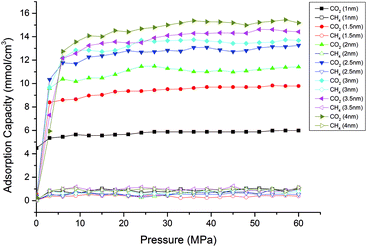 | ||
| Fig. 10 Competitive adsorption isotherms of CO2/CH4 in shale organic nanopores with different pore sizes. | ||
Fig. 11 shows the adsorption selectivity of CO2/CH4 as a function of pore size at the temperature of 318 K. It can be seen that the selectivity increases at the beginning stage when the pore size is extremely small (∼1 nm). This is because the pore size of 1 nm is comparative the size of CH4 and CO2 molecule, the kinetic diameters of which are 3.8 Å and 3.3 Å, respectively. The very small adsorption amount leads to a relatively small selectivity. However, a further increase of the pore size leads to a decline of the selectivity. The optimum selectivity occurs at the pore size of 1.5–2 nm. It can be seen from the figure, all selectivities of the studied cases in the present work are larger than 6 for the nanopores ranging from 1.5 nm to 4 nm in pore size. In addition, the result shows that the selectivity decreases as the increasing CO2 injection pressures. These findings indicate that carbon dioxide is suitable for enhancing shale gas recovery with the CO2 sequestration.
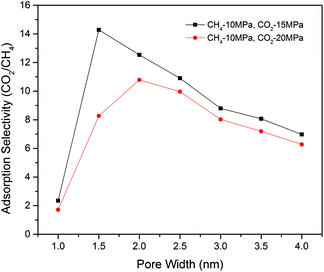 | ||
| Fig. 11 Adsorption selectivity of CO2/CH4 in shale organic nanopore as a function of pore size at different pressures. | ||
Effects of moisture content on competitive adsorption behaviours
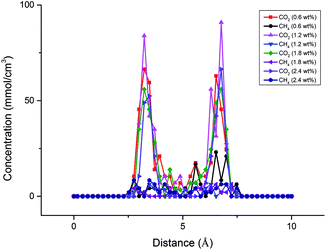
In this section, the effects of moisture content on CO2/CH4 mixture adsorption performances were explored. A certain number of H2O molecules, which was determined by eqn (5), were pre-loaded in shale nanopore to achieve the desired moisture content. The concentration profile of CO2/CH4 mixture in the nanopore at the temperature of 318 K with different moisture contents is shown in Fig. 12. It is observed that the peak concentration for CO2 near the pore wall declines with the increasing moisture content. The effect of moisture content on CH4 is less significant. Fig. 13 demonstrates the isotherms for CO2/CH4 binary mixture under different moisture contents. It can be noted that compared with dry shale organic nanopore, the adsorption capacity of CO2 drops from ∼14 mmol cm−3 to ∼8 mmol cm−3 at the moisture content of 2.4 wt%. It can be attributed to the competitive adsorption occurred between H2O molecules and CO2/CH4 mixture in shale nanopores. The H2O molecules, which could be potentially adsorbed by CO2/CH4 in the dry state, adsorb to shale organic matter.Fig. 14 presents the adsorption selectivity of CO2/CH4 as a function of moisture contents. It can be seen that compared with the nanopore in dry condition, moisture content would lower the selectivity of CO2/CH4 at all pressure conditions. The influences are different at low and high moisture contents. Specifically, the selectivity increases with the increasing moisture content at low moisture condition. However, an opposite trend has been found at high moisture condition, i.e., >1.2 wt% in the present study. It is due to the tendency of selectivity is more determined by the decrease of CH4 adsorption capacity at low moisture, which causes an increase of selectivity when the moisture is less than 1.2 wt%. A further increment of moisture content leads to a more notable decrease in the adsorption capacity of CO2 than that of CH4, causing a decrease in the adsorption selectivity of CO2/CH4. It is also observed that an increase of CO2 injection pressure would decrease the selectivity. These findings would be useful for formulating the plans on enhancing shale gas recovery and CO2 sequestration efficiency in CS-EGR project.
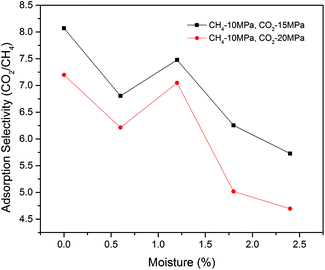 | ||
| Fig. 14 Adsorption selectivity of CO2/CH4 in shale organic nanopore as a function of moisture contents at different pressures. | ||
Conclusions
In this work, the competitive adsorption properties of CO2/CH4 binary mixture in shale organic nanopores were investigated by using a series of GCMC simulations. The effects of temperature, pressure, pore size and moisture content on CO2/CH4 competitive adsorption behaviours have been discussed in detail. Major conclusions are summarized as follows.(1) The results show that CO2 is preferentially adsorbed over CH4 in the silt organic nanopore of shale formations. A lower temperature is favourable for both the adsorption capacity and selectivity of CO2/CH4 binary mixture.
(2) The increasing CO2 injection pressure leads to an increase of adsorption capacity but a decline of selectivity. Therefore, an appropriate CO2 injection pressure should be chosen to take into account the adsorption capacity and selectivity.
(3) Except extremely small pore size (∼1 nm), the increase of pore size causes a decline of selectivity of CO2/CH4 binary mixture in shale organic nanopores. The optimum selectivity has been observed at the pore size of 1.5–2 nm.
(4) Compared with the dry state of shale organic matter, the presence of moisture content decreases the adsorption capacity of CO2 and CH4. The effect of moisture is more remarkable on CO2 than that on CH4. A fluctuation has been observed in selectivity with the increasing moisture content. Based on the results in this work, different strategies could be developed depending their real-time conditions of shale reservoirs in CS-EGR project.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51706018) and the Fundamental Research Funds for the Central Universities (Grant No. FRF-BD-18-015A).References
- Y. Yang, L. Wang, Y. Fang and C. Mou, Renewable Sustainable Energy Rev., 2016, 76, 1465–1478 CrossRef.
- L. Wang, S. Wang, R. Zhang, C. Wang, Y. Xiong, X. Zheng, S. Li, K. Jin and Z. Rui, J. Nat. Gas Sci. Eng., 2017, 37, 560–578 CrossRef CAS.
- M. Guo, X. Lu, C. P. Nielsen, M. B. McElroy, W. Shi, Y. Chen and Y. Xu, Renewable Sustainable Energy Rev., 2016, 66, 742–750 CrossRef.
- G. A. Kahrilas, J. Blotevogel, P. S. Stewart and T. Borch, Environ. Sci. Technol., 2015, 49, 16–32 CrossRef CAS PubMed.
- A. Vengosh, R. B. Jackson, N. Warner, T. H. Darrah and A. Kondash, Environ. Sci. Technol., 2014, 48, 8334–8348 CrossRef CAS PubMed.
- R. Xu, R. Li, J. Ma, D. He and P. Jiang, Acc. Chem. Res., 2017, 50, 2056–2066 CrossRef CAS PubMed.
- Y. Zhang, T. Li, B. Chen, M. Nishio and Y. Song, J. Chem. Eng. Data, 2016, 61, 873–880 CrossRef CAS.
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo, L. A. Hackett, J. P. Hallett, H. J. Herzog, G. Jackson, J. Kemper, S. Krevor, G. C. Maitland, M. Matuszewski, I. S. Metcalfe, C. Petit, G. Puxty, J. Reimer, D. M. Reiner, E. S. Rubin, S. A. Scott, N. Shah, B. Smit, J. P. M. Trusler, P. Webley, J. Wilcox and N. Mac Dowell, Energy Environ. Sci., 2018, 11, 1062–1176 RSC.
- C. Chen, Z. Chai, W. Shen, W. Li and Y. Song, Energy Fuels, 2017, 31, 7317–7324 CrossRef CAS.
- J. B. Curtis, AAPG Bull., 2002, 86, 1921–1938 CAS.
- C. Qin, Y. Jiang, Y. Luo, X. Xian, H. Liu and Y. Li, Energy Fuels, 2017, 31, 493–503 CrossRef CAS.
- L. Huang, Z. Ning, Q. Wang, W. Zhang, Z. Cheng, X. Wu and H. Qin, Appl. Energy, 2018, 210, 28–43 CrossRef CAS.
- T. H. Kim, J. Cho and K. S. Lee, Appl. Energy, 2017, 190, 1195–1206 CrossRef CAS.
- L. Chen, Q. Kang, R. Pawar, Y.-L. He and W.-Q. Tao, Fuel, 2015, 158, 650–658 CrossRef CAS.
- R. Xu, K. Zeng, C. Zhang and P. Jiang, Appl. Therm. Eng., 2016, 115, 1306–1314 CrossRef.
- B. Yan, L. Mi, Y. Wang, H. Tang, C. An and J. E. Killough, J. Pet. Sci. Eng., 2018, 160, 498–509 CrossRef CAS.
- P. Weniger, W. Kalkreuth, A. Busch and B. M. Krooss, Int. J. Coal Geol., 2010, 84, 190–205 CrossRef CAS.
- S. Duan, M. Gu, X. Du and X. Xian, Energy Fuels, 2016, 30, 2248–2256 CrossRef CAS.
- P. Charoensuppanimit, S. A. Mohammad and K. A. M. Gasem, Energy Fuels, 2016, 30, 2309–2319 CrossRef CAS.
- T. F. T. Rexer, M. J. Benham, A. C. Aplin and K. M. Thomas, Energy Fuels, 2013, 27, 3099–3109 CrossRef CAS.
- M. Gasparik, P. Bertier, Y. Gensterblum, A. Ghanizadeh, B. M. Krooss and R. Littke, Int. J. Coal Geol., 2014, 123, 34–51 CrossRef CAS.
- D. J. K. Ross and R. Marc Bustin, Mar. Pet. Geol., 2009, 26, 916–927 CrossRef CAS.
- Y. Jia, Y. Lu, D. Elsworth, Y. Fang and J. Tang, J. Pet. Sci. Eng., 2018, 165, 284–297 CrossRef CAS.
- X. Zhang, Y. Lu, J. Tang, Z. Zhou and Y. Liao, Fuel, 2017, 190, 370–378 CrossRef CAS.
- B.-B. Wang, X.-D. Wang, W.-M. Yan and T.-H. Wang, Langmuir, 2015, 31, 7457–7462 CrossRef CAS PubMed.
- S. Wang, Q. Feng, M. Zha, F. Javadpour and Q. Hu, Energy Fuels, 2018, 32, 169–180 CrossRef CAS.
- B. Liu, C. Qi, T. Mai, J. Zhang, K. Zhan, Z. Zhang and J. He, J. Nat. Gas Sci. Eng., 2018, 53, 329–336 CrossRef CAS.
- H. Zhang, X. Zeng, Z. Zhao, Z. Zhai and D. Cao, J. Nat. Gas Sci. Eng., 2017, 39, 82–89 CrossRef CAS.
- M. Pathak, H. Huang, P. Meakin and M. Deo, J. Nat. Gas Sci. Eng., 2018, 51, 1–8 CrossRef CAS.
- K. Lin, Q. Yuan and Y.-P. Zhao, Comput. Mater. Sci., 2017, 133, 99–107 CrossRef CAS.
- H. Yu, J. Fan, J. Chen, Y. Zhu and H. Wu, Int. J. Heat Mass Transfer, 2018, 123, 657–667 CrossRef CAS.
- J. Xiong, K. Liu, X. Liu, L. Liang and Q. Zeng, RSC Adv., 2016, 6, 110808–110819 RSC.
- H. Sun, W. Sun, H. Zhao, Y. Sun, D. Zhang, X. Qi and Y. Li, RSC Adv., 2016, 6, 32770–32778 RSC.
- T. Zhao, X. Li, Z. Ning, H. Zhao and M. Li, J. Pet. Sci. Eng., 2018, 161, 302–310 CrossRef CAS.
- B. Zhang, J. Kang and T. Kang, Appl. Surf. Sci., 2018, 439, 792–800 CrossRef CAS.
- M. Firouzi, E. C. Rupp, C. W. Liu and J. Wilcox, Int. J. Coal Geol., 2014, 121, 123–128 CrossRef CAS.
- H. Sun, H. Zhao, N. Qi and Y. Li, J. Phys. Chem. C, 2017, 121, 10233–10241 CrossRef CAS.
- J. Xiong, X. Liu, L. Liang and Q. Zeng, Energy Fuels, 2017, 31, 1489–1501 CrossRef CAS.
- P. M. Mathias and T. W. Copeman, Fluid Phase Equilib., 1983, 13, 91–108 CrossRef CAS.
- J. Xiong, X. Liu, L. Liang and Q. Zeng, Fuel, 2017, 200, 299–315 CrossRef CAS.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- L. Huang, Z. Ning, Q. Wang, R. Qi, Y. Zeng, H. Qin, H. Ye and W. Zhang, Fuel, 2018, 211, 159–172 CrossRef CAS.
- C. B. Pradip Chowdhury and S. Gumma, J. Phys. Chem. C, 2009, 113, 6616–6621 CrossRef.
- X. Xu, X. Zhao, L. Sun and X. Liu, J. Nat. Gas Chem., 2008, 17, 391–396 CrossRef CAS.
- F. Yang, Z. Ning, R. Zhang, H. Zhao and B. M. Krooss, Int. J. Coal Geol., 2015, 146, 104–117 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |