Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Efficient ammonia synthesis over a Ru/La0.5Ce0.5O1.75 catalyst pre-reduced at high temperature†
Yuta
Ogura
 a,
Katsutoshi
Sato
a,
Katsutoshi
Sato
 ab,
Shin-ichiro
Miyahara
a,
Yukiko
Kawano
a,
Takaaki
Toriyama
c,
Tomokazu
Yamamoto
d,
Syo
Matsumura
cd,
Saburo
Hosokawa
ab,
Shin-ichiro
Miyahara
a,
Yukiko
Kawano
a,
Takaaki
Toriyama
c,
Tomokazu
Yamamoto
d,
Syo
Matsumura
cd,
Saburo
Hosokawa
 b and
Katsutoshi
Nagaoka
b and
Katsutoshi
Nagaoka
 *a
*a
aDepartment of Integrated Science and Technology, Faculty of Science and Technology, Oita University, 700 Dannoharu, Oita 870-1192, Japan. E-mail: nagaoka@oita-u.ac.jp
bElements Strategy Initiative for Catalysts and Batteries, Kyoto University, 1-30 Goryo-Ohara, Nishikyo-ku, Kyoto 615-8245, Japan
cThe Ultramicroscopy Research Center, Kyushu University, Motooka 744, Nishi-ku, Fukuoka 819-0395, Japan
dDepartment of Applied Quantum Physics and Nuclear Engineering, Kyushu University, Motooka 744, Nishi-ku, Fukuoka 819-0395, Japan
First published on 15th January 2018
Abstract
Ammonia is an important feedstock for producing fertiliser and is also a potential energy carrier. However, the process currently used for ammonia synthesis, the Haber–Bosch process, consumes a huge amount of energy; therefore the development of new catalysts for synthesising ammonia at a high rate under mild conditions (low temperature and low pressure) is necessary. Here, we show that Ru/La0.5Ce0.5O1.75 pre-reduced at an unusually high temperature (650 °C) catalysed ammonia synthesis at extremely high rates under mild conditions; specifically, at a reaction temperature of 350 °C, the rates were 13.4, 31.3, and 44.4 mmol g−1 h−1 at 0.1, 1.0, and 3.0 MPa, respectively. Kinetic analysis revealed that this catalyst is free of hydrogen poisoning under the conditions tested. Electron energy loss spectroscopy combined with O2 absorption capacity measurements revealed that the reduced catalyst consisted of fine Ru particles (mean diameter < 2.0 nm) that were partially covered with partially reduced La0.5Ce0.5O1.75 and were dispersed on a thermostable support. Furthermore, Fourier transform infrared spectra measured after N2 addition to the catalyst revealed that N2 adsorption on Ru atoms that interacted directly with the reduced La0.5Ce0.5O1.75 weakened the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond and thus promoted its cleavage, which is the rate-determining step for ammonia synthesis. Our results indicate that high-temperature pre-reduction of this catalyst, which consists of Ru supported on a thermostable composite oxide with a cubic fluorite structure and containing reducible cerium, resulted in the formation of many sites that were highly active for N2 reduction by hydrogen.
N bond and thus promoted its cleavage, which is the rate-determining step for ammonia synthesis. Our results indicate that high-temperature pre-reduction of this catalyst, which consists of Ru supported on a thermostable composite oxide with a cubic fluorite structure and containing reducible cerium, resulted in the formation of many sites that were highly active for N2 reduction by hydrogen.
Introduction
Ammonia is an important chemical feedstock, and more than 80% of the ammonia that is synthesised is used to produce fertiliser.1 Ammonia also has potential utility as an energy carrier and a hydrogen source2–5 (1) because it has a high energy density (12.8 GJ m−3) and a high hydrogen content (17.6 wt%), (2) because infrastructure for ammonia storage and transportation already exists, and (3) because carbon dioxide is not emitted when ammonia is decomposed to produce hydrogen.2,4,6,7 If ammonia could be efficiently produced from a renewable energy source, such as solar or wind energy, problems related to the global energy crisis could be mitigated.Ammonia is usually synthesised by the energy-intensive Haber–Bosch process, which is performed at very high temperatures (>450 °C) and high pressures (>20 MPa) and which accounts for 1–2% of global energy consumption. Approximately 60% of the energy consumed by the process is recovered and stored as enthalpy in the ammonia molecule; but the remaining energy is lost, mostly during hydrogen production from natural gas, ammonia synthesis, and gas separation. The development of methods for reduction of the energy used by this process has been the goal of a considerable amount of research.8 One way to accomplish this would be to replace the iron-based catalysts used in the Haber–Bosch process with a catalyst that would permit the use of milder conditions (lower temperatures and pressures).9–12
Ammonia has been synthesised under ambient conditions with organometallic catalysts, but strong reducing agents and proton sources are generally needed, and the ammonia production rate is too low for practical applications.13–15 Supported ruthenium catalysts are good candidates for ammonia synthesis because they are more active at low temperature and pressure than iron-based catalysts are. The rate-determining step in ammonia synthesis is generally cleavage of the high-energy N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond of N2 (945 kJ mol−1).13,16 One effective way to accelerate this step is to modify the Ru electronic states.17,18 This can be accomplished by the use of basic catalyst supports and by the addition of a strongly basic promoter; these modifications have been shown to enhance ammonia synthesis activity17,18 by means of a mechanism that involves the transfer of electrons to the Ru metal from the basic components and subsequent transfer of electrons from Ru to the antibonding π-orbitals of N2, which weakens the N
N bond of N2 (945 kJ mol−1).13,16 One effective way to accelerate this step is to modify the Ru electronic states.17,18 This can be accomplished by the use of basic catalyst supports and by the addition of a strongly basic promoter; these modifications have been shown to enhance ammonia synthesis activity17,18 by means of a mechanism that involves the transfer of electrons to the Ru metal from the basic components and subsequent transfer of electrons from Ru to the antibonding π-orbitals of N2, which weakens the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond and promotes its cleavage.19 The most effective promoter has been reported to be Cs2O.19 The combination of Cs+, Ru, and MgO possesses high ammonia-synthesis activity19,20 and has been used as a benchmark in many studies.9,21 BaO is also an effective promoter, and the combination of Ba2+, Ru, and activated carbon has been used in industrial-scale commercial processes.22 Notably, Ru catalysts supported on non-oxides, such as Ru-loaded electride [Ca24Al28O64]4+(e−)4 (Ru/C12A7:e−) and Ru/Ca(NH2)2, also show high ammonia-synthesis activity.9,23,24 In fact, the ammonia-synthesis activity of Ru/Ca(NH2)2 is higher than the activities of any previously reported Ru catalysts, as well as the activities of 3d transition metal–LiH composites, which are a new class of non-Ru ammonia-synthesis catalysts.25 The high activities of catalysts supported on non-oxides have been attributed to the strong electron-donating ability of the supports. However, the practical utility of these catalysts might be limited by the sophisticated procedures required to prepare them and by their air and moisture sensitivity.
N bond and promotes its cleavage.19 The most effective promoter has been reported to be Cs2O.19 The combination of Cs+, Ru, and MgO possesses high ammonia-synthesis activity19,20 and has been used as a benchmark in many studies.9,21 BaO is also an effective promoter, and the combination of Ba2+, Ru, and activated carbon has been used in industrial-scale commercial processes.22 Notably, Ru catalysts supported on non-oxides, such as Ru-loaded electride [Ca24Al28O64]4+(e−)4 (Ru/C12A7:e−) and Ru/Ca(NH2)2, also show high ammonia-synthesis activity.9,23,24 In fact, the ammonia-synthesis activity of Ru/Ca(NH2)2 is higher than the activities of any previously reported Ru catalysts, as well as the activities of 3d transition metal–LiH composites, which are a new class of non-Ru ammonia-synthesis catalysts.25 The high activities of catalysts supported on non-oxides have been attributed to the strong electron-donating ability of the supports. However, the practical utility of these catalysts might be limited by the sophisticated procedures required to prepare them and by their air and moisture sensitivity.
In the 1990s, Aika et al. found that rare earth oxides, such as CeO2 and La2O3, are effective supports for Ru catalysts.26 In addition, we recently reported that a Ru catalyst supported on the rare earth oxide Pr2O3 exhibits high ammonia-synthesis activity.27 Aika et al. reported that the rate of ammonia synthesis over Ru/CeO2 is high when the catalyst has been pre-reduced at 500 °C.26 During pre-reduction, some of the Ce4+ is reduced to Ce3+, and thus an electron is transferred to Ru and then to adsorbed N2 molecules. However, the ammonia synthesis rate is slower over a catalyst that has been pre-reduced at a temperature higher than 500 °C, owing to structural changes associated with sintering of the support. To increase the specific surface area of the catalysts, as well as the reducibility of the Ce4+, various investigators have used composite-oxide supports, such as CeO2–La2O3,28 MgO–CeO2,29,30 BaO–CeO2,31 CeO2–ZrO2,32 and Sm2O3–CeO2,33 for Ru catalysts. However, the ammonia-synthesis rates achieved with these catalysts remain insufficient for practical use. As suggested by Aika et al., the pre-reduction temperature for these catalysts is kept below 500 °C to minimize aggregation of the Ru particles.26
Herein, we report the ammonia-synthesis activity of Ru/La0.5Ce0.5O1.75, a catalyst consisting of Ru supported on a La0.5Ce0.5O1.75 solid solution, which is a composite oxide of CeO2 and La2O3. After pre-reduction at the unusually high temperature of 650 °C, the catalyst exhibited high ammonia-synthesis activity at reaction temperatures from 300 to 400 °C; the activity was the highest among oxide supported Ru catalysts and comparable to that of the most active Ru catalysts reported to date. The thermostable oxide support, which had an average composition of La0.5Ce0.5O1.64 after pre-reduction at 650 °C, consisted of fine Ru particles strongly anchored to the reduced support and had numerous active Ru sites. The dependence of the catalyst structure and state on the reduction temperature was elucidated by means of various characterisation techniques, including energy electron loss spectroscopy (EELS) and scanning transmission electron microscopy (STEM). This catalyst has the advantages of being easy to prepare and stable in the atmosphere, which makes it easy to load into a reactor.
Results and discussion
Ammonia-synthesis activity of Ru/La0.5Ce0.5O1.75
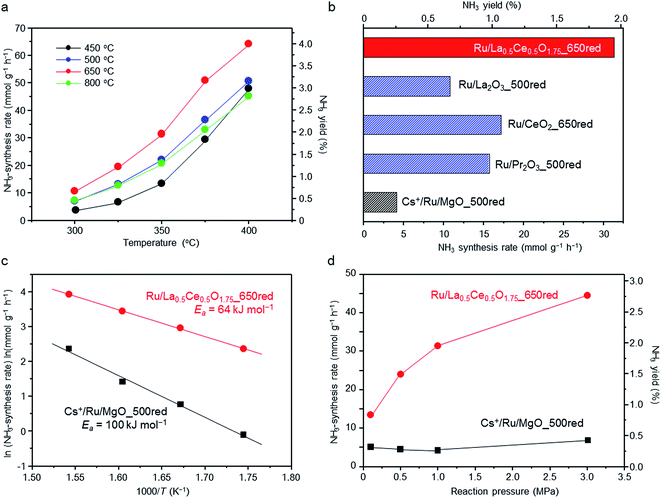
The reaction temperature dependence of the ammonia-synthesis rate over Ru/La0.5Ce0.5O1.75 was measured at 1.0 MPa after pre-reduction of the catalyst at 450, 500, 650, or 800 °C. Under the reaction conditions, the equilibrium ammonia-synthesis rate and the ammonia yield at 400 °C are 127 mmol g−1 h−1 and 7.91%, respectively. At all reaction temperatures, the ammonia-synthesis rate was markedly higher over the catalyst pre-reduced at 650 °C than over the catalysts pre-reduced at 450 °C (a temperature that was used in a previously reported study28) or 500 °C (Fig. 1a). However, increasing the pre-reduction temperature to 800 °C sharply decreased the rate. That is, the optimal pre-reduction temperature was 650 °C, which is considerably higher than the reaction temperatures usually used for Ru-catalysed ammonia synthesis (≤400 °C).We also compared the ammonia-synthesis rates over various other supported 5 wt% Ru catalysts at 350 °C and 1.0 MPa (Fig. 1b). Each of the catalysts had been pre-reduced at a temperature between 500 and 800 °C, and the ammonia-synthesis rates after reduction at the optimal pre-reduction temperature are displayed. The ammonia-synthesis rate over Ru/La0.5Ce0.5O1.75_650red (“650red” indicates that the catalyst had been reduced at 650 °C before the activity tests) reached 31.3 mmol g−1 h−1 and was much higher than the rates over the other tested catalysts, such as Ru/CeO2_650red (17.2 mmol g−1 h−1) and Ru/La2O3_500red (10.8 mmol g−1 h−1), whose supports each contain one of the rare earth elements in La0.5Ce0.5O1.75, and Ru/Pr2O3_500red (15.7 mmol g−1 h−1),27 which is one of the most active of the oxide-supported Ru catalysts. Furthermore, the ammonia-synthesis rate over Ru/La0.5Ce0.5O1.75_650red was approximately 7.6 times that over Cs+/Ru/MgO_500red (4.1 mmol g−1 h−1), a well-known catalyst that is often used as a benchmark and that is more active than Ba2+/Ru/activated carbon9,34 which is used commercially in ammonia-synthesis processes.22 Note also that the ammonia-synthesis rate over 5 wt% Ru/La0.5Ce0.5O1.75_650red was comparable to that over 10 wt% Ru/Ca(NH2)2 (31.7 mmol g−1 h−1, measured under similar reaction conditions [340 °C, 0.9 MPa]).24
We prepared Arrhenius plots for ammonia-synthesis reactions catalysed by Ru/La0.5Ce0.5O1.75_650red and Cs+/Ru/MgO_500red with the use of the rates at 300, 325, 350, and 375 °C (Fig. 1c). To avoid the contribution of the reverse reaction to the ammonia-synthesis rate, the rate at 400 °C was not used in the plots. The apparent activation energy (Ea) calculated for Ru/La0.5Ce0.5O1.75_650red (64 kJ mol−1) was much lower than that for Cs+/Ru/MgO_500red (100 kJ mol−1), and was comparable to that reported for 10 wt% Ru/Ca(NH2)2 (59 kJ mol−1).24 These results demonstrate that the low apparent activation energy for the reaction over Ru/La0.5Ce0.5O1.75_650red was responsible for the high ammonia-synthesis rate.
We also investigated the effect of reaction pressure on ammonia-synthesis rates at 350 °C (Fig. 1d). Increasing the reaction pressure from 0.1 to 1.0 MPa reportedly has no effect on the ammonia-synthesis rate over Cs+/Ru/MgO_500red.9,24 This result implies that hydrogen atoms strongly adsorbed on the Ru interfere with the activation of N2 molecules (a phenomenon referred to as hydrogen poisoning), which is a typical drawback of conventional Ru catalysts.35,36 In contrast, we observed that at 0.1 MPa, the ammonia-synthesis rate over Ru/La0.5Ce0.5O1.75_650red was 13.4 mmol g−1 h−1, which is the highest value reported for Ru catalysts to date; and the rate increased to 31.3 and 44.4 mmol g−1 h−1 when the pressure was increased to 1.0 and 3.0 MPa, respectively. Hence, we assumed that hydrogen poisoning did not occur over Ru/La0.5Ce0.5O1.75_650red at the tested temperature. To confirm this assumption, we performed kinetic analysis at 350 °C and 0.1 MPa. For that purpose, reaction orders for N2, H2, and NH3 were determined with the assumption of the rate expression (1) (reaction conditions and obtained results are shown in Table S1†).37,38
| r = kPN2nPH2hPNH3a | (1) |
As shown in Fig. S1,† H2 reaction orders for Cs+/Ru/MgO_500red and Ru/La0.5Ce0.5O1.75_650red were estimated to be −0.76 and 0.15, respectively. These results indicate that the surface of Cs+/Ru/MgO_500red is strongly poisoned by hydrogen. In contrast, Ru/La0.5Ce0.5O1.75_650red is not poisoned by hydrogen. These results are in good agreement with the observations shown in Fig. 1d. Furthermore, the N2 reaction order for Cs+/Ru/MgO_500red was 1.07, which is in accordance with earlier work.9,37,39 In contrast, it was 0.76 for Ru/La0.5Ce0.5O1.75_650red, indicating that N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond cleavage, which is the rate-determining step for ammonia synthesis, is relatively promoted over Ru/La0.5Ce0.5O1.75_650red. Moreover, stability of Ru/La0.5Ce0.5O1.75_650red at 350 °C under 3.0 MPa was examined. When an inline gas purifier was installed for cleaning the H2/N2 mixture (Fig. S2†), the ammonia-synthesis rate was stable for 50 h, indicating that Ru/La0.5Ce0.5O1.75 shows long-term stability.
N bond cleavage, which is the rate-determining step for ammonia synthesis, is relatively promoted over Ru/La0.5Ce0.5O1.75_650red. Moreover, stability of Ru/La0.5Ce0.5O1.75_650red at 350 °C under 3.0 MPa was examined. When an inline gas purifier was installed for cleaning the H2/N2 mixture (Fig. S2†), the ammonia-synthesis rate was stable for 50 h, indicating that Ru/La0.5Ce0.5O1.75 shows long-term stability.
Direct observation of Ru/La0.5Ce0.5O1.75_650red without exposure to air
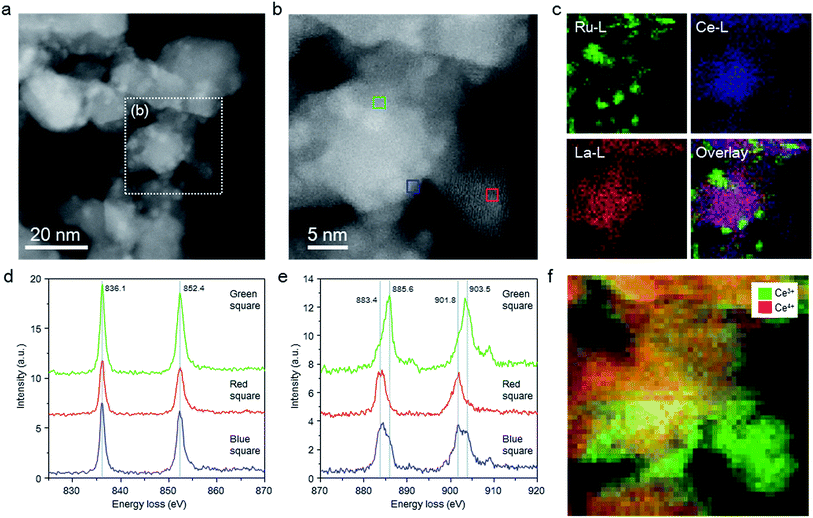
The structure of the Ru/La0.5Ce0.5O1.75_650red catalyst was investigated by means of aberration-corrected transmission electron microscopy (TEM), and the elemental distributions and valence states of the Ce ions were evaluated by means of STEM spectrum imaging of simultaneous energy dispersive X-ray (EDX) mapping and EELS. Because the elemental states and the structure of the catalyst might be changed by exposure to air, we carried out these analyses in the absence of air using a special holder with a gas cell to transfer the sample from an inert gas environment to the inside of the TEM column. Comparison of the high-angle annular dark-field (HAADF) STEM images (Fig. 2a and b) and EDX maps (Fig. 2c) of the catalyst indicated that Ce and La were homogeneously dispersed in the oxide support and that Ru particles were loaded on the support.Fig. 2d and e shows EEL spectra extracted from the spectrum imaging data for the centre region (Fig. 2b, green square) of a thick catalyst particle (information about both the surface and the bulk of the particle), the edge (blue square) of the same catalyst particle (information mainly about the particle surface), and the centre (red square) of a thin catalyst particle (information about the particle surface). In all of the EEL spectra, two La M4,5 peaks assignable to La3+ were observed, one at 836.1 and the other at 852.4 eV.40 In addition, all of the EEL spectra showed Ce M4,5 peaks ascribed to Ce3+ and Ce4+ at around 883.4 (as split peaks when the intensity was strong) and 901.8 eV and at 885.6 and 903.5 eV, respectively.40–42 Ce4+ predominated in the centre region (green square) of the thick catalyst particle, whereas Ce3+ predominated at the edge (blue square) of the thick catalyst particle, and the proportion of Ce3+ was highest at the centre (red square) of the thin catalyst particle. EELS maps of Ce in the thick and thin particles clearly showed the same tendency; that is, Ce3+ was enriched near the surface of the catalyst particles (Fig. 2f). These results indicate that a substantial proportion of the Ce4+ atoms located near the surface of the catalyst particles were reduced to Ce3+ at 650 °C.
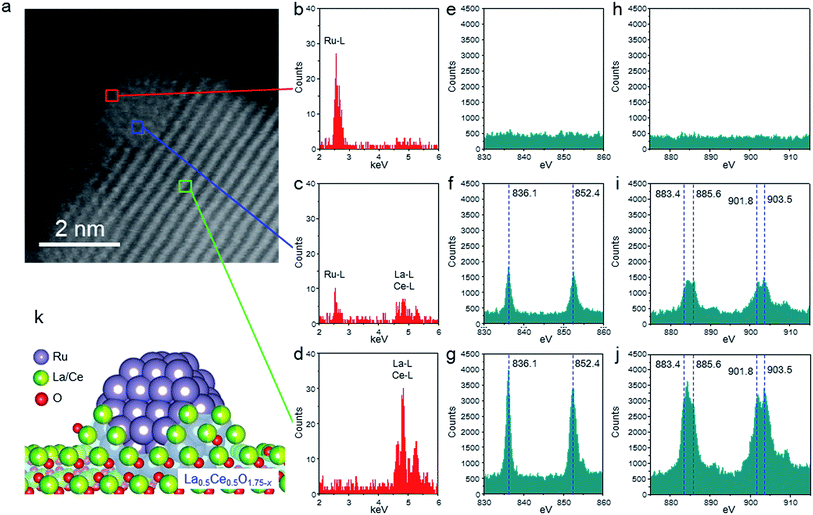
We used HAADF-STEM imaging and simultaneous EDX and EELS measurements at a higher magnification to study the interaction between the fine Ru particles and the support (Fig. 3). In the HAADF-STEM images shown in Fig. 3a (see Fig. S3† for additional images of the catalyst), we observed fine Ru particles (diameter ≈ 2 nm) dispersed on the composite-oxide support. Spot EDX and EEL spectra were measured for the detection of Ru and the rare earth elements (La and Ce) and the valence state of the rare earth elements, respectively. In the area indicated by the red square in Fig. 3a, the only observable peak, occurring in the EDX spectrum, was assignable to Ru (Fig. 3b, e and h). In contrast, the middle part of the Ru particle (blue square) showed a Ru peak in the EDX spectrum and peaks for La3+, Ce3+, and Ce4+ in both the EDX and the EEL spectra (Fig. 3c, f and i). The EDX and EEL spectra of the support material (green square) showed only peaks for the constituents of the support, that is, La3+, Ce3+, and Ce4+ (Fig. 3d, g and j). These results revealed that the Ru particles were partially covered by partially reduced support material; this result is consistent with a strong metal-support interaction (SMSI).26,43,44 In addition, these observations clearly indicate that fine Ru particles were anchored to the reduced La0.5Ce0.5O1.75 after pre-reduction at the unusually high temperature of 650 °C (Fig. 3k).
Explanation of the high ammonia-synthesis ability of Ru/La0.5Ce0.5O1.75_650red
We investigated the reason for the high ammonia-synthesis rate over Ru/La0.5Ce0.5O1.75_650red. At 1.0 MPa and 350 °C, the ammonia-synthesis rate over Ru/La0.5Ce0.5O1.75_500red was approximately 1.7 times the rate over Ru/CeO2_500red and approximately 2 times the rate over Ru/La2O3_500red (Fig. S4†). These results indicate that use of the La2O3–CeO2 composite support increased the ammonia-synthesis rate. In the X-ray diffraction (XRD) pattern of Ru/La2O3, many peaks assignable to LaOOH and La(OH)3 were observed, in addition to small peaks assigned to La2O3 (Fig. S5†), compounds that are produced by the adsorption of water vapour from the atmosphere onto La2O3. Note that adsorption of water decreases the basicity of the support and thus should be avoided. In contrast, the XRD pattern of Ru/La0.5Ce0.5O1.75 was consistent with a cubic fluorite structure like that of CeO2, although the peaks were shifted to much lower angles than for the corresponding peaks for Ru/CeO2. The XRD pattern contained no peaks assignable to impurities. A plot of the lattice constant as a function of La/(Ce + La) molar ratios for fresh Ru/LayCe1−yO2−0.5y (0 ≤ y ≤ 0.5) was linear (Fig. S6†), in accordance with Vegard’s law, which indicates that the Ru/La0.5Ce0.5O1.75 catalyst was a solid solution of La species homogeneously dissolved in a cubic fluorite structure. Note also that the peaks for Ru/La0.5Ce0.5O1.75 were broader and less sharp than those for Ru/CeO2. These results indicate that formation of the composite oxide interfered with water adsorption by La2O3 and with the crystal growth of the oxidic support. CeO2 reportedly tends not to form hydroxide or carbonate, owing to the symmetric octahedrally coordinated O2− ions surrounding the Ce4+ in the cubic fluorite structure, whereas La2O3 does tend to form hydroxide or carbonate, owing to the asymmetric heptahedral coordination.45 It is likely that the water adsorption observed over Ru/La2O3 was inhibited by incorporation of La3+ into the cubic fluorite structure. Furthermore, the specific surface area of Ru/La0.5Ce0.5O1.75_500red (Table 1) was much higher than the surface areas of Ru/CeO2_500red and Ru/La2O3_500red (Table S2†). The co-existence of Ce4+ and La3+ cations on the oxide surface probably prevented sintering of the oxide.46 Because of this enhancement of the stability of the material, the Ru particles that formed on La0.5Ce0.5O1.75 (mean diameter = 1.8 nm) after pre-reduction at 500 °C were finer than those that formed on La2O3 (mean diameter = 7.8 nm) and on CeO2 (mean diameter = 2.4 nm) (see Tables 1 and S2† and the TEM images in Fig. S8 and S9†). In addition, the H/Ru ratio, a measure of Ru dispersion, for Ru/La0.5Ce0.5O1.75_500red (Table 1) was 3.5 and 1.7 times the ratios for Ru/La2O3_500red and Ru/CeO2_500red, respectively (Table S2†). These results revealed that the use of the La2O3–CeO2 composite support increased the number of Ru active sites and thus increased the ammonia-synthesis rate over Ru/La0.5Ce0.5O1.75_500red relative to the rates over Ru/La2O3_500red and Ru/CeO2_500red.| Reduction temperature (°C) | Specific surface area (m2 g−1) | H/Rua (—) | Degree of Ce4+ reductionb (%) | Mean Ru particle sizec (nm) | TOFd (s−1) | NH3-synthesis rate at 350 °C and 1.0 MPa (mmol g−1 h−1) |
|---|---|---|---|---|---|---|
| a Estimated from the H2 chemisorption capacity. b Calculated from the O2 absorption capacity shown in Fig. S7 for the reduced catalysts. c Estimated from the STEM images in Fig. S8. d TOF, turnover frequency. Calculated from the H/Ru value and the ammonia-synthesis rate. | ||||||
| 500 | 47 | 0.46 | 23 | 1.8 | 0.027 | 22.1 |
| 650 | 42 | 0.35 | 43 | 1.7 | 0.051 | 31.3 |
| 800 | 21 | 0.11 | 63 | 2.7 | 0.108 | 20.6 |
We also investigated the influence of the catalyst pre-reduction temperature on the ammonia-synthesis rate and on the properties of Ru/La0.5Ce0.5O1.75 (Fig. 1a and Table 1). Increasing the pre-reduction temperature from 500 to 650 °C had little effect on the mean Ru particle diameter (see Fig. S8† for TEM and EDX mapping images of Ru/La0.5Ce0.5O1.75 after pre-reduction at the various temperatures; note that although the TEM image of Ru/La0.5Ce0.5O1.75_650red in Fig. S8† was obtained after exposure to air, the mean Ru particle diameter was similar to that measured in the absence of air [Fig. 2]). However, increasing the reduction temperature from 650 to 800 °C increased the mean diameter of the Ru particles to 2.7 nm (owing to sintering of the La0.5Ce0.5O1.75 support) and decreased the specific surface area of the catalyst from 42 to 21 m2 g−1. On the other hand, the H/Ru ratio decreased gradually as the pre-reduction temperature was increased from 500 to 800 °C. Note that when the reduction temperature was increased from 500 to 650 °C, the H/Ru ratio decreased from 0.46 to 0.35, but the mean diameter of the Ru particles remained unchanged. These results indicate that the surface Ru atoms were partially covered with partially reduced support material, at least after reduction at 650 °C, owing to the SMSI phenomenon, which is consistent with the EDX and EEL spectra (Fig. 3). The driving force for the SMSI is considered to be reduction of a support, such as TiO2−x and CeO2−x, bearing a coordinately unsaturated metal cation.26,43,44 We estimated the degree of Ce4+ reduction to Ce3+ by measuring the O2 absorption capacity of the reduced Ru/La0.5Ce0.5O1.75; the degrees of reduction were determined to be 23% and 43% after pre-reduction at 500 and 650 °C, respectively, revealing that SMSI occurred, especially at the higher temperature. The degree of Ce4+ reduction for Ru/La0.5Ce0.5O1.75_650red indicates that the average composition of the reduced support was Ce0.5La0.5O1.64. We also observed that the lattice was expanded by pre-reduction, owing both to the formation of Ce3+, which has a larger ionic radius than Ce4+ (1.14 Å versus 0.97 Å in eight coordination), and to the formation of oxygen vacancies. Specifically, the lattice parameter of the cubic fluorite structure of La0.5Ce0.5O1.75, as measured by in situ XRD analysis, increased from 0.5577 nm at room temperature to 0.5596 and 0.5603 nm after treatment with H2 at 500 and 650 °C, respectively (the XRD patterns are compared in Fig. S10†). Note that we confirmed that the lattice expansion that occurred upon treatment with H2 was larger than the thermal expansion observed upon simple heat treatment in air (Fig. S10†). Furthermore, both the SMSI effect and sintering of the Ru particles were greater after reduction at 800 °C than after reduction at the lower temperatures, which we attributed to the drastic decrease in the H/Ru ratio (to 0.11) and to the increase both in the degree of Ce4+ reduction (to 63%) and in the mean diameter of the Ru particles (to 2.7 nm) (Table 1).
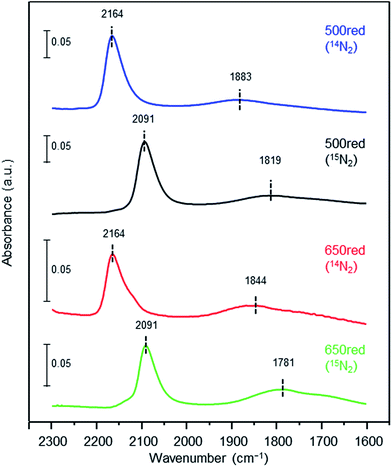
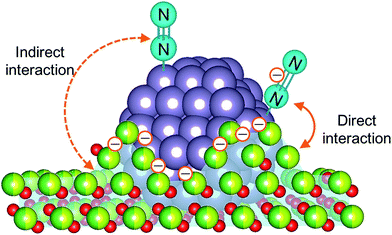
To elucidate the influence of the pre-reduction temperature on N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond cleavage, which is the rate-determining step for ammonia synthesis over Ru/La0.5Ce0.5O1.75, we determined the state of the adsorbed N2 molecules by means of Fourier transform infrared (IR) spectroscopy. The IR spectra measured after addition of 14N2 or 15N2 to Ru/La0.5Ce0.5O1.75_500red and Ru/La0.5Ce0.5O1.75_650red at room temperature are shown in Fig. 4 (the highest temperature at which our IR cell could be used was 650 °C). Both spectra show a peak at 2164 cm−1 and a broader peak at around 1700–1900 cm−1. Note that the wavenumber of the broader peak decreased from 1883 to 1844 cm−1 when the pre-reduction temperature was increased from 500 to 650 °C. In the spectra measured after 15N2 adsorption, the two peaks were observed at lower wavenumbers (2091 and 1819 cm−1) relative to those for the 14N2 spectra, and the wavenumbers were in good agreement with those estimated by consideration of the isotope effect:20,47 2164 cm−1 × (14/15)1/2 = 2091 cm−1 and 1883 cm−1 × (14/15)1/2 = 1819 cm−1. Similar peak shifts ascribable to the isotope effect were observed in the spectrum after adsorption of 15N2 on Ru/La0.5Ce0.5O1.75_650red. Therefore, all of the peaks were assignable to the stretching vibration mode of N2 adsorbed in an end-on orientation on the Ru particles. The peak at 2164 cm−1, the location of which was independent of reduction temperature, was assigned to N2 adsorbed on Ru atoms that interacted only weakly with the reduced support (Fig. 5, indirect interaction). The broader peaks at around 1700–1900 cm−1 were assigned to N2 adsorbed on Ru atoms that interacted directly with the reduced support formed by SMSI (Fig. 5, direct interaction). The peak broadening may reflect the heterogeneous character of the metal-support boundary.
N bond cleavage, which is the rate-determining step for ammonia synthesis over Ru/La0.5Ce0.5O1.75, we determined the state of the adsorbed N2 molecules by means of Fourier transform infrared (IR) spectroscopy. The IR spectra measured after addition of 14N2 or 15N2 to Ru/La0.5Ce0.5O1.75_500red and Ru/La0.5Ce0.5O1.75_650red at room temperature are shown in Fig. 4 (the highest temperature at which our IR cell could be used was 650 °C). Both spectra show a peak at 2164 cm−1 and a broader peak at around 1700–1900 cm−1. Note that the wavenumber of the broader peak decreased from 1883 to 1844 cm−1 when the pre-reduction temperature was increased from 500 to 650 °C. In the spectra measured after 15N2 adsorption, the two peaks were observed at lower wavenumbers (2091 and 1819 cm−1) relative to those for the 14N2 spectra, and the wavenumbers were in good agreement with those estimated by consideration of the isotope effect:20,47 2164 cm−1 × (14/15)1/2 = 2091 cm−1 and 1883 cm−1 × (14/15)1/2 = 1819 cm−1. Similar peak shifts ascribable to the isotope effect were observed in the spectrum after adsorption of 15N2 on Ru/La0.5Ce0.5O1.75_650red. Therefore, all of the peaks were assignable to the stretching vibration mode of N2 adsorbed in an end-on orientation on the Ru particles. The peak at 2164 cm−1, the location of which was independent of reduction temperature, was assigned to N2 adsorbed on Ru atoms that interacted only weakly with the reduced support (Fig. 5, indirect interaction). The broader peaks at around 1700–1900 cm−1 were assigned to N2 adsorbed on Ru atoms that interacted directly with the reduced support formed by SMSI (Fig. 5, direct interaction). The peak broadening may reflect the heterogeneous character of the metal-support boundary.
Our results indicate that the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond of N2 was weakened by the contribution of SMSI even after reduction at 500 °C, and when the reduction temperature was increased to 650 °C, the contribution of SMSI was greater. That is, the partially reduced support, which is enriched in electrons owing to the reduction of Ce4+ to Ce3+ and to the formation of oxygen vacancies, partially covered the Ru particles. As a result, electron transfer from the reduced support to the Ru metal was greatly enhanced, and the electrons were transferred to the antibonding π-orbitals of N2; thus, the N
N bond of N2 was weakened by the contribution of SMSI even after reduction at 500 °C, and when the reduction temperature was increased to 650 °C, the contribution of SMSI was greater. That is, the partially reduced support, which is enriched in electrons owing to the reduction of Ce4+ to Ce3+ and to the formation of oxygen vacancies, partially covered the Ru particles. As a result, electron transfer from the reduced support to the Ru metal was greatly enhanced, and the electrons were transferred to the antibonding π-orbitals of N2; thus, the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bonds of N2 adsorbed on Ru atoms that interacted directly with the reduced support were further weakened. The ratio of the peak area of the higher-wavenumber peak to that of the lower-wavenumber peak decreased when the pre-reduction temperature was increased from 500 to 650 °C, which is consistent with an increase in the contribution of the SMSI.
N bonds of N2 adsorbed on Ru atoms that interacted directly with the reduced support were further weakened. The ratio of the peak area of the higher-wavenumber peak to that of the lower-wavenumber peak decreased when the pre-reduction temperature was increased from 500 to 650 °C, which is consistent with an increase in the contribution of the SMSI.
These results demonstrate that pre-reduction at high temperature induced SMSI and enhanced the turnover frequency (TOF) but decreased the number of Ru active sites because the Ru particles became partially covered by partially reduced support. The fact that active Ru sites (TOF = 0.051 s−1) were abundant (H/Ru = 0.35) after pre-reduction at 650 °C explains the high ammonia-synthesis rate (31.3 mmol g−1 h−1) over Ru/La0.5Ce0.5O1.75_650red. In contrast, after pre-reduction at 800 °C, the Ru sites were very active (TOF = 0.108 s−1), but the number of active Ru sites was small (H/Ru = 0.11); thus the ammonia-synthesis rate over Ru/La0.5Ce0.5O1.75_800red (20.6 mmol g−1 h−1) was lower than that over Ru/La0.5Ce0.5O1.75_650red. Note that the specific surface area of Ru/CeO2_650red was only 20 m2 g−1, the mean diameter of the Ru particles was 3.1 nm, and H/Ru was 0.17, which indicates that sintering of the Ru particles and La0.5Ce0.5O1.75 was retarded in the case of Ru/La0.5Ce0.5O1.75_650red, and thus the H/Ru ratio for this catalyst remained high.
Conclusions
Pre-reduction of conventional supported-metal catalysts is crucial for their activation, because active metal sites are formed on the surface by reduction of metal oxides, and because adsorbates (such as H2O and CO2) on the surface of the fresh catalyst are removed. However, pre-reduction at an excessively high temperature results in sintering, which decreases the number of active sites. Here, we found that 400–450 °C was usually sufficient to reduce Ru3+. However, pre-reduction of Ru/La0.5Ce0.5O1.75 at the unusually high temperature of 650 °C produced a catalyst that showed a high ammonia-synthesis rate under mild reaction conditions (300–400 °C, 0.1–3.0 MPa). This catalyst consisted of fine Ru particles anchored on a heat-tolerant complex-oxidic support. During pre-reduction, the particle size of the Ru particles remained unchanged, but the particles became partially covered with partially reduced La0.5Ce0.5O1.75. A strong interaction between the Ru active sites and the reduced support accelerated the rate-determining step of ammonia synthesis, that is, N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond cleavage. We suggest that this simple strategy for the design of Ru catalysts—that is, using a thermostable composite oxide containing a redox-active rare earth element in a cubic fluorite structure as a support, and pre-reducing the supported catalyst at high temperature—will lead to the development of a more energy efficient ammonia-synthesis process, thus reducing global energy consumption and facilitating the eventual use of ammonia as an energy carrier.
N bond cleavage. We suggest that this simple strategy for the design of Ru catalysts—that is, using a thermostable composite oxide containing a redox-active rare earth element in a cubic fluorite structure as a support, and pre-reducing the supported catalyst at high temperature—will lead to the development of a more energy efficient ammonia-synthesis process, thus reducing global energy consumption and facilitating the eventual use of ammonia as an energy carrier.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by a grant from the CREST, JST program (no. JPMJCR1341). STEM observations were performed as part of a program conducted by the Advanced Characterization Nanotechnology Platform Japan, sponsored by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. K. Sato and S. Hosokawa thank the Program for Elements Strategy Initiative for Catalysts & Batteries (ESICB) commissioned by MEXT. The authors thank Mr Y. Wada (Oita University) for assistance with characterisation techniques.References
- J. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont and W. Winiwarter, Nat. Geosci., 2008, 1, 636–639 CrossRef CAS.
- A. Klerke, C. H. Christensen, J. K. Nørskov and T. Vegge, J. Mater. Chem., 2008, 18, 2304–2310 RSC.
- R. Schlogl, ChemSusChem, 2010, 3, 209–222 CrossRef PubMed.
- F. Schüth, R. Palkovits, R. Schlögl and D. S. Su, Energy Environ. Sci., 2012, 5, 6278–6289 Search PubMed.
- J. W. Makepeace, T. J. Wood, H. M. A. Hunter, M. O. Jones and W. I. F. David, Chem. Sci., 2015, 6, 3805–3815 RSC.
- K. Eguchi, in Energy Technology Roadmaps of Japan, ed. Y. Kato, M. Koyama, Y. Fukushima and T. Nakagaki, Springer, Japan, 2016 Search PubMed.
- K. Nagaoka, T. Eboshi, Y. Takeishi, R. Tasaki, K. Honda, K. Imamura and K. Sato, Sci. Adv., 2017, 3, e1602747 CrossRef PubMed.
- C. W. Hooper, Catalytic Ammonia Synthesis, Fundamentals and Practice, Springer, US, Boston, MA, 1991 Search PubMed.
- M. Kitano, Y. Inoue, Y. Yamazaki, F. Hayashi, S. Kanbara, S. Matsuishi, T. Yokoyama, S. W. Kim, M. Hara and H. Hosono, Nat. Chem., 2012, 4, 934–940 CrossRef CAS PubMed.
- F. Hayashi, M. Kitano, T. Yokoyama, M. Hara and H. Hosono, ChemCatChem, 2014, 6, 1317–1323 CAS.
- Y. Inoue, M. Kitano, S.-W. Kim, T. Yokoyama, M. Hara and H. Hosono, ACS Catal., 2014, 4, 674–680 CrossRef CAS.
- M. Hara, M. Kitano and H. Hosono, ACS Catal., 2017, 7, 2313–2324 CrossRef CAS.
- J. A. Pool, E. Lobkovsky and P. J. Chirik, Nature, 2004, 427, 527–530 CrossRef CAS PubMed.
- K. Arashiba, Y. Miyake and Y. Nishibayashi, Nat. Chem., 2011, 3, 120–125 CrossRef CAS PubMed.
- A. Eizawa, K. Arashiba, H. Tanaka, S. Kuriyama, Y. Matsuo, K. Nakajima, K. Yoshizawa and Y. Nishibayashi, Nat. Commun., 2017, 8, 14874 CrossRef CAS PubMed.
- S. Gambarotta and J. Scott, Angew. Chem., Int. Ed., 2004, 43, 5298–5308 CrossRef CAS PubMed.
- K. Aika, H. Hori and A. Ozaki, J. Catal., 1972, 27, 424–431 CrossRef CAS.
- K. Aika, M. Kumasaka, T. Oma, O. Kato, H. Matsuda, N. Watanabe, K. Yamazaki, A. Ozaki and T. Onishi, Appl. Catal., 1986, 28, 57–68 CrossRef CAS.
- K. Aika, A. Ohya, A. Ozaki, Y. Inoue and I. Yasumori, J. Catal., 1985, 92, 305–311 CrossRef CAS.
- J. Kubota and K.-i. Aika, J. Phys. Chem., 1994, 98, 11293–11300 CrossRef CAS.
- K. Aika, Catal. Today, 2017, 286, 14–20 CrossRef CAS.
- D. E. Brown, T. Edmonds, R. W. Joyner, J. J. McCarroll and S. R. Tennison, Catal. Lett., 2014, 144, 545–552 CrossRef CAS.
- M. Kitano, Y. Inoue, H. Ishikawa, K. Yamagata, T. Nakao, T. Tada, S. Matsuishi, T. Yokoyama, M. Hara and H. Hosono, Chem. Sci., 2016, 7, 4036–4043 RSC.
- Y. Inoue, M. Kitano, K. Kishida, H. Abe, Y. Niwa, M. Sasase, Y. Fujita, H. Ishikawa, T. Yokoyama, M. Hara and H. Hosono, ACS Catal., 2016, 6, 7577–7584 CrossRef CAS.
- P. Wang, F. Chang, W. Gao, J. Guo, G. Wu, T. He and P. Chen, Nat. Chem., 2017, 9, 64–70 CAS.
- Y. Niwa and K. Aika, Chem. Lett., 1996, 3, 3–4 CrossRef.
- K. Sato, K. Imamura, Y. Kawano, S.-i. Miyahara, T. Yamamoto, S. Matsumura and K. Nagaoka, Chem. Sci., 2017, 8, 674–679 RSC.
- X. Luo, R. Wang, J. Ni, J. Lin, B. Lin, X. Xu and K. Wei, Catal. Lett., 2009, 133, 382–387 CrossRef CAS.
- M. Saito, M. Itoh, J. Iwamoto, C. Li and K. Machida, Catal. Lett., 2006, 106, 107–110 CrossRef CAS.
- X. Wang, J. Ni, B. Lin, R. Wang, J. Lin and K. Wei, Catal. Commun., 2010, 12, 251–254 CrossRef CAS.
- X. Yang, W. Zhang, C. Xia, X.-M. Xiong, X.-Y. Mu and B. Hu, Catal. Commun., 2010, 11, 867–870 CrossRef CAS.
- Z. Ma, X. Xiong, C. Song, B. Hu and W. Zhang, RSC Adv., 2016, 6, 51106–51110 RSC.
- L. Zhang, J. Lin, J. Ni, R. Wang and K. Wei, Catal. Commun., 2011, 15, 23–26 CrossRef CAS.
- K. Aika, T. Takano and S. Murata, J. Catal., 1992, 136, 126–140 CrossRef CAS.
- Y. Kadowaki and K. Aika, J. Catal., 1996, 161, 178–185 CrossRef CAS.
- S. Siporin, J. Catal., 2004, 225, 359–368 CrossRef CAS.
- K. Aika, J. Kubota, Y. Kadowaki, Y. Niwa and Y. Izumi, Appl. Surf. Sci., 1997, 121–122, 488–491 CrossRef.
- R. Kojima and K. Aika, Appl. Catal., A, 2001, 218, 121–128 CrossRef CAS.
- F. Rosowski, A. Hornung, O. Hinrichsen, D. Herein, M. Muhler and G. Ertl, Appl. Catal., A, 1997, 151, 443–460 CrossRef CAS.
- C. C. Ahn and O. L. Krivanek, EELS atlas: a reference collection of electron energy loss spectra covering all stable elements, Gatan, Inc., 1983 Search PubMed.
- S. Turner, S. Lazar, B. Freitag, R. Egoavil, J. Verbeeck, S. Put, Y. Strauven and G. Van Tendeloo, Nanoscale, 2011, 3, 3385–3390 RSC.
- S. M. Collins, S. Fernandez-Garcia, J. J. Calvino and P. A. Midgley, Sci. Rep., 2017, 7, 5406 CrossRef PubMed.
- S. J. Tauster, Acc. Chem. Res., 1987, 20, 389–394 CrossRef CAS.
- A. Lewera, L. Timperman, A. Roguska and N. Alonso-Vante, J. Phys. Chem. C, 2011, 115, 20153–20159 CAS.
- S. Bernal, F. J. Botana, R. García and J. M. Rodríguez-Izquierdo, React. Solids, 1987, 4, 23–40 CrossRef CAS.
- J. Kašpar, P. Fornasiero and M. Graziani, Catal. Today, 1999, 50, 285–298 CrossRef.
- J. Kubota and K. Aika, J. Chem. Soc., Chem. Commun., 1991, 1544 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed procedures for each method, catalytic performance, STEM-EDX images, and detailed characterizations. See DOI: 10.1039/c7sc05343f |
| This journal is © The Royal Society of Chemistry 2018 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: