Solid phase extraction based on the phase transition of poly(N-isopropylacrylamide): the extraction behaviour of lanthanide(III) ions in highly acidic solutions†
Ki Chul
Park
 ,
Haruka
Tateno
and
Takehiko
Tsukahara
*
,
Haruka
Tateno
and
Takehiko
Tsukahara
*
Laboratory for Advanced Nuclear Energy, Institute of Innovative Research in Tokyo Institute of Technology, 2-12-1-N1-6 O-okayama, Meguro-ku, Tokyo, 152-8550, Japan. E-mail: ptsuka@lane.iir.titech.ac.jp
First published on 13th December 2017
Abstract
The present study investigates the applicability of poly(N-isopropylacrylamide) (PNIPAAm) as an extraction medium for trivalent lanthanide (Ln(III)) ions in high concentrations of nitric acid solutions by using octyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide (CMPO) as an extracting agent. The extraction is based on the hydrophobic, co-aggregative binding of the Ln-CMPO nitrate complex formed in situ onto the PNIPAAm aggregates growing above the phase-transition temperature. The resulting co-aggregates enabled easy separation of the liquid and solid phases. The extraction of Ln(III) ions was affected by the efficiency and extent of complexation and the stability of the complex. Furthermore, the difference in the stability of Ln-CMPO complex species allowed for the stripping of Ln(III) ions in hydrochloric acid solutions.
Introduction
The development of new technologies for spent nuclear fuel reprocessing to reduce social and environmental burdens has been a major goal of chemists participating in nuclear chemical engineering. In general, spent nuclear fuels dissolved in highly acidic solutions are treated via reprocessing, partitioning and separation mainly consisting of multi-step solvent extraction processes.1,2 In the course of the processes, trivalent lanthanides (Ln(III)) and actinides are extracted via the transuranic element extraction (TRUEX) process, which is carried out in high concentrations (0.5–6 mol L−1) of nitric acid solution.2,3 In the TRUEX process, an electroneutral bidentate chelating ligand, octyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide (CMPO, Fig. 1) is used as the extracting agent in combination with tri-n-butylphosphate (TBP) as the phase modifier. Typically, the TRUEX process uses n-dodecane solutions composed of 0.2 mol L−1 CMPO and 1.2–1.4 mol L−1 TBP. In spent fuel reprocessing based on solvent extraction, including the TRUEX process, a relatively small amount of solvent is used while being continuously recycled to minimize the generation of secondary waste. However, the number of recycling cycles is limited due to the radiolytic and hydrolytic degradation of the solvent,4,5 which has caused the actual issue of secondary waste treatment. In addition, the use of flammable organic solvents carries the potential risks of serious accidents.6Solid phase extraction (SPE) based on a clouding phenomenon (phase transition) of surfactant micelles has been extensively studied as a green, safe and economical process for separation of metal ions. Cloud point extraction (CPE) using mainly non-ionic (or zwitterionic) surfactants is based on the trapping of metal complexes into the hydrophobic cores of micelles and subsequent phase separation by controlling the temperature above (or below) the cloud points.7–10 The great advantage of CPE lies in the high preconcentration of solutes from aqueous solutions to a far smaller volume of micellar phases, compared with conventional liquid phase extraction. The solubilization of hydrophobic solutes in aqueous solutions is attained by the interaction with micellar binding sites, forming an equilibrium between free solutes and micelle-bound solutes. The equilibrium (binding) constant11 is directly related to the extraction efficiency of CPE. Therefore, various experimental factors affecting the equilibrium, as well as those related to micellization and cloud points, are required to be optimized to enhance the extractability of CPE.7
Another approach similar to CPE includes SPE utilizing the phase transition of poly(N-isopropylacrylamide) (PNIPAAm, Fig. 1) as the extraction medium.12–14 PNIPAAm is a well-known smart polymer exhibiting a thermo-responsive property of reversibly switching hydrophilicity and hydrophobicity at a lower critical solution temperature (LCST) of about 32 °C.15–17 Thus far, a wide variety of PNIPAAm-based materials including homo- and copolymers have been applied to the adsorption and detection of metal ions, i.e., Ag(I),18 Cu(I),18,19 Cs(I),20 Li(I),21 Fe(II),12,13 Cu(II),22–24 Pd(II),18 Co(II),19 Ni(II),25 Cd(II),26 Hg(II),27 Pb(II),27,28 Au(III),18,29 In(III),14 Ln(III).30 The application of PNIPAAm as an extraction medium was first reported for the extraction of a water-soluble cationic chelate, tris(1,10-phenanthroline)iron(II), as an ion pair with perchlorate anions.12,13 On the other hand, Tokuyama and Iwama reported the first application of PNIPAAm to the direct extraction of a water-insoluble complex species, the In(III)-organophosphate complex.14 The SPE of In(III) ions is composed of two main processes, that is, the complexation of In(III) ions with organophosphate ligands in the presence of dissolved PNIPAAm below the LCST, and hydrophobic binding of the metal complex onto the PNIPAAm precipitates formed above the LCST. The extraction of In(III) ions was successfully achieved at a high extractability of 89.1% (0.854 mmol per g dry polymer). Furthermore, based on the dependence of extractability on the initial ligand amount and pH, it was indicated that the amount of In(III) extracted to PNIPAAm precipitates was determined by the degree of complex formation, which was enhanced with increasing organophosphate anions as coordination species in the complexation equilibrium. Inversely, the stripping of In(III) ions was achieved by decreasing the anionic species of ligands in more acidic solutions.
We have explored the applicability of PNIPAAm-based SPE to the extraction of metal ions in highly acidic solutions, aiming to develop a new technology for spent nuclear fuel reprocessing. In this study, we investigate its applicability to the extraction of Ln(III) ions in high concentrations of nitric acid (1.0–3.0 mol L−1 HNO3). PNIPAAm-based SPE is more advantageous in respect that the extraction process is simply based on the hydrophobic binding between PNIPAAm and metal complexes, compared with CPE with complicated experimental parameters. Above the LCST, PNIPAAm dissolved in an aqueous solution initiates intramolecular hydrophobic interaction to form globular particles by a conformational change from the coil state,16 further growing to larger aggregates of hydrophobic particles. From another perspective, an inherently difficult molecular-level dispersion of hydrophobic macromolecules is considered to be achieved in the early stage of phase transition. Therefore, we expect that a high dispersion of PNIPAAm molecules realizes a highly efficient binding with the metal complex coexisting in the system. In this study, we report the PNIPAAm-based extraction of Ln(III) ions using CMPO ligands in nitric acid solutions of mixed ions (La3+, Nd3+, Eu3+, Lu3+, Cs+ and Sr2+).
Experimental
Materials
PNIPAAm was synthesized by atom transfer radical polymerization (ATRP) using a copper chloride complex with tris[2-(dimethylamino)ethyl]amine as the catalyst and 4-ethylbenzyl chloride as the ATRP initiator according to the literature,30 and purified three times by solidification using acetone and diethyl ether. CMPO (purity 97%) was purchased from Strem Chemicals, Inc. and used as received. La(NO3)3·6H2O (purity ≥99.9%), Nd(NO3)3·6H2O (purity >99.5%), CsNO3 (purity 99.9%) and Sr(NO3)2 (purity >98.0%) were purchased from Wako Pure Chemical Industries, Ltd., and Eu(NO3)3·6H2O (99.95% purity) and Lu(NO3)3·4H2O (purity 99.95%) were obtained from Kanto Chemical Co., Inc. The nitrate solutions of Ln(III) ions including Cs+ and Sr2+ were prepared by diluting a stock solution with ultrapure water (Direct-Q3 system, Millipore Co.) to a suitable concentration, when the final concentration of nitric acid was adjusted to 1.0–3.0 mol L−1 by adding appropriate amounts of 60% nitric acid. The stock solution was prepared by dissolving appropriate amounts of La(NO3)3·6H2O, Nd(NO3)3·6H2O, Eu(NO3)3·6H2O, Lu(NO3)3·4H2O, CsNO3 and Sr(NO3)2 in diluted nitric acid solutions of known concentrations.Determination of the LCST of PNIPAAm in the solutions of nitric acid and hydrochloric acid
The light transmittance of PNIPAAm solutions at 500 nm was measured using a UV–Vis spectrophotometer (V-630, JASCO Co.) equipped with a Peltier thermostatic cell holder (control accuracy: ±0.1 °C). The aqueous solution of PNIPAAm was prepared by dissolving a given amount of PNIPAAm into each concentration of acid solution (HNO3: 1.0, 2.0 and 3.0 mol L−1, HCl: 1.0 and 3.0 mol L−1, polymer concentration: 25 mg/10 mL except for 28 mg/16 mL for 3.0 mol L−1 HNO3). The LCST in each solution was determined from the temperature-dependent plot of optical transmittance.Extraction and stripping procedure based on the phase transition of PNIPAAm
Extraction was conducted basically at 20 °C in a jacketed beaker connected to a temperature-controlled circulator. The nitrate solution of the mixed metal ions (15 mL) was loaded to the jacketed beaker containing PNIPAAm and CMPO, and vigorously stirred at 800 rpm with a magnetic stirrer. PNIPAAm was completely dissolved by stirring, while colorless granular CMPO was initially converted to colorless oily drops and then became difficult to recognize visually. After stirring for 1 h, the mixture solution was rapidly heated to 40 °C by switching the flow line to the circulator pre-controlled at the temperature. Within a few tens of seconds, the mixture solution became clouded due to the precipitation of PNIPAAm. The white turbidity of the solution changed gradually from a translucent to a transparent state under vigorous stirring at the temperature for 1 h, due to the complete aggregation of PNIPAAm. The extraction equilibrium was regarded to be reached at the time when the phase separation was achieved. The liquid phase was recovered, and a given portion of the recovered solution was diluted by using ultrapure water and nitric acid to a suitable concentration for measurement by inductively coupled plasma-mass spectrometry (ICP-MS, NexION 300X, PerkinElmer Co.). The extraction percentage, E(%), of metal ions was determined from mass-balance calculation before and after the extraction.The stripping of metal ions was carried out using the same procedure as the extraction process, except for the additional washing operations. The glutinous, gel-like aggregates of PNIPAAm left in the jacketed beaker at 40 °C were washed four times with nitric acid (1.0 mol L−1, 30 mL × 3 and 15 mL × 1) pre-heated at the temperature. After washing, the aggregates were re-dissolved in hydrochloric acid (1.0 mol L−1, 15 mL) under vigorous stirring at 20 °C. The stirring was continued for 1 h after the dissolution of the aggregates. The subsequent phase separation was carried out at 40 °C under vigorous stirring for 1 h. After the recovery of the liquid phase, the same stripping operation was repeated twice. The recovered solutions were also diluted using ultrapure water and nitric acid for ICP-MS analysis.
According to the above-mentioned procedure, the extraction was conducted basically by varying the metal concentrations (0.0378–1.21 mmol L−1 for each metal ion, solution volume: 15 mL) under constant amounts of PNIPAAm (30.8 mg, NIPAAm-monomer unit: 0.272 mmol) and CMPO (11.1 mg, 0.0272 mmol). Each batch experiment was carried out three times in total, and the average values of the metal amounts determined by ICP-MS analysis were used to plot the extraction and stripping percentages. The error bar in each plot represents the standard deviation of three batch experiments.
Results and discussion
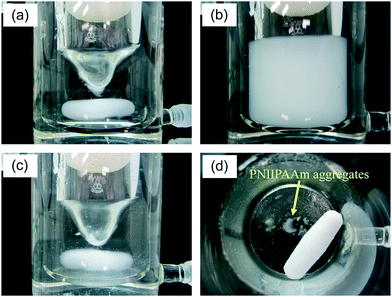
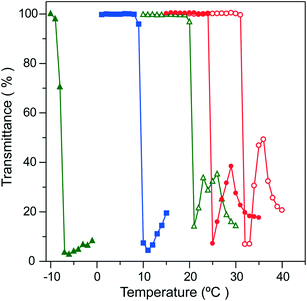
The extraction process based on the phase transition of PNIPAAm is shown in Fig. 2. The complexation process of Ln(III) ions with CMPO was carried out in the nitric acid solution containing a dissolved state of PNIPAAm below the LCST (Fig. 2(a)). The mixture solution became clouded immediately beyond the LCST, due to the particle formation and aggregation of PNIPAAm (Fig. 2(b)). The extraction process would be achieved in the course of particle formation by hydrophobic, co-aggregative binding of the Ln-CMPO complex onto the PNIPAAm solid phase. Furthermore, the continuous stirring of the mixture solution caused a gelation-like aggregation of PNIPAAm, which enabled the easy separation of the liquid and solid phases (Fig. 2(c) and (d)). Prior to the extraction experiments, it was confirmed that PNIPAAm provided almost no extraction of any of the metal ions considered in this study in the absence of CMPO (see Fig. S1 in the ESI†).As shown in Fig. 3, the extractability was dependent on the nitric acid concentration. The extraction was conducted at 20 °C for 1 h, followed by 40 °C for 1 h (open symbols in Fig. 3, [Ln] = [Cs] = [Sr] = 0.0755 mmol L−1). The higher extractability of La3+, Nd3+ and Eu3+ was observed at 1.0 mol L−1 nitric acid, while it declined at higher acid concentrations. The LCST of PNIPAAm at nitric acid concentrations of more than 2.0 mol L−1 was observed below 10 °C (Fig. 4). Therefore, the decrease in extractability is attributed mainly to the decrease in extraction efficiency of the Ln-CMPO complex, due to the insolubility of PNIPAAm at 20 °C. Actually, the extractability at 2.0 mol L−1 nitric acid was improved in the experiment conducted at 4 °C below the LCST (filled symbols in Fig. 3), although it stayed lower than that at 1.0 mol L−1 nitric acid. The abrupt decrease of LCST in nitric acid solutions indicates that nitrate anions would more remarkably reduce the hydrogen-bond energy around the amide groups of PNIPAAm, compared with chloride anions.30
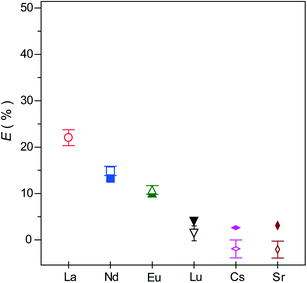
The effect of polymer amount on the extraction efficiency was examined in the presence of an excess amount of Ln ions against CMPO. The molar ratios of CMPO against Ln ions and total metals correspond to [CMPO]/[Ln]total = 0.375 and [CMPO]/[M]total = 0.250, respectively ([Ln] = [Cs] = [Sr] = 1.21 mmol L−1, [HNO3] = 1.0 mol L−1). As shown in Fig. 5, the electroneutral CMPO exhibited almost no extractability to the alkali and alkaline earth metal ions. In contrast, Ln(III) ions were extracted to PNIPAAm (30.8 mg) at the total extraction percentage of 12.3% (22.0, 14.9, 10.8, 1.4% for La3+, Nd3+, Eu3+ and Lu3+, respectively). The total extraction capacity calculated from the extraction percentage corresponds to the high value of 41.8 mg per g dry PNIPAAm (18.0, 12.6, 9.6 and 1.5 mg per g dry PNIPAAm for La3+, Nd3+, Eu3+ and Lu3+, respectively). The presence of excess Ln ions induced the selective coordination of CMPO, probably due to the competitive complexation among Ln ions with CMPO. The lighter La3+, Nd3+ and Eu3+ exhibited much higher extractability than the heavy Lu3+. This tendency is consistent with that of conventional SPE.31,32 In general, the extraction of Ln(III) ions increases for lighter Ln, and then, with a break at Gd(III), decreases with increasing charge density.33 Therefore, the observed tendency appears to suggest that the coordination of CMPO becomes harder as the hydration energy of Ln(III) ions increases with increasing charge density. In particular, the lower extractability for Lu3+ could result from its more stable hydration which hindered the coordination of CMPO. This appears to be supported by Fig. 3, where only the extractability for Lu3+ increased with increasing concentration of nitric acid. A higher concentration of nitric acid would be required for the disruption of the stable hydration shells of Lu3+ to facilitate the coordination of CMPO. As seen from Fig. 5, three times the amount of PNIPAAm exhibited no significant difference in extractability. This indicates that almost all the Ln-CMPO complex was extracted with a smaller amount of PNIPAAm. The calculated molar ratios of CMPO against the extracted Ln ions were [CMPO]/[Ln]ext = 3.06 and 3.04 for the small and large amounts of PNIPAAm, respectively. It has been reported that Ln(III) ions in nitrate solutions are extracted to organic phases in the form of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 complex with CMPO, as presented in eqn (i).31,32,34–36
3 complex with CMPO, as presented in eqn (i).31,32,34–36
| Ln3+ + 3CMPOorg + 3NO3− ⇄ Ln(NO3)3·(CMPOorg)3 | (i) |
Therefore, the obtained values of [CMPO]/[Ln]ext which are consistent with the stoichiometry of eqn (i) support that almost all CMPO formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 complex with Ln(III) ions, which was extracted to the PNIPAAm solid phase. Furthermore, the extractability was almost constant, independent of the stirring time (0.5–15 h) in the complexation process (see Fig. S2 in the ESI†). This suggests that the complexation reaction proceeds fast enough.
3 complex with Ln(III) ions, which was extracted to the PNIPAAm solid phase. Furthermore, the extractability was almost constant, independent of the stirring time (0.5–15 h) in the complexation process (see Fig. S2 in the ESI†). This suggests that the complexation reaction proceeds fast enough.
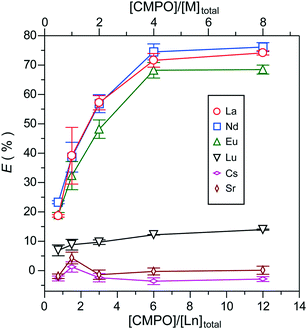
The variation of extractability with increasing total molar ratios of [CMPO]/[Ln]total in the lower concentration region of each Ln ion ([Ln]: 0.0378–0.604 mmol L−1) is shown in Fig. 6. The molar ratios were controlled by adjusting the total metal concentration ([M]total) to the constant amount of CMPO (0.0272 mmol, 11.1 mg) in order to ensure the quantitative sufficiency of PNIPAAm for the extraction of all the Ln-CMPO complex species. The increase in extractability with increasing [CMPO]/[Ln]total ratio is attributed to the enhancement of complex formation. However, the extractabilities of La3+, Nd3+ and Eu3+ were almost saturated at molar ratios of more than 6. The maximum extraction percentages were ca. 74% for La3+, 76% for Nd3+, 68% for Eu3+ and 14% for Lu3+. Furthermore, the extraction selectivity of Ln ions became ambiguous compared with the extraction conducted in a large excess molar amount of Ln ions (Fig. 5). The results in Fig. 5 and 6 suggest that the extractability is largely affected by the concentration range of Ln ions. In the present extraction method, the complex formation proceeds in a heterogeneous system due to the water insolubility of CMPO, where the reaction efficiency of complexation is a significant factor to determine the extractability. Therefore, increasing not only the high relative molar ratios but also the large absolute amounts of metal ions or ligands would be required to increase the efficiency of complex formation. In particular, in terms of increasing the extraction percentage of Ln ions, a large excess amount of ligands should be required to enhance the complexation efficiency.
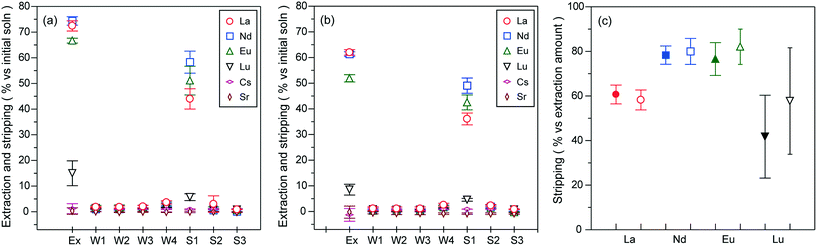
The stripping of Ln(III) ions was achieved by the complete re-dissolution of PNIPAAm co-aggregates (ion-adsorbing species) at 20 °C for 1 h in the presence of hydrochloric acid (1.0 mol L−1) and the subsequent phase separation between PNIPAAm co-aggregates (ion-desorbing species) and bulk solutions at 40 °C above the LCST. The extraction was carried out at [CMPO]/[Ln]total ratios of 3 and 6 ([HNO3] = 1.0 mol L−1). As seen from Fig. 7(a) ([CMPO]/[Ln]total = 6), no significant stripping was observed in the nitric acid solutions used for washing the co-aggregates after the extraction process. In contrast, hydrochloric acid achieved the stripping percentage of ca. 44% for La3+, 58% for Nd3+, 51% for Eu3+ and 5.7% for Lu3+ against the initial concentration of the test solution. The stripping for the extraction conducted at a [CMPO]/[Ln]total ratio of 3 also afforded Ln recoveries of ca. 36%, 49%, 43% and 4.6%, respectively, by using 1.0 mol L−1 hydrochloric acid (Fig. 7(b)). The stripping percentages converted against the extracted amounts are shown in Fig. 7(c). Except for Lu3+, the stripping effect of hydrochloric acid on each Ln ion was almost the same, independent of the extraction amounts, exhibiting the inverse tendency to the order of extraction and adsorption selectivity shown in Fig. 5. It has been reported that nitrate ions form an inner-sphere Ln-complex in moderate concentrations (2 mol L−1) of nitric acid solutions, while chloride ions coordinate to Ln(III) ions in the outer sphere even in concentrated hydrochloric acid (6 mol L−1).37 In hydrochloric acid solutions, therefore, the stability of the Ln-CMPO chloride complex would be lower than that of the nitrate complex for higher hydration energies of the Ln(III) ions (Eu3+ > Nd3+ > La3+). In addition, more hydrophilic chloride ions than nitrate ions might facilitate the exchange reaction between the complex and water. However, only Lu3+ with the highest hydration energy among the Ln ions considered deviated from the above tendency. This might be attributed to the small difference in the stability constants of nitrate and chloride complexes (although the stability constant of the Lu nitrate complex is larger than that of the chloride complex).38 The almost the same stripping efficiency observed for each Ln ion of different extraction amounts could result from the concentrations of the Ln ions stripped in hydrochloric acid solutions being not high enough to cause the quantitative difference in the formation of the Ln-CMPO chloride complex in the complexation equilibrium (note that the concentration region of the extracted Ln ions was almost comparable to that in the [CMPO]/[Ln]total ratio ranging from 6 to 12 in Fig. 6, where the extractability of each Ln ion has no large difference). From the above discussion, the stripping amount of Ln ions would reflect the difference in the stability and amounts between the nitrate complex formed in the extraction process and the chloride complex formed in the stripping process.
Conclusions
The solid phase extraction using PNIPAAm as the extraction medium was proved to be applicable to the extraction of Ln(III) ions in high concentrations of nitric acid by using CMPO as the extracting agent. The extraction is based on the hydrophobic binding of the Ln-CMPO nitrate complex onto PNIPAAm aggregates formed above the phase-transition temperature. In the present method, the efficiency and extent of complexation and the stability of the resulting complex species are significant factors in determining the extractability of Ln ions. The difference in the stability of the complex species has enabled the stripping of Ln ions in hydrochloric acid solutions. The present method provided a high maximum extraction capacity of 41.8 mg per g dry PNIPAAm for Ln ions under the quantitative condition that almost all CMPO molecules complex with Ln ions ([CMPO]/[Ln]total = 0.375). The highest extractability of the examined Ln ions remained 76% or less under the given amounts of PNIPAAm and CMPO. From the findings of this study, in principle, to enhance the extractability of metal ions in the present extraction method, the high stability of the complex is essential, and additionally, a large excess amount of ligands against metal ions should be used in respect that the complexation efficiency is compulsorily enhanced. The results of this study suggest the potential applicability of PNIPAAm as an extraction medium for metal ions in high concentrations of acid solutions. However, detailed investigations of the reaction in a heterogeneous system between insoluble hydrophobic ligands (as well as metal complexes) and bulk solutions should be indispensable for the expansion of PNIPAAm-based solid phase extraction to the level of practical use.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present work was partially supported by “the Funding Program for Next Generation World-Leading Researchers (NEXT program)” of the Cabinet Office and “the Initiatives for Atomic Energy Basic and Generic Strategic Research 260402” from MEXT (Ministry of Education, Culture, Sports, Science and Technology).Notes and references
- G. Modolo, A. Wilden, A. Geist, D. Magnusson and R. Malmbeck, Radiochim. Acta, 2012, 100, 715–725 CrossRef CAS.
- A. P. Paiva and P. Malik, J. Radioanal. Nucl. Chem., 2004, 261, 485–496 CrossRef CAS.
- C. H. Oh, Hazardous and radioactive waste treatment technologies handbook, CRC press, 2001, ch. 3.3, pp. 1–12 Search PubMed.
- H. Ikeda, M. Tokeshi and T. Kitamori, Radioisotopes, 2005, 54, 35–40 CrossRef CAS.
- D. R. Peterman, L. G. Olson, R. G. McDowell, G. Elias and J. D. Law, Idaho National Laboratory (INL) Technical Report, INL/EXT-12-25311, 2012 Search PubMed.
- Q. Sun, L. Jiang, L. Gong and J.-H. Sun, J. Hazard. Mater., 2016, 314, 230–236 CrossRef CAS PubMed.
- F. H. Quina and W. L. Hinze, Ind. Eng. Chem. Res., 1999, 38, 4150–4168 CrossRef CAS.
- E. K. Paleologos, D. L. Giokas and M. I. Karayannis, TrAC, Trends Anal. Chem., 2005, 24, 426–436 CrossRef CAS.
- N. R. Bader, K. Edbey and U. Telgheder, J. Chem. Pharm. Res., 2014, 6, 496–501 CAS.
- M. A. Bezerra, M. A. Z. Arruda and S. Luis, Appl. Spectrosc. Rev., 2005, 40, 269–299 CrossRef CAS.
- F. H. Quina, E. O. Alonso and J. P. S. Farah, J. Phys. Chem., 1995, 99, 11708–11714 CrossRef CAS.
- T. Saitoh, T. Ohyama, K. Takamura, T. Sakurai, T. Kaise and C. Matsubara, Anal. Sci., 1997, 13, 1–4 CrossRef CAS.
- K. Fujinaga, Y. Yamoto, Y. Seike and M. Okumura, Anal. Sci., 1997, 13, 141–144 CrossRef CAS.
- H. Tokuyama and T. Iwama, Sep. Purif. Technol., 2009, 68, 417–421 CrossRef CAS.
- S. Fujishige, K. Kubota and I. Ando, J. Phys. Chem., 1989, 93, 3311–3313 CrossRef CAS.
- X. Wang, X. Qiu and C. Wu, Macromolecules, 1998, 31, 2972–2976 CrossRef CAS.
- Z. Tong, F. Zeng, X. Zheng and T. Sato, Macromolecules, 1999, 32, 4488–4490 CrossRef CAS.
- K. Chayama, Y. Morita and S. Iwatsuki, J. Chromatogr. A, 2010, 1217, 6785–6790 CrossRef CAS PubMed.
- N. N. Reddy, S. Ravindra, N. M. Reddy, V. Rajinikanth, K. M. Raju and V. S. Vallabhapurapu, J. Magn. Magn. Mater., 2015, 394, 237–244 CrossRef CAS.
- D. Nagai and T. Daimon, Polym. Bull., 2016, 73, 1553–1564 CrossRef CAS.
- Y. S. Kim, H. M. Lee, J. H. Kim, J. Joo and I. W. Cheong, RSC Adv., 2015, 5, 10656–10661 RSC.
- J. Cheng, G. Shan and P. Pan, Ind. Eng. Chem. Res., 2017, 56, 1223–1232 CrossRef CAS.
- C.-H. Huang, T.-H. Hsieh and W.-Y. Chiu, Carbohydr. Polym., 2015, 116, 249–254 CrossRef CAS PubMed.
- H. Tokuyama and T. Iwama, Langmuir, 2007, 23, 13104–13108 CrossRef CAS PubMed.
- F. Yan, M. Wang, D. Cao, S. Guo and L. Chen, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2401–2408 CrossRef CAS.
- W. Watanabe, T. Maekawa, Y. Miyazaki, T. Kida, K. Takeshita and A. Mori, Chem. – Asian J., 2012, 7, 1679–1683 CrossRef CAS PubMed.
- V. P. Mahida and M. P. Patel, Desalin. Water Treat., 2016, 57, 13733–13746 CrossRef CAS.
- X.-J. Ju, S.-B. Zhang, M.-Y. Zhou, R. Xie, L. Yang and L.-Y. Chu, J. Hazard. Mater., 2009, 167, 114–118 CrossRef CAS PubMed.
- H. Tokuyama and G. Kato, Ind. Eng. Chem. Res., 2014, 53, 8215–8220 CrossRef CAS.
- K. C. Park, N. Idota and T. Tsukahara, React. Funct. Polym., 2014, 79, 36–46 CrossRef CAS.
- K. Nakashima, F. Kubota, T. Maruyama and M. Goto, Ind. Eng. Chem. Res., 2005, 44, 4368–4372 CrossRef CAS.
- Y. Koma, T. Koyama and Y. Tanaka, J. Nucl. Sci. Technol., 1999, 36, 934–939 CrossRef CAS.
- K. Kumar, C. A. Chang and M. F. Tweedle, Inorg. Chem., 1993, 32, 587–593 CrossRef CAS.
- K. Nakashima, F. Kubota, T. Maruyama and M. Goto, Anal. Sci., 2003, 19, 1097–1098 CrossRef CAS PubMed.
- Y. Koma, M. Watanabe, S. Nemoto and Y. Tanaka, J. Nucl. Sci. Technol., 1998, 35, 130–136 CrossRef CAS.
- A. Rout, K. A. Venkatesan, T. G. Srinivasan and P. R. Vasudeva Rao, Sep. Purif. Technol., 2011, 76, 238–243 CrossRef CAS.
- T. Yaita, H. Narita, Sh. Suzuki, Sh. Tachimori, H. Motohashi and H. Shiwaku, J. Radioanal. Nucl. Chem., 1999, 239, 371–375 CrossRef CAS.
- D. F. Peppard, G. W. Mason and I. Hucher, J. Inorg. Nucl. Chem., 1962, 24, 881–888 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7re00060j |
| This journal is © The Royal Society of Chemistry 2018 |