Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Preparative microdroplet synthesis of carboxylic acids from aerobic oxidation of aldehydes†
Xin
Yan
 ,
Yin-Hung
Lai
,
Yin-Hung
Lai
 and
Richard N.
Zare
and
Richard N.
Zare
 *
*
Department of Chemistry, Stanford University, Stanford, CA 94305-5080, USA. E-mail: rnz@stanford.edu
First published on 16th May 2018
Abstract
Single liquid-phase and liquid–liquid phase reactions in microdroplets have shown much faster kinetics than that in the bulk phase. This work extends the scope of microdroplet reactions to gas–liquid reactions and achieves preparative synthesis. We report highly efficient aerobic oxidation of aldehydes to carboxylic acids in microdroplets. Molecular oxygen plays two roles: (1) as the sheath gas to shear the aldehyde solution into microdroplets, and (2) as the sole oxidant. The dramatic increase of the surface-area-to-volume ratio of microdroplets compared to bulk solution, and the efficient mixing of gas and liquid phases using spray nozzles allow effective mass transfer between aldehydes and molecular oxygen. The addition of catalytic nickel(II) acetate is shown to accelerate further microdroplet reactions of this kind. We show that aliphatic, aromatic, and heterocyclic aldehydes can be oxidized to the corresponding carboxylic acids in a mixture of water and ethanol using the nickel(II) acetate catalyst, in moderate to excellent yields (62–91%). The microdroplet synthesis is scaled up to make it preparative. For example, aerobic oxidation of 4-tert-butylbenzaldehyde to 4-tert-butylbenzoic acid was achieved at a rate of 10.5 mg min−1 with an isolated product yield of 66%.
Introduction
Recent findings indicate that reactions in microdroplets created by spray-based ionization/aerosols are extremely attractive, as microdroplet reactions can be many orders of magnitude faster than their conventional bulk-phase counterparts.1,2 This phenomenon stimulates strong interest in using microdroplets as a chemical synthetic tool. Remarkable acceleration has been observed in single liquid-phase reactions and liquid–liquid phase reactions. For example, an accelerated Pomeranz–Fritsch synthesis of isoquinoline in methanolic solution was detected by on-line mass spectrometry (MS) in charged droplets generated by electrospray.3 The rate of this droplet reaction was reported to be 106 times faster than that in the bulk. Liquid–liquid bulk-phase Stevens oxidation of alcohols to the corresponding aldehydes/ketones is inhibited if no phase-transfer-catalyst is used, because of the inability of reagents to come together. In sharp contrast, non-catalytic oxidation can be achieved in microdroplets formed by dual sonic sprays within milliseconds in moderate to good yields.4 Other reactions involving C–C,5–7 C–N,6,8–10 and C–O11 bond formation have been reported to be accelerated by factors of 10 to 106 in single liquid-phase solutions.Gas–liquid reactions are of great chemical, biological, physiological, and ecological importance.12,13 An important question is whether gas–liquid reactions can be accelerated in microdroplets generated by spray-based ionization methods. Such methods of forming microdroplets often apply a sheath gas (commonly nitrogen gas) to pneumatically assist the formation of the sprayed droplets. An extra advantage of replacing sheath gas with reagent gas will be gained by its dual role as an assistant in droplet formation and as a reagent.
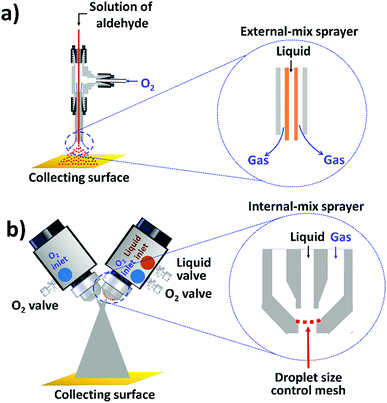
The oxidation of aldehydes to carboxylic acids has been of long-standing interest in synthetic organic chemistry,14 and is an industrially important process.15 Compared to different oxidizing reagents used in conventional methods such as Cr(IV)-based Jones oxidation,16,17 Ag(I)-based Tollen's reaction,18 and Cu(II)-based Fehling's reaction,19 molecular oxygen is considered as an ideal oxidant because it is inexpensive, environmentally friendly,20 and exhibits highly atom-efficient oxidation per weight (100% atom efficiency).21 Methods to achieve direct and efficient oxidation of aldehydes to carboxylic acids using molecular oxygen as the oxidant under mild conditions are relatively scarce and highly needed,22 although recently, progress has been made in the development of less expensive transition-metal catalysts for oxidation of aldehydes to carboxylic acids in the bulk.22–24 In this work, we report a highly efficient aerobic oxidation of aldehydes to carboxylic acids in microdroplets generated by sonic spray ionization (Fig. 1a). Molecular oxygen plays dual roles of being the oxidant as well as the sheath gas to generate microdroplets. Mixing of two phases occurs during microdroplet formation. The effect of the surface-area-to-volume ratio (SA/V ratio) of microdroplets on the yield of the reactions is also studied.
Another question well worth investigation is the scale of microdroplet reactions, as it determines the practicality for chemical synthesis. Previous studies on “preparative electrospray” employed four or eight spray sources at the same time, and products were generated at rates of ca. 1.2–1.6 milligrams per minute for Claisen–Schmidt condensations, benzoin condensations, and Stevens oxidations.4,5 Further scale-up of microdroplet reactions by paralleling more spray sources might not be practical and economical owing to complicated arrangements of splitting gas and liquid, as well as the large demand for duplicated spray sources. Here, we developed a device applying a high flux of liquid droplets colliding with gas molecules while maintaining suitable microdroplet sizes for fast and large-scale microdroplet synthesis (Fig. 1b).
Results and discussion
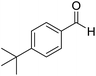
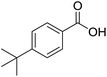
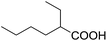
We began our investigation by examining (Scheme 1) the oxidation of 4-tert-butylbenzaldehyde 1 in a water–ethanol solvent (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
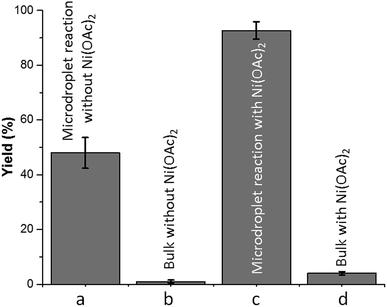
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2) with molecular oxygen (O2) and without any metal catalyst to form 4-tert-butylbenzoic acid 2 at room temperature. A water–ethanol solution of 1 (0.1 M) introduced through a fused silica capillary (i.d. 50 μm) at a rate of 15 μL min−1 was atomized into microdroplets (average size ca. 3.1 μm; see the ESI† for the method of droplet measurement) with a coaxial flow of oxygen, which was used as the turbulent nebulizing gas operated at 120 psi as well as the sole oxidant. The oxidation of 1 was initiated by the interactions between 1 in microdroplets with molecular oxygen at the interface. The resulting products 2 were collected for 30 min using a microdroplet trapping system (Fig. S1†). The reaction mixture was extracted with dichloromethane and analysed by 1H NMR. The yield of 2 from the oxidation of 1 was found to be 48% (Fig. 2a). A control experiment was performed in bulk solution (O2 was supplied in a balloon). The reaction mixture was vigorously stirred at room temperature, and less than 1% of the product was detected after 30 min (Fig. 2b). An acceleration factor of 50 was achieved for the reaction in microdroplets compared to the bulk reaction calculated based on the yields obtained after 30 min. Reaction is expected to continue on the surface of the collected film, but the film surface area is so small compared to the surface area of the microdroplets that this contribution to the calculation of the acceleration factor has been neglected.
1.2) with molecular oxygen (O2) and without any metal catalyst to form 4-tert-butylbenzoic acid 2 at room temperature. A water–ethanol solution of 1 (0.1 M) introduced through a fused silica capillary (i.d. 50 μm) at a rate of 15 μL min−1 was atomized into microdroplets (average size ca. 3.1 μm; see the ESI† for the method of droplet measurement) with a coaxial flow of oxygen, which was used as the turbulent nebulizing gas operated at 120 psi as well as the sole oxidant. The oxidation of 1 was initiated by the interactions between 1 in microdroplets with molecular oxygen at the interface. The resulting products 2 were collected for 30 min using a microdroplet trapping system (Fig. S1†). The reaction mixture was extracted with dichloromethane and analysed by 1H NMR. The yield of 2 from the oxidation of 1 was found to be 48% (Fig. 2a). A control experiment was performed in bulk solution (O2 was supplied in a balloon). The reaction mixture was vigorously stirred at room temperature, and less than 1% of the product was detected after 30 min (Fig. 2b). An acceleration factor of 50 was achieved for the reaction in microdroplets compared to the bulk reaction calculated based on the yields obtained after 30 min. Reaction is expected to continue on the surface of the collected film, but the film surface area is so small compared to the surface area of the microdroplets that this contribution to the calculation of the acceleration factor has been neglected.
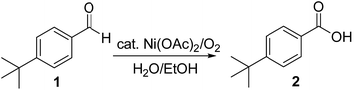 | ||
Scheme 1 Oxidation of 4-tert-butylbenzaldehyde 1 with molecular oxygen and 5 mol% Ni(OAc)2 in water–ethanol (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2) to form 4-tert-butylbenzoic acid 2. 1.2) to form 4-tert-butylbenzoic acid 2. | ||
Next, we screened for possible catalysts without adding any ligand or additive which would promote this reaction in microdroplets, with an emphasis on widely available and inexpensive metal catalysts. Nickel(II) acetate (5 mol%) showed the best efficiency among all the screened catalysts (Fig. S2†); a yield of 91% was achieved. In contrast, the addition of nickel(II) acetate improved the yield of bulk reaction to 4% in 30 min (Fig. 2d, ESI† Section 6). We rationalized the observation as follows: the liquid-phase oxidation of organic compounds with O2 can be affected by a complex set of factors which include intrinsic parameters (aldehyde reactivity, solvent, etc.) and extrinsic parameters (catalyst, initiators/inhibitors, etc.), as well as physical phenomena such as gas to liquid mass transfer.25 When oxygen transfer becomes the rate limiting step, the rate of the overall process is no longer controlled by chemical mechanisms but rather by physical transport.25
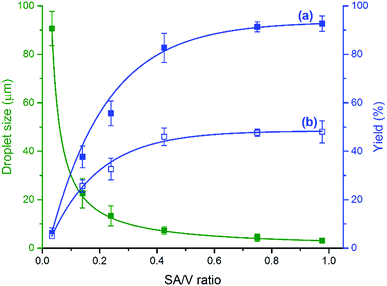
Mass transfer across the interface is the rate-controlling step in most two-phase reactions.26 The surface effect has also been observed in atmospheric halogen chemistry,27 reactions with Criegee intermediates at the air–aqueous interface,28 and catalytic oxidation of p-xylene to produce high-purity terephthalic acid,29 as well as biphasic reactions in flow systems.24,30–33 These considerations prompted us to investigate the effect of SA/V ratio on the product yield. We controlled the droplet size by varying the pressure of sheath gas and using capillaries with different inner and outer diameters. The SA/V ratio of microdroplets was calculated based on the droplet size measured by micro-particle image velocimetry (μPIV, see the ESI for details†). The experiment started with dripping droplets with a SA/V ratio of 0.002 through the capillary (i.d. 250 μm, o.d. 365 μm) with no sheath gas supply but in an oxygen environment protected by an O2 balloon. The flow rate was kept at 15 μL min−1, and less than 6% product was formed in 30 min. We increased the SA/V ratio of droplets by up to 500 times by increasing the O2 sheath gas pressure from 30 to 120 psi through the capillary (i.d. 50 μm, o.d. 365 μm). The yield of product 4-tert-butylbenzoic acid was largely enhanced with an increase of the SA/V ratio of droplets from 0.033 to 1 (Fig. 3a), and reached the maximum yield when the droplet size decreased to about 3 μm. Similar phenomena were also observed using compressed air as the oxidant with less product formation (Fig. 3b). The closely related effect of SA/V ratio on the yield was found for reactions with different aldehydes (Fig. S3†).
The solvent system was investigated, because it not only serves as the reaction medium but also affects the formation of microdroplets.10 We found that water–ethanol (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2) gave the best yields for the droplet reactions among various organic solvents as well as miscible aqueous organic solvents for the microdroplet oxidation of 4-tert-butylbenzaldehyde (Fig. S4†).
1.2) gave the best yields for the droplet reactions among various organic solvents as well as miscible aqueous organic solvents for the microdroplet oxidation of 4-tert-butylbenzaldehyde (Fig. S4†).
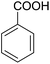
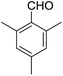
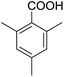
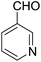
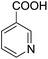
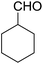
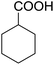
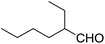
Encouraged by these results, various aldehydes including aromatic, heterocyclic and aliphatic aldehydes (Table 1) were tested under optimized conditions. The corresponding carboxylic acids were obtained in moderate to good yields (62–91%).
The highly efficient transformation of aldehydes into carboxylic acids described above inspired us to explore the possibility of scaling up these reactions in microdroplets. Regular sprayers (electrospray, sonic spray source, etc.) applied in previous microdroplet work use concentric capillaries (for liquid reagents) inserted into a sheath gas tubing with a length of 1 mm staying outside (Fig. 1a inset). The sheath gas comes into contact with the liquid outside the sprayer and shears the liquid into microdroplets. Simply enlarging the capillary size and increasing the liquid flow rate from previous spray sources (Fig. S5a†) resulted in incomplete atomization of the liquid (especially for the liquid in the middle of the flow), as well as a large distribution of droplet sizes, causing little product (<1%) to be formed. In our design, an internal-mix nozzle (from Unist Co., Grand Rapids, MI) was used in which the sheath gas comes into contact with the fluid inside the nozzle and disperses it into microdroplets flying throughout the spray hole (Fig. 1b inset). Such a nozzle uses less atomizing gas and generates droplets with a smaller size distribution compared to the previous external mix spray of liquids at the same flow rate. It is also better suited to higher viscosity streams.
The problems with direct use of commercialized internal-mix nozzles for microdroplet reactions are (1) the droplets generated from this nozzle are too large (ca. 90 μm) for accelerated microdroplet reactions (Fig. 5), and (2) an increased flow rate (8 mL min−1) does not allow 4-tert-butylbenzaldehyde to make good contact with the oxidant, leading to a reaction yield of less than 5%.
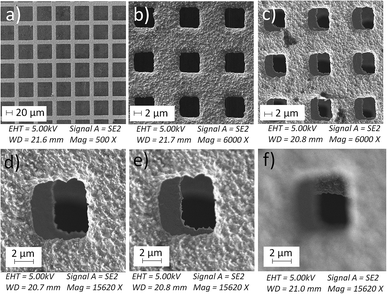
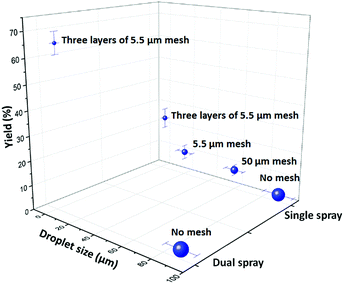
We tried various methods to reduce the droplet size including using electrified droplet fission, and acceleration of droplet desolvation by heating the droplet flying path and extending the droplet flying distance (Fig. S6†). We found that the most efficient method was to mount meshes in front of the spray hole (Fig. 1b). Large droplets were broken into small droplets through size-guided Ni wire meshes. Scanning electron microscopy (SEM) images (Fig. 4) show meshes of 50 μm, 5.5 μm and three layers of 5.5 μm used in the study. PIV was used to measure the sizes of microdroplets generated by the internal-mix nozzle mounted with these meshes in a water–ethanol solution. The meshes effectively reduced the droplet sizes (Fig. 5), and by overlapping three layers of 5.5 μm mesh (the minimum size we purchased commercially), the droplet size was reduced to about 3 μm, which can be comparable to the size of microdroplets generated in the small sonic sprayer.
Another important factor that allows the reaction to have high yield is the mixing efficacy of gas and microdroplets. In order to increase the interactions between 4-tert-butylbenzaldehyde and O2, we introduced another stream of O2 through a similar nozzle but without infusing the liquid. The optimized angle between the two nozzles was set between 60° and 80°. Rapid mixing at the cross section of two fluid streams allows efficient mass transfer between the two phases. Finally, the aerobic oxidation of 4-tert-butylbenzaldehyde to 4-tert-butylbenzoic acid was achieved in a mixture of water and ethanol (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2) at a product formation rate of 10.5 mg min−1 with a yield of 66% for the pure product isolated by liquid chromatography. As Fig. 5 shows, the highest yield was obtained with small droplets in dual spray.
1.2) at a product formation rate of 10.5 mg min−1 with a yield of 66% for the pure product isolated by liquid chromatography. As Fig. 5 shows, the highest yield was obtained with small droplets in dual spray.
Conclusions
In summary, we have demonstrated that aerobic oxidation can be carried out in microdroplets much more rapidly and with higher yield compared with their bulk-phase counterpart. Addition of catalytic nickel(II) acetate further accelerated microdroplet reaction and increased the yield by about a factor of two. Aromatic, heterocyclic, and aliphatic aldehydes were oxidized to their corresponding carboxylic acids. O2 plays the dual role of being the sheath gas to generate microdroplets as well as the sole oxidant in the reaction. We also developed a high flux device for scaling up microdroplet synthesis using an internal-mix nozzle mounted with size-controlled meshes. We achieved a preparative synthesis of 4-tert-butylbenzoic acid at a rate of 10.5 mg min−1 with an isolated product yield of 66%, which demonstrates the possible practical utility of the present method.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Dr Wei Li for the help in taking SEM images. This work was supported by the Air Force Office of Scientific Research through a Basic Research Initiative grant (AFOSR FA9550-16-1-0113).References
- X. Yan, R. M. Bain and R. G. Cooks, Angew. Chem., Int. Ed., 2016, 55, 12960–12972 CrossRef PubMed.
- S. Banerjee, E. Gnanamani, X. Yan and R. N. Zare, Analyst, 2017, 142, 1399–1402 RSC.
- S. Banerjee and R. N. Zare, Angew. Chem., Int. Ed., 2015, 54, 14795–14799 CrossRef PubMed.
- X. Yan, H. Cheng and R. N. Zare, Angew. Chem., Int. Ed., 2017, 56, 3562–3565 CrossRef PubMed.
- T. Müller, A. Badu-Tawiah and R. G. Cooks, Angew. Chem., Int. Ed., 2012, 51, 11832–11835 CrossRef PubMed.
- M. Girod, E. Moyano, D. I. Campbell and R. G. Cooks, Chem. Sci., 2011, 2, 501–510 RSC.
- Y. Li, X. Yan and R. G. Cooks, Angew. Chem., Int. Ed., 2016, 55, 3433–3437 CrossRef PubMed.
- X. Yan, R. Augusti, X. Li and R. G. Cooks, ChemPlusChem, 2013, 78, 1142–1148 CrossRef.
- R. M. Bain, C. J. Pulliam and R. G. Cooks, Chem. Sci., 2015, 6, 397–401 RSC.
- A. K. Badu-Tawiah, D. I. Campbell and R. G. Cooks, J. Am. Soc. Mass Spectrom., 2012, 23, 1461–1468 CrossRef PubMed.
- R. M. Bain, C. J. Pulliam, S. A. Raab and R. G. Cooks, J. Chem. Educ., 2016, 93, 340–344 CrossRef.
- W. Li, K. Liu, R. Simms, J. Greener, D. Jagadeesan, S. Pinto, A. Günther and E. Kumacheva, J. Am. Chem. Soc., 2012, 134, 3127–3132 CrossRef PubMed.
- M. Sharma, Chem. Eng. Sci., 1983, 38, 21–28 CrossRef.
- J. March, Advanced organic chemistry: reactions, mechanisms, and structure, John Wiley & Sons, 1992 Search PubMed.
- J. R. McNesby and C. A. Heller Jr, Chem. Rev., 1954, 54, 325–346 CrossRef.
- R. Shriner and E. Kleiderer, Org. Synth., 1930, 82 Search PubMed.
- J. R. Ruhoff, Org. Synth., 1936, 39 Search PubMed.
- K. Oshima and B. Tollens, Eur. J. Inorg. Chem., 1901, 34, 1425–1426 Search PubMed.
- H. v. Fehling, Eur. J. Org. Chem., 1849, 72, 106–113 Search PubMed.
- E. V. Johnston and J.-E. Bäckvall, in Modern Oxidation Methods, Wiley-VCH Verlag GmbH & Co. KGaA, 2010, ch. 10, pp. 353–369, DOI:10.1002/9783527632039.
- Z. Shi, C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3381–3430 RSC.
- M. Liu and C.-J. Li, Angew. Chem., Int. Ed., 2016, 55, 10806–10810 CrossRef PubMed.
- H. Yu, S. Ru, G. Dai, Y. Zhai, H. Lin, S. Han and Y. Wei, Angew. Chem., Int. Ed., 2017, 56, 3867–3871 CrossRef PubMed.
- L. Vanoye, M. Pablos, N. Smith, C. de Bellefon and A. Favre-Réguillon, RSC Adv., 2014, 4, 57159–57163 RSC.
- L. Vanoye, A. Favre-Réguillon, A. Aloui, R. Philippe and C. de Bellefon, RSC Adv., 2013, 3, 18931–18937 RSC.
- R. Klaewkla, M. Arend and W. F. Hoelderich, in Mass Transfer-Advanced Aspects, InTech, 2011 Search PubMed.
- S. Enami, M. R. Hoffmann and A. J. Colussi, J. Phys. Chem. A, 2016, 120, 6242–6248 CrossRef PubMed.
- S. Enami and A. J. Colussi, Phys. Chem. Chem. Phys., 2017, 19, 17044–17051 RSC.
- M. Li, F. Niu, X. Zuo, P. D. Metelski, D. H. Busch and B. Subramaniam, Chem. Eng. Sci., 2013, 104, 93–102 CrossRef.
- L. Vanoye, A. Aloui, M. Pablos, R. Philippe, A. Percheron, A. Favre-Réguillon and C. de Bellefon, Org. Lett., 2013, 15, 5978–5981 CrossRef PubMed.
- S. J. Pye, S. J. Dalgarno, J. M. Chalker and C. L. Raston, Green Chem., 2018, 20, 118–124 RSC.
- M. Movsisyan, E. I. P. Delbeke, J. K. E. T. Berton, C. Battilocchio, S. V. Ley and C. V. Stevens, Chem. Soc. Rev., 2016, 45, 4892–4928 RSC.
- J. Britton and C. L. Raston, Chem. Soc. Rev., 2017, 46, 1250–1271 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sc01580e |
| This journal is © The Royal Society of Chemistry 2018 |