Open Access Article
Open Access ArticleProduction of cyclopentanone from furfural over Ru/C with Al11.6PO23.7 and application in the synthesis of diesel range alkanes†
Tao Shenab,
Ruijia Huab,
Chenjie Zhu *abc,
Ming Liab,
Wei Zhuangab,
Chenglun Tangab and
Hanjie Ying
*abc,
Ming Liab,
Wei Zhuangab,
Chenglun Tangab and
Hanjie Ying *abc
*abc
aCollege of Biotechnology and Pharmaceutical Engineering, Nanjing Tech University, Nanjing, China. E-mail: zhucj@njtech.edu.cn; yinghanjie@njtech.edu.cn
bNational Engineering Technique Research Center for Biotechnology, Nanjing, China
cJiangsu National Synergetic Innovation Center for Advanced Bio-Manufacture, Nanjing, China
First published on 12th November 2018
Abstract
The bio-based platform molecule furfural was converted to the high value chemical cyclopentanone over Ru/C (0.5 wt%) and Al11.6PO23.7 catalysts in good yield (84%) with water as the medium. After screening the reaction conditions, the selectivity for cyclopentanone and cyclopentanol could be controlled by adjusting the hydrogen pressure at the temperature of 433 K. Herein, we propose a new mechanism for the synergistic catalysis of a Bronsted acid and Lewis acid for the conversion of furfural to cyclopentanone through the cyclopentenone route, which is catalyzed by Ru/C and Al11.6PO23.7. In addition, based on cyclopentanone, higher octane number cyclic alkanes (>85% selectivity), which are used as hydrocarbon fuels, were synthesized via a C–C coupling reaction followed by hydrodeoxygenation.
Introduction
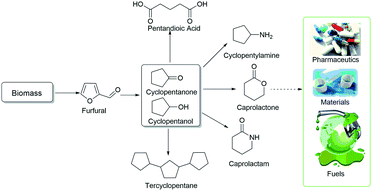
With the depletion of petroleum resources, the exploitation of renewable resources has drawn increasing attention.1,2 Biomass is a renewable resource due to its abundant sources and short growth periods.3,4 Recently, accompanied by the successful synthesis of platform molecules from lignocellulose, the catalytic conversion technology of transforming biomass into high-value chemicals has shown great progress. Various furan-based derivatives can be produced via the acid-catalyzed dehydration of cellulose and hemicellulose.5–9 For example, furfural (FFA) can be produced on an industrial scale from biomass.10 Based on this, the catalytic hydrogenation and dehydration of furfural to a range of nonpetroleum-derived chemicals is significant, including 2-methyl furan (2-MF),11 furfural alcohol (FAL),12 tetrahydrofurfuryl alcohol (THFA),13 tetrahydrofuran (THF),14 2-methyl tetrahydrofuran (MTHF),15 levulinic acid (LA),16 and pentanediol.17,18 These chemical intermediates can be used in the fields of materials, fuels and pharmaceuticals.19–23Cyclopentanone (CPO) is another hydrogenation product of furfural, and cyclopentanol (CPL) is the depth hydrogenation product of CPO. CPO and CPL are versatile chemical intermediates that can be used to synthesize medicines, rubber chemicals and fragrance chemicals, which are applied in pharmaceuticals, fuel energy and materials (Scheme 1).24–29 Traditionally, CPO and CPL are synthesized via the ketonic decarboxylation of adipic acid or catalytic cyclization of 1, 6-hexanediol, followed by hydrogenation.30,31 Recently, based on lignocellulose, a new route was proposed, in which furfural was converted to CPL with no petroleum compounds as the substrates.
Inspired by the previous work of the Hronec group,32–34 new methods have been reported regarding furfural or furfuryl alcohol after hydrogenation and following rearrangement in aqueous media to synthesize CPO. These studies showed that hydrogenation metal catalysts (e.g., Pt/C, Pd–Cu/C and Ni-based catalysts) have good selectivity for CPO. Besides, hydrogenation metal-based solid acid and solid base were also found to have good performance for the synthesis of CPO and CPL from furfural, for example, Ru/MIL-101 (Materials of Institute Lavoisier),35 Ru/carbon nanotubes (CNTs),36 Au/TiO2,37 Ni/CNTs,38 and Ni/Cu–Mg–Al hydrotalcite.39 In addition, alloy catalysts were also found to be efficient catalysts for the conversion of furfural converted CPO (e.g., Ni–Cu/SBA-15,40 Cu–Co alloy,41 CuZnAl and CuNiAl hydrotalcite42,43). Considering the reported catalysts, higher selectivity for CPL can be obtained while in catalytic systems consisting of hydrogenation catalyst and solid acid.
Although some pioneering works have been performed in this area, this field is still lacking regarding some detailed processes. For example, the understanding of the mechanism in the cyclization process of the product is still in its infancy. Herein, we report the design of a new catalyst, aluminium phosphate (Al11.6PO23.7) and Ru/C, which is used in an aqueous environment to give a good yield of CPO and CPL. Additionally, the selectivity of the targeted product could be controlled by adjusting the hydrogen pressure. Al11.6PO23.7 is a bifunctional catalyst that has both an acid site and base site;44 thus, we could adjust the mole ratio of Al and P to control the amount of acid and base, and also control the ratio of Lewis acid and Bronsted acid. According to these properties, different mole ratios of Al and P catalysts were prepared to study the influence factors and cyclization mechanism during the hydrogenation of furfural in aqueous media. This is a novel and efficient catalytic system in this process, which, to the best of our knowledge, has not been previously reported in this area.
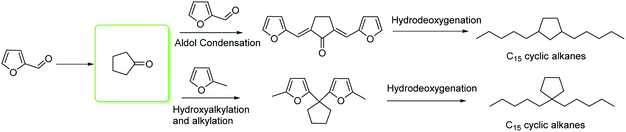
CPO and 2-MF are novel products from the hydrogenation of furfural. Both are C5 feedstocks for the synthesis of long carbon cyclic alkanes. In our previous reports, carbonyl compounds through the C–C coupling reaction consisting of aldol condensation or HAA (hydroxyalkylation and alkylation) reaction with furan-based derivatives were prepared as fuel precursors, which were applied to the synthesis of liquid hydrocarbon fuels through hydrodeoxygenation.45–47 Here, we design a new route for the synthesis of cyclic alkanes for use in diesel range fuels from the hydrogenation product of furfural.
Results and discussion
Hydrogenation of furfural to CPO or CPL
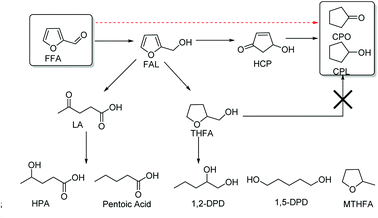
Building on the result of furfural after hydrogenation, FAL and THFA are the normal hydrogenation products.17,18 Under acidic and basic reaction conditions, dehydroxylation and ring opening rearrangement products (e.g., FAL, THFA, MTHFA, LA, and pentanediol) can be obtained, respectively.16,19–23 CPO is the hydrogenation and Piancatelli rearrangement product of furfural.37 In this process (Scheme 2), furfural is firstly hydrogenated to furfuryl alcohol. If furfuryl alcohol undergoes a Piancatelli rearrangement, the product will be 4-hydroxy-2-cyclopentenone (HCP), which will then undergo in-depth hydrogenation and dehydroxylation to produce CPO or CPL. However, furfuryl alcohol in a Bronsted acid environment is dehydroxylated and undergoes a ring opening rearrangement reaction, and then, levulinic acid and in-depth hydrogenation compounds are obtained as the products.49 Additionally, self-condensation of CPO can occur while in an acidic environment. These are the major factors that limit the yield of CPO. The hydrogenation metal catalyst is another factor that limits the yield of the product in this reaction. A higher hydrogenation efficiency catalyst would convert furfural into THFA, which is irreversibly transformed into CPO.36 To our delight, after screening the hydrogenation metal and solid acid catalyst (entries 1–10), we found that aluminium phosphate and Ru/C were favorable catalysts for this reaction.According to the previous research, solid acid is an important factor that influences the selectivity of LA and others. Considering all of the factors in the rearrangement process, we screened HfO2 and TFA/SiO2 as well as different mole ratios of Al and P aluminium phosphate solid acid catalysts as ring opening and rearrangement catalysts. Among them, HfO2 has a pure Lewis acid site, TFA/SiO2 is representative Bronsted acid catalyst, and aluminium phosphate has an adjustable ratio of L/B acid sites. From the results presented in Table 1 (entries 1–7), we found that HfO2 and TFA/SiO2 had poor selectivity for CPO and CPL. This phenomenon illustrated that the cyclization rearrangement is not a simple Lewis or Bronsted acid catalyzed rearrangement reaction. However, after screening different mole ratios of Al- and P-type aluminium phosphate catalysts, we found that aluminium phosphate well-catalyzed the rearrangement. Additionally, the higher the molar ratio of the Al- and P-type aluminium phosphate catalyst, the better the selectivity for CPL and CPO. To determine the ultimate mole ratio of Al and P for this catalyst, we used 20 portions of Al(NO3)3·9H2O and 1 portion of (NH4)2HPO4 to prepare the aluminium phosphate catalyst. After XPS analysis of these catalysts (Fig. S1†), the maximum mole ratio of Al and P was determined to be 11.6 and the atomic levels of O 1s, Al 2p and P 2p were 50.33%, 24.75% and 2.12%, respectively (Fig. S1†).
| Entry | Catalyst | Solvent | Temp. | Con. (FFA) | Product (selectivity) | ||||
|---|---|---|---|---|---|---|---|---|---|
| FAL | CPO | CPL | THFA | LA + others | |||||
| a Reaction conditions: Furfural (20 mmol), hydrogenation metal catalyst (0.5 wt%, 0.25 g) and solid acid (0.25 g), 4 MPa H2, reacted at the given temperature for 4 h. The products were analyzed and detected via HPLC.b Reacted for 8 hours.c Reacted at 2 MPa H2 pressure.d Hydrogenation metal catalyst respect to 0.25 wt%.e Substrate furfural respect to 40 mmol. | |||||||||
| 1 | Ru/C + TFA/SiO2 | H2O | 433 K | 100% | 0% | 0% | 34% | 27% | 39% |
| 2 | Ru/C + HfO2 | H2O | 433 K | 100% | 0% | 5% | 46% | 32% | 17% |
| 3 | Ru/C + AlP1.2O4 | H2O | 433 K | 100% | 0% | 4% | 47% | 13% | 36% |
| 4 | Ru/C + Al1.1PO4.2 | H2O | 433 K | 100% | 0% | 3% | 64% | 17% | 19% |
| 5 | Ru/C + Al2.1PO6.4 | H2O | 433 K | 100% | 0% | 0% | 72% | 11% | 17% |
| 6 | Ru/C + Al5.3PO12 | H2O | 433 K | 100% | 0% | 0% | 74% | 10% | 16% |
| 7 | Ru/C + Al11.6PO23.7 | H2O | 433 K | 100% | 0% | 0% | 84% | 6% | 10% |
| 8 | RANEY® Ni + Al11.6PO23.7 | H2O | 433 K | 100% | 0% | 0% | 56% | 24% | 20% |
| 9 | Pd/C + Al11.6PO23.7 | H2O | 433 K | 100% | 0% | 1% | 68% | 24% | 17% |
| 10b | Pt/C + Al11.6PO23.7 | H2O | 433 K | 90% | 0% | 90% | 0% | 2% | 8% |
| 11c | Ru/C + Al11.6PO23.7 | H2O | 433 K | 100% | 0% | 78% | 4% | 7% | 11% |
| 12d | Ru/C + Al11.6PO23.7 | H2O | 433 K | 100% | 0% | 3% | 74% | 4% | 19% |
| 13e | Ru/C + Al11.6PO23.7 | H2O | 433 K | 96% | 0% | 70% | 5% | 4% | 21% |
| 14 | Ru/C + Al11.6PO23.7 | MeOH | 433 K | 100% | 0% | 0% | 0% | 98% | 2% |
| 15 | Ru/C + Al11.6PO23.7 | THF | 433 K | 100% | 0% | 0% | 0% | 96% | 4% |
| 16b | Ru/C + Al11.6PO23.7 | H2O | 413 K | 100% | 0% | 3% | 78% | 6% | 13% |
| 17b | Ru/C + Al11.6PO23.7 | H2O | 393 K | 100% | 0% | 0% | 3% | 76% | 21% |
| 18 | Ru/C + Al11.6PO23.7 | H2O | 453 K | 100% | 0% | 0% | 72% | 12% | 16% |
Aluminium phosphate is a solid acid-base bifunctional catalyst, as discussed in our previous reports.45 After NH3-TPD and CO2-TPD tests (Fig. S2†), the amount of acidic and basic Al11.6PO23.7 was found to be 1.211 mmol g−1 and 0.705 mmol g−1, respectively. Also, the ratio of Lewis/Bronsted acid can be adjusted by changing the mole ratio of Al and P. Different mole ratios of Al and P (e.g., 0.5, 1, 2, 5, and 20) aluminium phosphate catalysts were prepared to test in this reaction. After XPS analysis of these catalysts, the molecular formula of the different mole ratio Al and P aluminium phosphate catalysts were found to be AlP1.2O4, Al1.1PO4.2, Al2.1PO6.4, Al5.3PO12, Al11.6PO23.7. From the results presented in Table 1 (entries 3–7), the maximum mole ratio Al and P catalyst, Al11.6PO23.7, showed the best selectivity for CPO and CPL. Considering the influence of the ratio of Lewis/Bronsted acid and that Al11.6PO23.7 had the best catalytic properties, the pyridine-adsorbed infrared spectra were analyzed to characterize the different molar ratio aluminium phosphate catalysts at temperatures of 313 K and 433 K (Fig. 1). From the results presented in Fig. 1 and Table S1,† we found that Al11.6PO23.7 had the highest ratio of L/B at the reaction temperature. This result showed that a high amount of Lewis acid with a small amount of Bronsted acid can promote the cyclization rearrangement reaction. However, the pure Lewis acid catalyst HfO2 had the poor selectivity for CPO. This phenomenon showed that the conversion of furfural to CPO occurs via the synergistic catalysis of a Lewis acid and Bronsted acid and that the catalyst must have a moderate ratio of L/B acid at the reaction temperature.
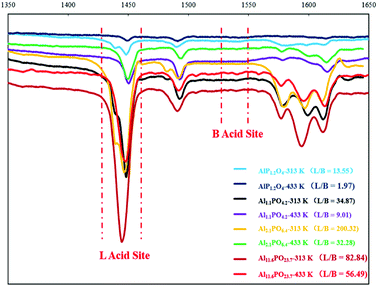 | ||
| Fig. 1 Pyridine adsorption FT-IR spectroscopy result of different mole ratios of Al and P aluminium phosphate catalyst. | ||
Besides the influence of the solid acid, the hydrogenation metal is another factor that limits the yield of CPL. As observed from Table 1 (entries 7–10), after comparing different hydrogenation metal catalysts, we found that Ru/C was the best catalyst for this reaction. RANEY® Ni and Pd/C had high hydrogenation activity, which was expressed as higher selectivity for THFA as the ultimate product. Furthermore, Pt/C had poor hydrogenation activity in this process since furfural was not completely converted and no CPL product was detected after 8 hours. In addition, different reaction conditions were tested for this reaction. From Table 1, Entry 11, we found CPO as the major product when the hydrogen pressure was controlled at 2 MPa, which indicates that the selectivity of CPL or CPO can be controlled by adjusting the hydrogen pressure. Also, we decreased the amount of hydrogenation metal catalyst to 0.25 wt% and increased the amount of substrate to 40 mmol, as seen from Table 1, entries 12–13, and we found that a change in the amount of hydrogenation metal catalyst has little influence on the yield of CPL. However, CPO was detected as the major product and furfural had poor conversion with an increase in the amount of substrate under the same reaction conditions. This phenomenon proved that hydrogen pressure is a major factor in the yield of CPO. Additionally, it was clearly found that the cyclization rearrangement occurred in the aqueous environment from the result in the Table 1, entries 8, 14–15. Herein, MeOH and THF, as a proton organic solvent and non-protonic organic solvent, were tested in this reaction, respectively. From the results, we found that THFA was the main product obtained in both solvents. Additionally, the reaction temperature is another factor that influenced the major products. From the result in Table 1, entries 8, 16–18, we found that the temperature determined the reaction speed and whether the reaction could be carried out or not. From Table 1, entries 16–17, we found that for the cyclization rearrangement to occur, the reaction temperature had to be over 393 K, and we also found that a higher temperature could increase the reaction speed.
Also, termination of this reaction did not occur during 4 hours at a temperature of 413 K. In addition, the higher temperature promoted the reaction process, but decreased the selectivity of the target product. For example, compared with the lower reaction temperature, more LA and other products were detected at the temperature of 453 K (Table 1, Entry 18). Hence, for the efficient catalysts Ru/C and Al11.6PO23.7 with water as the medium at the temperature of 433 K, CPL or CPO can be obtained as the main product. Furthermore, the catalysts can be recovered by filtration and washed with methanol after the reaction. From the results shown in Fig. S6,† it is observed that these catalysts can be reused six times and maintain their catalytic efficiency.
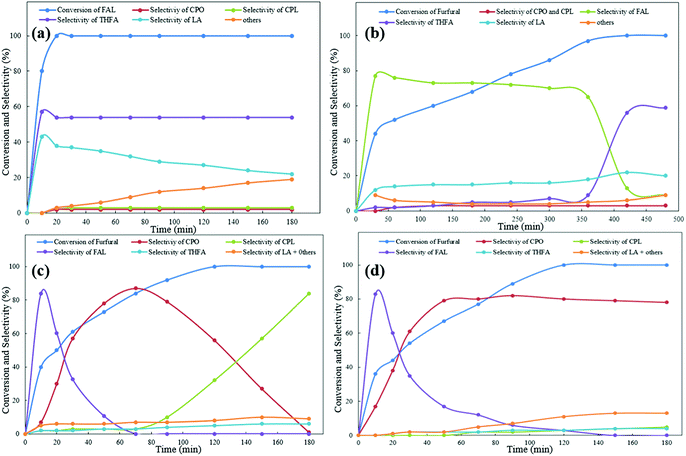
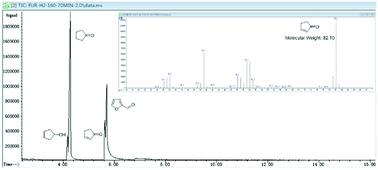
To research the in-depth process of the hydrogenation of furfural to CPO or CPL, different reaction conditions and substrates were used to examine the mechanism of the cyclization process at different reaction times. As observed from Fig. 2(a), an interesting phenomenon was found that differed from the previous report. In the previous work on the preparation of CPO and CPL, furfural was first converted to furfuryl alcohol, followed by Piancatelli rearrangement to HCP and converted to CPO via hydrogenation and dehydroxylation. However, from the result shown in Fig. 2(a), no CPO or CPL was obtained with furfuryl alcohol as the substrate at 433 K with Ru/C and Al11.6PO23.7 as the catalyst. The hydrogenation of furfuryl alcohol was very fast, and within 20 minutes all of the furfuryl alcohol was converted to levulinic acid (LA), tetrahydrofurfuryl alcohol (THFA) and others. This result is contrary with the previous mechanism of the hydrogenation of furfural to CPO. In addition, different temperatures and hydrogen pressures were tested using furfural as the substrate. From the results shown in Fig. 2(b), we found that at 393 K, furfural could not be converted to CPO. Also, we found that furfural was firstly completely converted to furfuryl alcohol in this process, followed by the conversion of furfuryl alcohol to THFA at a fast rate. Furthermore, conversion of furfuryl alcohol to LA and others was not restricted to these conditions, and the speed at which furfuryl alcohol was converted to LA and others was promoted by a high temperature. Comparing Fig. 2(a) and (b), we can infer that FAL converted to THFA and others at a fast speed under the heating reactor conditions, which is the reason why no targeted products were obtained using FAL as the substrate. In addition, from the result shown in Fig. 2(c) and (d), within 10 minutes, the slight conversion of furfural and higher selectivity for FAL were detected in this process. Furthermore, in the following few minutes, the selectivity of FAL decreased acutely, and the selectivity for CPO increased. This phenomenon demonstrated that furfural was converted to FAL, and then FAL converted to CPO immediately in this catalytic system. Additionally, the selectivity for CPO and CPL can be controlled by adjusting the hydrogen pressure.
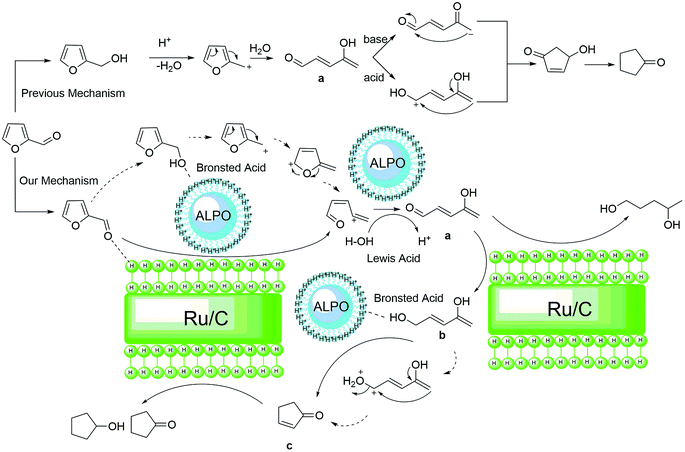
Encouraged by the previous report, we know that CPO is the hydrogenation and Piancatelli rearrangement product of furfural. During the rearrangement process, furfural is first hydrogenated to furfuryl alcohol. 4-Hydroxy-2-cyclopentenone is the intermediate product, which forms after the Piancatelli rearrangement of furfural alcohol, and then following in-depth hydrogenation and dehydroxylation, cyclopentanone or cyclopentanol is produced. From the previous report,50 there are two methods for the formation of 4-hydroxy-2-cyclopentenone (HCP) (Fig. 3). One is the acid catalyzed enol attack of the aldehyde carbocation, followed by cyclization to HCP, and the other is via base-catalyzed aldol condensation. However, from the result show in Fig. 2, there no HCP and a little FAL were detected in this process. Hence, furfuryl alcohol conversion to HCP during Piancatelli rearrangement may not be an exclusive method to prepare CPO from furfural.
According to the results shown in Fig. 2 and the characterization of the Al11.6PO23.7 catalyst, we inferred the mechanism of the hydrogenation of furfural to CPO as follows (Fig. 3). Initially, furfural is converted to FAL, which is catalyzed by Ru/C in the hydrogen environment, and then dehydroxylation of FAL occurs to form the furan methyl carbocation, which is catalyzed by the Bronsted acid site in Al11.6PO23.7. After that, intermediate a (4-hydroxypenta-2,4-dienal) is formed through the carbocation rearrangement, which is catalyzed by the Lewis acid site in Al11.6PO23.7. In the following step, intermediate a is catalyzed by Ru/C and converted to b (penta-2,4-diene-1,4-diol) in the hydrogen environment. Finally, dehydroxylation occurs and the hydroxyl group in b is catalyzed by Al11.6PO23.7. Then the cyclopentenone c intermediate is formed through the enol attack on the carbocation after dehydroxylation, and CPO is obtained via hydrogenation in this process at last. This mechanism is consistent with the result shown in Fig. 2. Additionally, the cyclopentenone c was detected at the reaction time of 70 min, and no HCP and FAL were detected at that time (see Fig. 4). This phenomenon is attributed to the Bronsted acid site in Al11.6PO23.7, which promoted the formation of the furan methyl carbocation. Also, the in-depth hydrogenation product (1,4-pentanediol) of intermediate b was detected by GC-MS (see Fig. S7.†) at a higher hydrogen pressure. This phenomenon demonstrated that intermediates b and c were formed during this process, and the process of rearrangement was not through the HCP route. This result is consistent with the previous report,34 where cyclopentenone c was a promising intermediate in the conversion of furfural to CPO. In addition, based on previous reports,51–54 HCP would have been formed at a high temperature with FAL as the substrate. However, as this process was carried out at 433 K during, it is hard to prove that furfuryl alcohol as the substrate through HCP was converted to CPO. Hence, this cyclization reaction is a Bronsted acid and Lewis acid synergistic catalytic process via dehydroxylation and hydrogenation.
Hydrodeoxygenation of C–C coupling reaction product
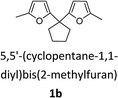
CPO and CPL are versatile chemical intermediates that are applied in various fields of daily life. Inspired by our previous work,45–47 cyclopentanone can be used as a substrate to synthesize diesel range fuels due to its carbonyl group. This process consists of two steps. The first step is a C–C coupling reaction (e.g., hydroxyalkylation and alkylation or aldol condensation) to increase the carbon number and octane value. Then, hydrocarbon fuels were obtained after the hydrodeoxygenation of the C–C coupling products (Scheme 3). According to the carbon numbers of the alkanes, we divided these products into light alkanes (C1–C4), gasoline range alkanes (C5–C8) and diesel range alkanes (C9–C16). Herein, we chose furfural and 2-methyl furan as the substrates to couple with CPO for the synthesis of diesel range hydrocarbon fuels, and all of the substrates came from hemicellulose.55Aldol condensation of bio-based carbonyl compounds and furfural was carried out in our previous report.47 After comparing mineral bases, organic bases and solid bases, we found that organic amines were efficient catalysts to control the single and double aldol condensation products. TBD (1,5,7-triazabicyclo[4.4.0]dec-5-ene) was identified as an efficient and reusable catalyst for this reaction. Herein, the experiments were carried out in a methanol environment at room temperature, and 10% equivalent catalyst to cyclopentanone was used to catalyze the aldol condensation. To our surprise, a greater than 95% yield of 1a was obtained after 10 minutes (Table 2, Fig. S9†), and the product was isolated after filtration with no further purification. Furthermore, the catalyst in the solvent could be reused for the next reaction. Hydroxyalkylation and alkylation of cyclopentanone with 2-methylfuran were carried out to synthesize another fuel precursor. Encouraged by the previous result,56–59 the hydroxyalkylation and alkylation reactions were sensitive to the acid strength of the catalyst. A strong acid was more active than weak acid for this reaction. Using para-toluenesulfonic acid as a commercially available and efficient catalyst for this reaction, more than 90% yield of 1b was obtained when 10% equivalent catalyst to cyclopentanone was used at 333 K for 6 hours (Table 2, Fig. S10†). After the reaction, the catalyst and product were separated after extraction with water and ethyl acetate. After concentrating the ethyl acetate and water phases, the catalyst and product were obtained, respectively.
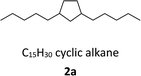
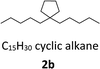
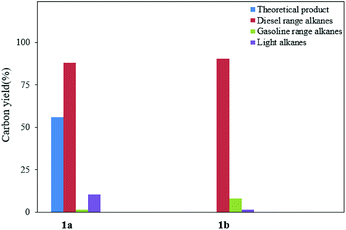
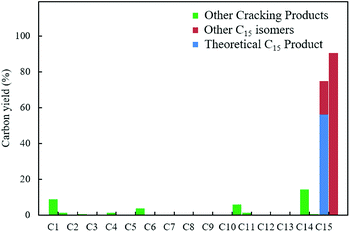
With the aim of synthesizing hydrocarbon fuels, hydrodeoxygenation of C–C coupling products was performed in a batch reactor. Hydrodeoxygenation is both hydrogenation and deoxygenation. This process requires synergistic catalysis between the hydrogenation catalyst and solid acid. Encouraged by our previous work,45–47 we found that the commercial catalyst Pd/C added zeolite (H-beta) had the best selectivity for alkanes. During this experiment, 5% equivalent Pd with 0.5 g H-beta (Si/Al = 40) to 5 mmol C–C coupling products was used as the catalyst and reacted at 473 K, and water as the solvent and 3 MPa hydrogen pressure were required simultaneously. After hydrodeoxygenation, the gas product was detected by GC with a TCD detector and the liquid product was extracted with ethyl acetate and detected with GC-MS (Fig. S8†). From the result in Fig. 5 and 6, hydrodeoxygenation of the aldol condensation product gives more than 50% yield of theoretical product, which is also accompanied by isomerization and decarbonylation. To our delight, a small amount of C11 and C14 cyclic alkanes was detected, which belong to the diesel range alkanes. However, it is unfortunate that there was no theoretical product produced after hydrodeoxygenation of the hydroxyalkylation and alkylation product. This phenomenon is attributed to the stability of the carbocation, where C–C cleavage of 2 at the carbon atom adjacent to the furan ring generated a tertiary carbocation, and the formation of the most stable tertiary carbocation led to the C–C cleavage products during hydrodeoxygenation. From the results shown in Fig. 5 and S12,† according to the different cracking cleavage, more than 85% yield of C15 isomer alkanes were obtained. To our delight, regarding the specificity of cyclopentyl, hexatomic ring cyclic alkanes were obtained from the cracking of cyclopentyl and subsequent rearrangement, and the final products C10 and C15 cyclic alkanes in the range of diesel fuels. Hence, based on cyclopentanone, the C–C coupling reaction followed by hydrodeoxygenation is an effective method to prepared cyclic alkanes. Compared to straight chain alkanes, cyclic alkanes have higher densities and volumetric heating values due to the strong ring strain. Also, cyclic alkanes are the second most abundant component in commercial and military jet fuels, at a mass percent of 20–50 wt%.60–63
 | ||
| Fig. 6 Product distribution of alkanes from the HDO of the fuel precursors (left: 1a and right: 1b). | ||
Experimental
Catalyst preparation
All reagents used in this work were of analytical regent grade, commercially available and used without further purification. Amorphous aluminium phosphate (AlPO) was prepared according to the reported procedure.48 In a typical synthesis of AlPO (Al/P = 1), Al(NO3)3·9H2O and (NH4)2HPO4 based on a mole ratio of Al/P = 1 were dissolved in water acidified with nitric acid. Concentrated ammonia was added to this solution, and a hydrogel was achieved at pH = 8. The above hydrogel solution was stirred for 1 h and then the solvent was filtered, and the hydrogel was washed with distilled water twice. The hydrogel was dried at 120 °C for 12 h and calcined at 550 °C at a heating rate of 5 °C min−1 in air for 30 min. The other AlPO catalyst with different mole ratios of Al/P (e.g., Al10PO, Al5PO, Al2PO, AlP2O) were prepared according to the abovementioned method, but using different mole ratios of Al(NO3)3·9H2O and (NH4)2HPO4.Hafnium oxide was prepared via calcination of hafnium hydroxide hydrate. Typically, the hydrogel was prepared via the dropwise addition of a concentrated ammonia solution to an aqueous solution of HfCl4 (10 mmol in 50 mL deionized water). The mixture was stirred for 2 h at room temperature, after which a pH of 8–9 was reached, and then aged at 120 °C for 6 h. The catalyst was obtained through filtration and then dried at 50 °C overnight after removing excess ions via thorough washing with water. The samples were then calcined at 550 °C for 4 h.
Trifluoromethanesulfonic acid-based silicon dioxide (TFA/SiO2 (30%)) was prepared using 7 g nano silica dispersed in 100 mL isobutanol and 3 g trifluoromethanesulfonic acid dissolved in 20 mL dichloromethane, which were added dropwise to aqueous isobutanol aqueous with violent stirring at a temperature of 353 K for 6 hours. Subsequently, the TFA/SiO2 (30%) catalyst was obtained after filtration and dried at 333 K under vacuum.
Experimental
Conclusions
In this study, the commercial hydrogenation catalyst Ru/C with Al11.6PO23.7 was validated as a novel and efficient catalyst system for the synthesis of cyclopentanone or cyclopentanol from furfural. In this process, a new mechanism for the conversion of furfural to cyclopentanone catalyzed by Ru/C and Al11.6PO23.7 was proposed. Additionally, with cyclopentanone as the building block, the C–C coupling reaction and hydrodeoxygenation was an excellent route to synthesize cyclic alkanes (diesel range cyclic alkanes, selectivity > 85%). Hence, based on furfural, we not only provide an efficient catalytic system to produce cyclopentanol, but also offer a novel route for the synthesis of cyclic alkanes as hydrocarbon fuels without phenyl compounds.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Program for the National Natural Science Foundation of China (Grant No: 21776132); the Young Elite Scientist Sponsorship Program by CAST; the Changjiang Scholars and Innovative Research Team in University (Grant No: IRT_14R28); the Major Research Plan of the National Natural Science Foundation of China (Grant No: 21390204); the Jiangsu National Synergetic Innovation Center for Advanced Bio-Manufacture.Notes and references
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed.
- D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493–1513 RSC.
- C. Zhou, X. Xia, C. Lin, D. Tong and J. Beltramini, Chem. Soc. Rev., 2011, 40, 5588–5617 RSC.
- Y. Lin and G. W. Huber, Energy Environ. Sci., 2009, 2, 68–80 RSC.
- Y. Román-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1937 CrossRef PubMed.
- T. S. Hansen, J. Mielby and A. Riisager, Green Chem., 2011, 13, 109–114 RSC.
- S. Q. Hu, Z. F. Zhang, J. L. Song, Y. X. Zhou and B. X. Han, Green Chem., 2009, 11, 1746–1749 RSC.
- G. Marcotullio and W. De Jong, Green Chem., 2010, 12, 1739–1746 RSC.
- J. N. Chheda, Y. Romaán-Leshkov and J. A. Dumesic, Green Chem., 2007, 9, 342–350 RSC.
- D. T. Win, Aust. J. Biotechnol., 2005, 8, 185–190 Search PubMed.
- Y. Roman-Leshkov, C. J. Barrett, Z. Y. Liu and J. A. Dumesic, Nature, 2007, 447, 982–985 CrossRef CAS PubMed.
- B. M. Nagaraja, A. H. Padmasri, B. David Raju and K. S. Rama Rao, J. Mol. Catal. A: Chem., 2007, 265, 90–97 CrossRef CAS.
- V. V. Ordomsky, J. C. Schouten, J. van der Schaaf and T. A. Nijhuis, Appl. Catal., A, 2013, 451, 6–13 CrossRef CAS.
- E. J. García-Suárez, A. M. Balu, M. Tristany, A. B. García, K. Philippot and R. Luque, Green Chem., 2012, 14, 1434–1439 RSC.
- K. Yan, J. Y. Liao, X. Wu and X. M. Xie, RSC Adv., 2013, 3, 3853–3856 RSC.
- X. Hu, S. Jiang, L. Wu, S. Wang and C. Z. Li, Chem. Commun., 2017, 53, 2938–2941 RSC.
- T. Mizugaki, T. Yamakawa, Y. Nagatsu, Z. Maeno, T. Mitsudome, K. Jitsukawa and K. Kaneda, ACS Sustainable Chem. Eng., 2014, 2, 2243–2247 CrossRef CAS.
- S. Liu, Y. Amada, M. Tamura, Y. Nakagawa and K. Tomishige, Green Chem., 2014, 16, 617–626 RSC.
- G. Li, N. Li, M. Zheng, S. Li, A. Wang, Y. Cong, X. Wang and T. Zhang, Green Chem., 2016, 18, 3607–3613 RSC.
- S. Dutta, RSC Adv., 2012, 2, 12575–12593 RSC.
- N. Li, G. A. Tompsett and G. W. Huber, ChemSusChem, 2010, 3, 1154–1157 CrossRef CAS PubMed.
- M. J. Climent, A. Corma and S. Iborra, Green Chem., 2014, 16, 516–547 RSC.
- A. B. Gawade and G. D. Yadav, Curr. Catal., 2017, 6, 193–200 CAS.
- L. Rokhum and G. Bez, Synth. Commun., 2011, 41, 548–552 CrossRef CAS.
- G. Liang, A. Wang, L. Li, G. Xu, N. Yan and T. Zhang, Angew. Chem., 2017, 129, 3096–3100 CrossRef.
- J. Zhu, Z. Tan and W. Yang, Macromol. Res., 2017, 25, 792–798 CrossRef CAS.
- S. Madabhushi, N. Chinthala, V. S. Vangipuram, K. R. Godala, R. Jillella, K. K. R. Mallu and C. R. Beeram, Tetrahedron Lett., 2011, 52, 6103–6107 CrossRef CAS.
- L. K. Sharma, K. B. Kim and G. I. Elliott, Green Chem., 2011, 13, 1546–1549 RSC.
- X. Sheng, N. Li, G. Li, W. Wang, J. Yang, Y. Cong, A. Wang, X. Wang and T. Zhang, Sci. Rep., 2015, 5, 9565 CrossRef CAS PubMed.
- M. Renz, Eur. J. Org. Chem., 2005, 6, 979–988 CrossRef.
- T. Akashi, S. Sato, R. Takahashi, T. Sodesawa and K. Inui, Catal. Commun., 2003, 4, 411–416 CrossRef CAS.
- M. Hronec, K. Fulajtarova and T. Liptaj, Appl. Catal., A, 2012, 437, 104–111 CrossRef.
- M. Hronec and K. Fulajtarova, Catal. Commun., 2012, 24, 100–104 CrossRef CAS.
- M. Hronec, K. Fulajtarova, I. Vavra, T. Sotak, E. Dobrocka and M. Micusik, Appl. Catal., B, 2016, 181, 210–219 CrossRef CAS.
- R. Fang, H. Liu, R. Luque and Y. Li, Green Chem., 2015, 17, 4183–4188 RSC.
- Y. Liu, Z. Chen, X. Wang, Y. Liang, X. Yang and Z. Wang, ACS Sustainable Chem. Eng., 2017, 5, 744–751 CrossRef CAS.
- G. Zhang, M. Zhu, Q. Zhang, Y. Liu, H. He and Y. Cao, Green Chem., 2016, 18, 2155–2164 RSC.
- M. Zhou, H. Zhu, L. Niu, G. Xiao and R. Xiao, Catal. Lett., 2014, 144, 235–241 CrossRef CAS.
- M. Zhou, Z. Zeng, H. Zhu, G. Xiao and R. Xiao, J. Energy Chem., 2014, 23, 91–96 CrossRef CAS.
- Y. Yang, Z. Du, Y. Huang, F. Lu, F. Wang, J. Gao and J. Xu, Green Chem., 2013, 15, 1932–1940 RSC.
- X. Li, J. Deng, J. Shi, T. Pan, C. Yu, H. Xu and Y. Fu, Green Chem., 2015, 17, 1038–1046 RSC.
- J. Guo, G. Xu, Z. Han, Y. Zhang, Y. Fu and Q. Guo, ACS Sustainable Chem. Eng., 2014, 2, 2259–2266 CrossRef CAS.
- H. Zhu, M. Zhou, Z. Zeng, G. Xiao and R. Xiao, Korean J. Chem. Eng., 2014, 31, 593–597 CrossRef CAS.
- M. J. Climent, A. Corma, H. Garcia, R. Guil-Lopez, S. Iborra and V. Fornés, J. Catal., 2001, 197, 385–393 CrossRef CAS.
- C. Zhu, T. Shen, D. Liu, J. Wu, Y. Chen, L. Wang, K. Guo, H. Ying and P. Ouyang, Green Chem., 2016, 18, 2165–2174 RSC.
- T. Shen, C. Zhu, C. Tang, Z. Cao, L. Wang, K. Guo and H. Ying, RSC Adv., 2016, 6, 62974–62980 RSC.
- T. Shen, J. Tang, C. Tang, J. Wu, L. Wang, C. Zhu and H. Ying, Org. Process Res. Dev., 2017, 21, 890–896 CrossRef CAS.
- T. Lindblad, B. Rebenstorf, Z.-G. Yan and S. L. T. Andersson, Appl. Catal., A, 1994, 112, 187–208 CrossRef CAS.
- Y. Xu, J. Shi, W. Wu, R. Zhu, X. Li, J. Deng and Y. Fu, Appl. Catal., A, 2017, 543, 266–273 CrossRef CAS.
- D. Ren, Z. Song, L. Li, Y. Liu, F. Jin and Z. Huo, Green Chem., 2016, 18, 3075–3081 RSC.
- T. Kumaraguru, P. Babita, G. Sheelu, K. Lavanya and N. W. Fadnavis, Org. Process Res. Dev., 2013, 17, 1526–1530 CrossRef CAS.
- K. Ulbrich, P. Kreitmeier and O. Reiser, Synlett, 2010, 2010, 2037–2040 CrossRef.
- M. Hronec, K. Fulajtárová and T. Soták, J. Ind. Eng. Chem., 2014, 20, 650–655 CrossRef CAS.
- G. Li, N. Li, M. Zheng, S. Li, A. Wang, Y. Cong, X. Wang and T. Zhang, Green Chem., 2016, 18, 3607–3613 RSC.
- K. J. Zeitsch, in The Chemistry and Technology of Furfural and its Many By-Products, Sugar Series, Elsevier Science, Dordrecht, 2000, vol. 13, pp. 229–230 Search PubMed.
- G. Li, N. Li, S. Li, A. Wang, Y. Cong, X. Wang and T. Zhang, Chem. Commun., 2013, 49, 5727–5729 RSC.
- S. Li, N. Li, G. Li, A. Wang, Y. Cong, X. Wang and T. Zhang, Catal. Today, 2014, 234, 91–99 CrossRef CAS.
- G. Li, N. Li, Z. Wang, C. Li, A. Wang, X. Wang, Y. Cong and T. Zhang, ChemSusChem, 2012, 5, 1958–1966 CrossRef CAS PubMed.
- K.-I. Shimizu and A. Satsuma, Energy Environ. Sci., 2011, 4, 3140–3153 RSC.
- E. Corporan, T. Edwards, L. Shafer, M. J. DeWitt, C. Klingshirn, S. Zabarnick, Z. West, R. Striebich, J. Graham and J. Klein, Energy Fuels, 2011, 25, 955–966 CrossRef CAS.
- P. Bi, J. Wang, Y. Zhang, P. Jiang, X. Wu, J. Liu, H. Xue, T. Wang and Q. Li, Bioresour. Technol., 2015, 183, 10–17 CrossRef CAS PubMed.
- H. A. Meylemans, R. L. Quintana, B. R. Goldsmith and B. G. Harvey, ChemSusChem, 2011, 4, 465–469 CrossRef CAS PubMed.
- J. Yang, N. Li, G. Li, W. Wang, A. Wang, X. Wang, Y. Cong and T. Zhang, Chem. Commun., 2014, 50, 2572–2574 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra08757a |
| This journal is © The Royal Society of Chemistry 2018 |