Evaluation of multiple cation/anion perovskite solar cells through life cycle assessment†
Jaume-Adrià
Alberola-Borràs
 ab,
Rosario
Vidal
ab,
Rosario
Vidal
 *a and
Iván
Mora-Seró
*a and
Iván
Mora-Seró
 *b
*b
aGrupo de Ingeniería de Diseño (GID), Departament d'Enginyeria Mecànica i Construcció, Universitat Jaume I, Av. SosBaynat s/n, 12071 Castelló, Spain. E-mail: vidal@uji.es
bInstitute of Advanced Materials (INAM), Universitat Jaume I, Av. Sos Baynat, s/n, 12006 Castelló, Spain. E-mail: sero@uji.es; Tel: +34 964387552
First published on 9th May 2018
Abstract
After the great initiation of perovskite as a photovoltaic material, laboratory efficiencies similar to those of other photovoltaic technologies already commercialised have been reached. Consequently, recent research efforts in perovskite solar cells have been directed towards improving their stability as well as making their industrialisation possible. Record efficiencies in perovskite solar cells (PSCs) have been achieved using as the active material a multiple cation/anion perovskite by combining not only methylammonium (MA) and formamidinium (FA), but also the Cs cation and I and Br as anions, materials that have also demonstrated superior stability. Herein, the environmental performance of the production of such perovskite films was evaluated via life cycle assessment. Our study points out that multiple cation/anion perovskite films show major detrimental environmental impacts for all categories assessed, except for abiotic depletion potential, when they are compared with a canonical perovskite, MAPbI3. In addition, a closer analysis of the materials utilised for the synthesis of the different multiple cation perovskite compositions revealed that lead halide reagents and chlorobenzene produced the most adverse results in terms of impact. However, the former is used in all perovskite compositions and the latter can be avoided by the use of alternative fabrication methods to the anti-solvent method. To this end, FAI, with the current synthesis procedures, is the most decisive compound as it increases significantly the impacts and the c ost in comparison with MAI. A further economic analysis revealed that multiple cation perovskites need a significantly higher photoconversion efficiency to produce the same payback times compared to the canonical perovskite.
1 Introduction
Perovskite solar cells (PSCs) have experienced an unprecedented growth since they were discovered in 2009 (ref. 1) and constitute a promising technology to collect energy from the Sun in the near future. The fundamental reasons for such success are the easy and cheap deposition of perovskite combined with efficiencies (PCE) comparable to those of the most expensive monocrystalline silicon solar cells.2 For now, perovskite compositions, solvents and deposition processes are under optimisation.3–5 However, in order to produce them on a large scale, stability and reproducibility issues must be overcome.6–9Thus far, much research on PSCs has been oriented towards compositional engineering.3–5,10 Perovskites with outstanding photovoltaic properties have a distinctive structure, composed of three elements according to the formula ABX3, where A corresponds to a monovalent organic/inorganic cation, B corresponds to a divalent inorganic cation (commonly Pb) and X corresponds to a halide anion (Cl, Br and I). As for the monovalent cationic position (A), the most efficient perovskite compositions introduce the formamidinium (FA) cation along with the traditional methylammonium (MA) cation and also Br partially substituting the I anion4,5,11–14 with published efficiencies as high as 22.1%.3 Recently, a caesium inorganic monovalent cation also showed good results when combined with MA and FA in the perovskite structure. Actually, the three cations combined produced an enhanced power conversion efficiency (PCE) of 21.1%.15 Not only that, but high reproducibility was achieved and the efficiency after 250 h was found to be quite stable, yielding an efficiency of 18%.
Lately, the so-called anti-solvent method has been used extensively to deposit high quality perovskite layers.14–17 This method is implemented into the conventional spin-coating method, which can use dimethylformamide (DMF), dimethylsulfoxide (DMSO) or γ-butyrolactone (GBL) as solvents for perovskite precursors. What makes this method different is the addition of a drop of a non-polar solvent into the mixture during the spin-coating stage in order to force the formation of nucleation centres. Thus far, the most used solvents for this method have been chlorobenzene (CB), toluene (TL) and diethyl ether (DE), also known as ether.18,19 Nevertheless, other solvents such as ethyl acetate (EA), xylene (XYL) and dichloromethane (DCM) are also being used, yielding good performance.19–21
The chemical and optoelectronic properties of the three cations are notably different. On one hand, the band gap of FAPbI3 is closer to the theoretical optimum.22 However, pure FAPbI3 presents low structural stability at room temperature, which is a disadvantage,11,23 thus needing MAPbI3 to reach a fair balance between efficiency and stability. On the other hand, inclusion of Cs enhances the stability of Br PSCs.24,25 In fact, caesium iodine perovskites forming CsPbI3 could provide a band gap of 1.73 eV, which is relatively close to the aforementioned theoretical optimum, but its bulk perovskite phase is solely stable at temperatures above 300 °C.26
Apparently, combining MA and FA cations also combines their advantages while avoiding their disadvantages. Nevertheless, a PSC with FA and Cs has been reported with enhanced thermal and humidity stability.27 Cs assists the crystallisation of FA faster and more effectively than MA does, due to a superior size difference.15 Although Cs may be deemed a low abundance element, its presence in the Earth's crust is comparable to other large-scale produced elements like Sn,24 thus demonstrating that the use of Cs is feasible. Indeed, the sufficient abundance of Cs in the Earth's crust compared with the rest of the elements is illustrated in a chart elaborated by the U.S. Geological Survey.28 Furthermore, Cs concentration in the Earth's crust is signally larger than that of other elements already used in photovoltaics, namely cadmium, tellurium, selenium and indium.
Environmental analyses of PSCs via the life cycle assessment (LCA) methodology have been reported to ensure an environmentally safer PSC development and assist PSC technology growth while respecting the environment.29–33 Recently, a comparison of PSCs with silicon solar cells and a tandem with both perovskite and silicon was performed.34 Furthermore, the four most common methods to produce PSCs were environmentally revised by us.35 This study also assessed a PSC regeneration method previously proposed36,37 and applied it to the four production methods considered.
Other studies addressed the inclusion of Cs and FA in PSCs. For instance, a LCA was individually applied to the whole life cycle of modules of five different types of perovskite, namely MAPbI2Cl, MAPbI3, FAPbI3, CsPbI3, and MASnI3 − xBrx.38 In this work, MAPbI3 and FAPbI3 were found to be the most harmful perovskites. However, results of the comparison of perovskites were clouded by the rest of the layer forming devices, such as the cathode and the anode made of fluorine-doped tin oxide (FTO). Another study collated a conventional MAPbX3 and a more stable CsFAPbX3 with other photovoltaic technologies.39
The aim of this work is to evaluate the ongoing trend of compositional engineering in PSCs through LCA from cradle to gate. Therefore, the focus is solely set on the perovskite layer. Furthermore, as layers apart from the perovskite layer would be roughly the same for every PSC studied, focusing the comparison on the perovskite layer is more meaningful. By isolating the perovskite layer, clear results of the environmental performance of the different compositions of perovskites combining the three Cs, FA and MA cations, as well as the reagents that contain them, are obtained here for the first time. Herein, the four compositions reported in the manuscript of Saliba et al.15 are contrasted with a canonical MAPbI3 perovskite synthesised and deposited according to Noh et al.40 As a consequence, the corresponding deposition methods for each type of perovskite are also contrasted. For a more realistic determination of the energy consumption, it has been directly measured for the preparation of cells at the laboratory scale. The energy used in the method to synthesise the multiple cation/anion perovskite was obtained by measuring the consumption in a laboratory environment. On the other hand, the energy consumption for the synthesis of the canonical perovskite was taken from our previous study, which was also based on direct measurements.35 Furthermore, the usage of materials for the synthesis of the multiple cation/anion perovskite is analysed to find the compounds responsible for the impact of the four compositions. A final economic analysis of the materials complements this assessment. Together with the economic cost of the materials, this analysis presents an economic payback time analysis of the materials used for the synthesis of all perovskites. The outcomes of this study are intended to support the scientific community to develop PSCs with the highest efficiencies and stabilities in a safe and environmentally respectful way, thus fulfilling one of the objectives of this technology. Furthermore, the results provided herein must give an approach about PSCs being able to compete with other well-established photovoltaic technologies in terms of environmental and economic performance. For the final application of a technology there are four important aspects that need to be considered: efficiency, stability, cost and impacts; while the implications of the two former ones are clear to the whole community, the repercussions of the latter ones are not as clear. This work focuses indeed on impact and cost, aiming to provide a more precise picture of the effective implementation of halide perovskite technology. Therefore, after this study PSCs are expected to be one step closer to industrial scale production.
2 Materials and methods
2.1 Goal and scope definition
Fundamental research on PSCs is mainly focused on efficiency enhancement and the increase of long term stability. This study is intended to assist researchers in the realm of perovskite-based photovoltaics on issues less investigated but significantly important for the further development of an industrial technology such as the effect of composition on cell impacts and cost. Furthermore, multiple cation/anion compositions enhance reproducibility as well as stability,15 therefore making them more suitable for industrial scale production. Hence, this analysis is intended to pave the way towards industrialisation of perovskites. For this purpose, four different combinations of the inorganic cation Cs with the most used organic cations FA and MA using Br/I anions were compared with the canonical MAPbI3 perovskite. The impacts generated by the perovskite layer were assessed from cradle to gate using LCA as a tool. A functional unit of 1 cm2 of PSCs was chosen as a representative for the comparison of several compositions of perovskite. It is very important to highlight that this work solely assesses the perovskite layer deposited, as the rest of the layers (substrate, and electron and hole selective contacts…) are considered to be similar for all the analysed cases and for that reason excluded from the LCA.41,42 A detailed effect of these other parts of the cell can be found in our previous LCA study.35 The corresponding efficiencies and lifetimes of the PSCs made of the five combinations of perovskite were extracted from the studies in which their syntheses are reported.15,402.2 System boundary
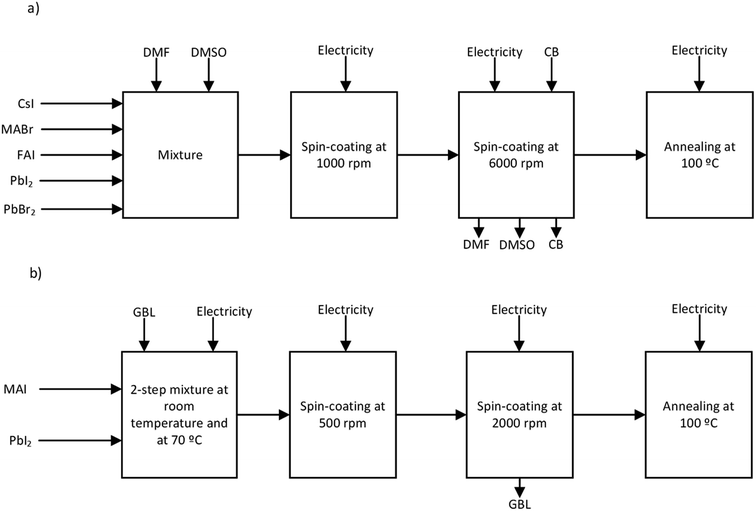
In order to compare the environmental performance of the highly efficient and more stable multiple cation/anion perovskite with a canonical perovskite, an LCA was conducted from cradle to gate. Therefore, the steps of the life cycle of PSCs included all the processes from the extraction of raw materials to the deposition of the perovskite layer. Since in this work just the perovskite layer is studied, the system ends when every step concerning the deposition of the perovskite layer is accounted for. Although perovskite is generally synthesised in a nitrogen glovebox, its energy consumption and the nitrogen it uses are dismissed, as it participates in both synthesis processes. The deposition methods modelled for this work are described in Fig. 1.In the work of Saliba et al.15 as well, a deposition using the anti-solvent method was simulated, which is illustrated in Fig. 1a. This method cannot easily be up-scaled for industrial applications;43 however we have decided to include it due to its extended use and to determine its impact from the point of view of LCA. In this study, for a perovskite deposition with an anti-solvent we consider a first step of spin-coating of the reagents with a mixture of (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) DMF and DMSO by volume at 1000 rpm for 10 s. This step was followed by another spin-coating step at 6000 rpm for 20 s, in which 100 μl of CB were dropped onto the mixture 5 s before the end of the step. Lastly, the substrate was annealed for 1 h at 100 °C. Energy consumption was directly measured from laboratory devices.
1) DMF and DMSO by volume at 1000 rpm for 10 s. This step was followed by another spin-coating step at 6000 rpm for 20 s, in which 100 μl of CB were dropped onto the mixture 5 s before the end of the step. Lastly, the substrate was annealed for 1 h at 100 °C. Energy consumption was directly measured from laboratory devices.
The deposition of the canonical perovskite, which is depicted in Fig. 1b, comprised first stirring the mixture of MAI and PbI2 reagents in GBL at room temperature for 10 minutes and then for 30 minutes at 70 °C. Then, the mixture was spin-coated at 500 rpm for 5 s and 2000 rpm for 60 s. Finally, the film was heated for 60 minutes at 100 °C. This procedure was extracted from our previous assessment.35
2.3 Inventory
Herein, combinations of the three cations and two anions follow a general formula (Csx[MA0.17FA0.83](1−x)Pb[I0.83Br0.17]3), and reached efficiencies of around 20% and a good stability during 250 h.15 Similar to the manuscript, the four combinations used herein were x = 0, 0.05, 0.10 and 0.15, where consequently x = 0 contains only MA and FA, the combination used for the currently published record PSC.3 According to that, the resulting stoichiometric coefficients of each precursor used for the synthesis are clarified in Table 1. As for the canonical perovskite an equal mixture of MAI and PbI2 was considered,40 known for being a general recipe from the early days of PSCs.| Composition | CsI | FAI | MABr | MAI | PbI2 | PbBr2 |
|---|---|---|---|---|---|---|
| 0.00 | 0.0000 | 0.8300 | 0.1700 | — | 0.8300 | 0.1700 |
| 0.05 | 0.0500 | 0.7885 | 0.1615 | — | 0.8258 | 0.1743 |
| 0.10 | 0.1000 | 0.7470 | 0.1530 | — | 0.8215 | 0.1785 |
| 0.15 | 0.1500 | 0.7055 | 0.1445 | — | 0.8173 | 0.1828 |
| Canonical | — | — | — | 1.0000 | 1.0000 | — |
Most of the inputs were calculated from datasets in Ecoinvent,44 namely electricity, transport, solvents and most of the reagents. However, FAI and CsI production processes could not be found in databases; they were therefore modelled from information in the literature. In particular, the synthesis of FAI was modelled from several reactions with hydrogen cyanide, hydroxylamine, acetic acid and hydroiodic acid as reagents.4,45,46 At the same time, CsI was modelled from a process of recovery of Cs from pollucite with sulphuric acid and hydroiodic acid as reagents.47 In addition, the characterisation factor of Cs for the abiotic depletion potential category was obtained from two different methods to compare them. The most utilised characterisation factor in this work was extracted from the literature.48,49 For the sake of presenting a comparison, an updated factor was estimated based on data from 2017,50,51 according to the methodology description.48
From the stoichiometric coefficients (Table 1) and the amount of perovskite, the amount of each reagent was obtained, which is reported in Table 2. The mass of the perovskite was calculated by multiplying the perovskite's density23 by the volume of perovskite deposited in the cell. The volume was obtained by multiplying 25 cm2 of the substrate area and 500 nm thickness of the perovskite layer, which were assumed.
| Reagents/solvents | 0.00 | 0.05 | 0.10 | 0.15 | Canonical |
|---|---|---|---|---|---|
| CsI | 0 | 4.38 | 8.76 | 13.1 | |
| FAI | 48.1 | 45.7 | 43.3 | 40.9 | |
| MABr | 6.42 | 6.09 | 5.77 | 5.45 | |
| MAI | 52.4 | ||||
| PbI2 | 129 | 128 | 128 | 127 | 152 |
| PbBr2 | 21.0 | 21.6 | 22.1 | 22.6 | |
| DMF | 799 | 791 | 783 | 774 | |
| DMSO | 233 | 230 | 228 | 226 | |
| CB | 4440 | ||||
| GBL | 307 | ||||
The electric energy consumption of the multiple cation/anion perovskite used in the steps detailed in Fig. 1 was experimentally determined. The inventory of the overall electricity usage for the two methods is shown in Table 3. During the mixing and annealing steps 16 devices processed simultaneously were assumed.
| Step | Anti-solvent method (J cm−2) | Conventional method (J cm−2) |
|---|---|---|
| Mixing | 36 | 171 |
| Spin-coating (500 rpm) | 20 | |
| Spin-coating (1000 rpm) | 144 | |
| Spin-coating (2000 rpm) | 252 | |
| Spin-coating (6000 rpm) | 288 | |
| Annealing | 1647 | 1647 |
| Total energy consumption | 2115 | 2090 |
Finally, there are two classes of inventory flows left to account for. These are the amount of transportation and the outputs released during the deposition. The amount of transportation was obtained from the distance of the supplier to Castelló (Spain), where the laboratories are located. Solvent releases during perovskite deposition were assumed to be similar to the amounts of solvent used. A complete inventory is described in ESI Tables S1–S7.†
2.4 Anti-solvent method analysis
In order to provide more insight into the environmental consequences stemming from the anti-solvent method, an analysis of the different compounds used is performed. The most used chemicals analysed herein are CB, DE, XYL, TL, EA and DCM.18–21 The processes of production of these chemicals were obtained from the Ecoinvent database.44 The volume of solvent used is assumed to be 1 μl, which corresponds to a single drop deposited. Finally, this volume is multiplied by the density of each compound to determine the mass utilised. The results are presented in the ESI (Section 2.2.2).†2.5 Economic analysis
An additional economic analysis was performed in order to support the environmental assessment. For this analysis, only the chemicals for the synthesis and deposition of the perovskite were considered as the transport price is embedded into the price of the chemicals. The energy consumption flow was not included in the economic analysis due to the great uncertainty of its measurement. It is important to emphasise that this was done exclusively for this economic analysis. Furthermore, the process outputs were considered as void of economic cost. The price in euros of each input was calculated from the amount of each used and its retail price from the main suppliers. Further details are provided in ESI Table S8.†2.6 Impact categories
For a comprehensive and thorough comparison, eleven impact categories were chosen, in which the most developed impact models and the most representative categories were used (see Table 4). Seven of this group of categories are included in CML baseline V3.02.48,52 These categories are abiotic depletion (ADP), abiotic depletion fossil fuels (ADPF), global warming (GWP), ozone layer depletion (ODP), photochemical oxidation (POP), acidification (AP) and eutrophication (EP). From these categories, one of the most significant for measuring the environmental performance of a solar energy collector device is global warming, as one of the main benefits of energy stemming from such devices is the mitigation of the greenhouse effect. Nonetheless, the other categories enlisted represent a broad panoply of the most concerning categories which must be taken into consideration in order to avoid environmental charge transference, from the global warming category to these categories.| Category | Abbreviation | Unit | Methodology |
|---|---|---|---|
| Abiotic depletion potential | ADP | kg Sb eq. | CML baseline V3.02 |
| Abiotic depletion potential, fossil fuels | ADPF | MJ | |
| Climate change potential | GWP | kg CO2 eq. | |
| Ozone layer depletion potential | ODP | kg CFC-11 eq. | |
| Photochemical oxidation potential | POP | kg C2H4 eq. | |
| Acidification potential | AP | kg SO2 eq. | |
| Eutrophication potential | EP | kg PO43− eq. | |
| Cumulative energy demand | CED | MJ | Cumulative energy demand V1.09 |
| Human toxicity, cancer effects | HTC | CTUh | Usetox V1.04 |
| Human toxicity, non-cancer effects | HTNC | CTUh | |
| Freshwater ecotoxicity | FET | CTUe |
Additionally, four determining categories were chosen. From the cumulative energy demand method (CED),53 the total cradle-to-gate energy invested in the perovskite layer is obtained by adding the cumulative energies obtained from renewable and non-renewable sources. This category allows contrasting the energy invested to produce it with the energy obtained from it. Owing to the concerning content of lead in PSCs, it is necessary to introduce into the assessment the impact categories human toxicity cancer (HTC), human toxicity non-cancer (HTNC) and freshwater ecotoxicity (FET) from the USEtox V1.04 method.54
The CML, CED and USEtox methods are incorporated within SimaPro® 8.0.3.14 software.55 In this manuscript, the abbreviations listed in Table 4 are used to name the selected impact categories.
3 Results and discussion
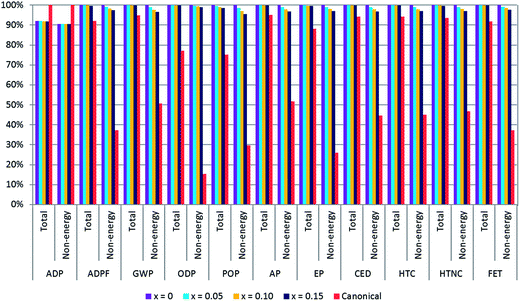
The environmental impact of the multiple cation/anion perovskites is compared with the environmental impact of a canonical perovskite in Fig. 2. These outcomes comprise the environmental impacts generated from the material extraction until the PSC is manufactured, although considering just the effect of perovskite layer as has been previously commented in Section 2.2 of this manuscript. The results are plotted in percentage considering the impact of the composition with the largest impact as 100%, this composition being the multiple cation/anion perovskite with x = 0, i.e. MA0.17FA0.83Pb[I0.83Br0.17]3, for every category but ADP. Along with the total impacts including the impact originating from the energy consumption for the preparation of the perovskite layer, results without accounting for the energy consumption are also plotted in Fig. 2. Energy consumption for the preparation of PSCs at the lab scale has been directly measured and in fact it accounts for most of the impact as can be appreciated in the distribution of impacts per type of flow in Fig. S1.† The contribution of energy consumption to the total impact will undoubtedly decrease with the industrial up-scaling process. Consequently, in order to take into account the direct impact of the materials themselves the impact without taking into account the energy consumption has also been calculated and represented in Fig. 2.Outcomes of the comparison of environmental impacts of the four compositions of perovskite containing Cs, FA, MA, I and Br with the canonical perovskite, which is composed of just MA and I, are quite homogeneous for all categories, except for the ADP category. In general, they show that multiple cation/anion perovskite compositions are more harmful than the canonical perovskite, except for ADP where the impact of the canonical perovskite surpasses that of the multiple cation/anion perovskites.
A comprehensive table with the absolute outcomes of the five different perovskite compositions compared here is available in ESI Tables S9–S13.† In addition, the impact distribution of the flows of inputs and outputs of the process of the perovskite synthesis and deposition is presented in the ESI (see Fig. S1†). The total impact results for multiple cation/anion perovskite impacts are very analogous among them. Approximately, the canonical perovskite impact is just 92% that of the multiple cation/anion perovskite with x = 0 impact, which is the most harmful among triple cation perovskites for all categories. This fact is true except for ODP, POP and EP categories, where the canonical perovskite reaches 77%, 75% and 87% of the x = 0 perovskite, respectively. The little deviation stems from a slightly larger impact of multiple cation/anion perovskite reagents and energy consumption.
However, for the ADP category, the multiple cation/anion perovskite impact is predominant, being roughly 92% with respect to the canonical perovskite impact. The reason behind this lies in the fact that the main group of inputs responsible for the impact of the ADP category is the synthesis reagents, mainly PbI2, whose impact is bigger for the canonical perovskite. There is little difference of impact amongst multiple cation/anion perovskites, as the amount of synthesis reagents used is identical. Generally, for this category, energy consumption is not as determinant as for the rest of the categories. Although Cs impact is negligible for the ADP category and unappreciable for the rest of the categories, its ADP characterisation factor (ADPF) needs a revision because it might be outdated. Because the ADPF utilised for this study is taken from the literature of 2002,48,49 ADPF was updated to 2017.50,51 Further details about the updating can be seen in the ESI.†
Nevertheless, the impact due to the different materials and processes employed in the fabrication of multiple cation/anion perovskites in comparison with the canonical perovskite is evident when energy consumption is not considered in the total impacts (see Fig. 2). MA0.17FA0.83Pb[I0.83Br0.17]3 is the most harmful perovskite, and the impact of multiple cation/anion perovskites decreases as the content of Cs increases. However, variations do not differ more than 5% for any category. On the other hand, the impacts of the canonical perovskite are just 15–55% of the impact of MA0.17FA0.83Pb[I0.83Br0.17]3 perovskite, the most harmful one, depending on the impact category except for ADP where the canonical perovskite has more impact, for the reason previously mentioned.
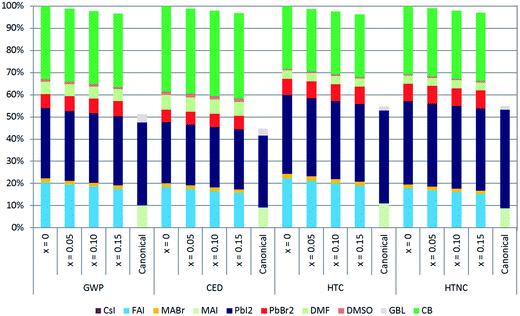
Fig. 2 clearly reflects that the canonical perovskite is significantly less harmful than the multiple cation/anion perovskite when energy use is not considered. A closer and more precise view of the contributions of the reagents and the solvents for the deposition of the here studied compositions of multiple cation/anion perovskites in comparison to the canonical perovskite is detailed in Fig. 3. In this analysis, the most determining categories to assess PSCs are selected, which are GWP, CED, HTC and HTNC.
Lead reagents are among the most pernicious compounds. For HTC and HTNC categories, PbI2 is the major contributor to the overall impact, which is attributed to the larger quantity of it used for the synthesis of the perovskites. However a similar impact is produced by the PbI2 in canonical samples than by the sum of the impacts originating from PbI2 plus PbBr2 in multiple cation/anion perovskite layers. The huge differences in the impacts among multiple cation/anion and canonical perovskite layers are due to the use in the former of CB in the anti-solvent method and FAI instead of MAI, where the latter generates lower impact. The CB used for the anti-solvent method has a slightly lower impact for HTC and HTNC categories. However, for GWP and CED categories CB is responsible for the highest contribution to the overall impact, where PbI2 is the second highest contributing compound. The great contribution of CB stems from the fact that the amount used is the highest among all compounds, despite its little impact per kg in comparison with PbI2. Impact improvements for a possible optimisation of CB are analysed in the ESI.† Results of this analysis reveal that despite the reductions in chlorobenzene usage, except for the ADP category, the overall impacts of the multiple cation/anion perovskites would not reach the impact extent of the canonical perovskite shown in Fig. 2. The up-scaling of anti-solvent technology to move from lab scale cells to large substrates is not straightforward at all from the technological point of view.43 Here we show that the anti-solvent method also has an important deleterious effect on the impacts generated and should consequently be exchanged by a lower impact method in the future commercialization of perovskites.
Among the reagents that supply the three cations, FAI emerges as the most adverse, which is also the reagent that varies the most with composition and therefore the reagent that eventually determines the result. Most of its impact stems from the energy used to synthesise it, as its synthesis from hydroiodic acid and formamidine acetate is performed in a laboratory environment. In particular, the most detrimental step of this process is a final treatment in a vacuum oven at 60 °C for 24 h.4 By reducing the operating time of this treatment, impacts of the multiple cation/anion perovskites would not decrease as much as those of the canonical perovskite. Nonetheless, for GWP, CED, HTC and HTNC categories, the impact of FAI is less adverse than that of the cation supplier reagent for canonical perovskite (MAI) when this operational time goes below 6 hours.
Meanwhile, the contribution of MABr is vague and the contribution of CsI is not visible because small amounts of them are used. Along with the fact that a greater amount of FAI is used for the synthesis of the multiple cation/anion perovskites, its impact per kg of reagent is the highest of the three reagents that supply cations, making the MA0.17FA0.83Pb[I0.83Br0.17]3 perovskite the most harmful, where the impact is reduced when x increases due to the fact that a smaller amount of FAI is used as it is partially substituted by CsI.
Usage of solvents produces a considerably smaller impact with respect to the reagents. DMF solvent causes a superior impact compared to DMSO, both considerably inferior to that of CB. In contrast to preceding studies,30,38 perovskite deposition solvent impacts are generally of trivial magnitude. This statement is true provided that CB would not be treated as such since it satisfies the extra function of removing solvents used for the deposition. DMF, PbBr2 and CsI are the only flows whose impact increases with the Cs content. Meanwhile, the remaining flow impacts decrease with the amount of Cs. Moreover, the CB impact remains constant because its amount does not vary with the different compositions.
3.1 Economic analysis
In order to complement the analysis of the four multiple cation/anion perovskites with the canonical MAPbI3 perovskite, the economic consequences of the usage of reagents and solvents for the perovskite deposition are analysed in Fig. 4. For this analysis, the use of energy flow is dismissed owing to an unrealistic usage in the laboratory environment. Another reason to dismiss the energy use flow lies in the fact that the cost of the energy used poses a significant cost, which would disguise the cost of reagents and solvents. In addition, as the energy usage of the canonical perovskite is lower than that of the multiple cation/anion perovskites, inclusion of this cost would not change the overall result. In this economic analysis the cost in euros of each reagent and solvent is obtained from its amount used and its retail price; see Table S8† for further details. Note that the economic analysis reported here is for cells at the lab scale and only considering the cost of the perovskite layer. This fact implies that the cost reported here is overestimated considering a future industrial application. However, it provides important clues about which parts affect the final price the most.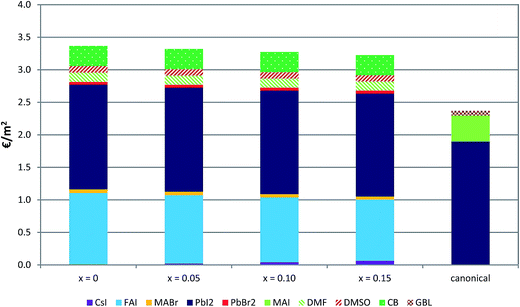 | ||
| Fig. 4 Cost in euros of materials for the deposition of the perovskite active layer for each of the PSCs analysed. Energy is not included in this analysis. | ||
From Fig. 4, the canonical perovskite is found to be the most economical type by virtue of the lower quantity of compounds used. The principal compound behind the higher cost of multiple cation/anion perovskites in comparison with the canonical composition is FAI. In fact, the cost of multiple cation/anion perovskites is roughly·1 € m−2 higher than that of the canonical perovskite, which matches approximately with the cost of FAI. Furthermore, this difference decreases with the Cs content used favoured by the subsequent reduction in the cost of FAI. Among the multiple cation/anion perovskites the cheapest composition is the one with x = 0.15 with the highest content of Cs and consequently the lowest content of FA. Despite the determining character of FAI and its highest cost per mass unit, PbI2 with the second highest cost per mass unit is the most expensive reagent used in the whole composition; however in the multiple cation/anion perovskites the cost is slightly mitigated by the introduction of the Br anion and the consequent use of the PbBr2 precursor, significantly cheaper than PbI2 (see Table S8†). DMF and DMSO solvent cost is lower than the CB cost; nonetheless the cost of these three compounds is neither important nor determining for the total cost.
Given that PSCs can be an economic source from the energy generated, a cost payback time analysis was performed for the perovskite layer. Because the canonical perovskite has the lowest perovskite layer cost with respect to the rest of the perovskites analysed here, the payback time will be lower for the canonical perovskite if the efficiencies of the cells are similar. Hence, the multiple cation/anion perovskites can only achieve the same payback time by an increase of cell efficiency. In Fig. 5, the efficiency of a multiple cation/anion perovskite with x = 0.10 (the perovskite with the highest reported efficiency in its original manuscript),15 left axis, is plotted against the efficiency of the canonical perovskite cell in order that both present the same payback time. In order to execute this cost payback time analysis, the money saved due to the electricity produced by two theoretical photovoltaic devices based on the perovskites studied counteracts the initial cost of the perovskite layer. It has been calculated considering the perovskite layer cost (see Fig. 4) and the price of 1 MJ of electricity in Spain. The amount of electricity generated by the theoretical photovoltaic devices was obtained by assuming a solar constant of 1 kW m−2 in Spain and no efficiency losses due to ambient temperature. This analysis calculates the efficiency that the perovskite with x = 0.10 of Cs should have to recover the money invested on its synthesis and deposition in the same time as that needed in the case of the canonical perovskite. The difference between the efficiencies of the multiple cation/anion and canonical perovskites to get the same payback time is represented by the right axis in Fig. 5, to facilitate the comprehension of this analysis.
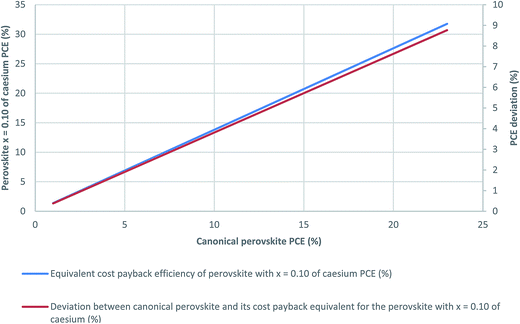 | ||
| Fig. 5 Equal cost payback time for the perovskite with x = 0.1 of caesium and canonical perovskite PSCs. | ||
The payback cost analysis in Fig. 5 reveals that the efficiency necessary to recover the money invested in the synthesis and deposition of a canonical perovskite with an efficiency of 16%, for a perovskite with x = 0.10 of Cs is a little below the current published record efficiency of 22.7%,4,56,57i.e. the efficiency of the multiple cation/anion perovskite has to be 1.38 times the efficiency of the canonical perovskite to make the payback cost equal. However, experimental results point to the fact that organic–inorganic perovskites are attaining increased stabilities in comparison to perovskites with utterly organic cations; whereas results also reveal that the former achieve lower efficiencies.58 If a 20% of efficiency in the canonical perovskite were assumed, a roughly 28% efficient multiple cation/anion would be necessary to get the same economic payback time, which is above the current record. Nevertheless, if a similar efficiency is attained, multiple cation/anion perovskite will present the upside of having a superior stability. As a result, the economic benefits from the multiple cation/anion will be much higher because both a higher efficiency and a longer lifetime. Furthermore, the time that could take to recover the money invested in the deposition of the perovskite layer with these aforementioned efficiencies is below 100 of minutes. Current perovskite efficiencies endure thousands of hours maintaining a quite similar efficiency,15,59 which is well above the time that takes to recover the money invested in the deposition of these perovskites.
4 Conclusions
The outcomes of the comparison of four perovskites containing Cs, formamidinium and methylammonium as cations and I and Br as anions to the canonical perovskite with the methylammonium cation and just I as the anion reveal that multiple cation/anion perovskites are harmful for all the impact categories analysed except for abiotic depletion potential (ADP). The impact of multiple cation/anion perovskite is even more pronounced if the energy used in the cell fabrication is not considered. There are two main reasons for the higher impact of multiple cation/anion perovskites: the use of the anti-solvent method and the utilization of FAI precursor. This work points out that the anti-solvent method, which produces outstanding results at the lab scale, is not adequate for industrial implementation. The reason behind this is not only the technical difficulties of its implementation on larger substrate sizes, but also the increase of impacts. On the other hand, the FA cation is present in the record efficiency PSCs.3–5,10 Nevertheless, the FAI chemical used in the multiple cation/anion perovskite synthesis increases significantly the impacts. Moreover, it is the most expensive precursor causing an important increase of the cell cost in comparison with canonical devices using just MA as the monovalent cation. The current synthesis of this precursor needs to be optimised in order to reduce these impacts and cost. In contrast, the partial substitution of I by Br anions has positive consequences as it has practically no effect in terms of impact, nonetheless reducing the device cost, as PbBr2 is remarkably cheaper than PbI2. The future utilisation of multiple cation/anion perovskites in the industrialisation of perovskite solar cells needs a detailed and balanced study of not only efficiency but also impact and cost. For example, here we highlight that a canonical MAPbI3 with 16% efficiency will be more attractive for industrialisation, as long as it can be prepared with enough stability, than multication cells with FA and with the current published record efficiency of 22.7% as both present the same payback time but the former exhibits reduced impacts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from MINECO of Spain under Project MAT2016-76892-C3-1-R.References
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef PubMed
.
- H. J. Snaith, J. Phys. Chem. Lett., 2013, 4, 3623–3630 CrossRef
.
- W. S. Yang, B.-W. Park, E. H. Jung, N. J. Jeon, Y. C. Kim, D. U. Lee, S. S. Shin, J. Seo, E. K. Kim, J. H. Noh and S. Il Seok, Science, 2017, 356, 1376–1379 CrossRef PubMed
.
- W. S. Yang, J. H. Noh, N. J. Jeon, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Science, 2015, 348, 1234–1237 CrossRef PubMed
.
- N. J. Jeon, J. H. Noh, W. S. Yang, Y. C. Kim, S. Ryu, J. Seo and S. Il Seok, Nature, 2015, 517, 476–480 CrossRef PubMed
.
- T. Leijtens, G. E. Eperon, N. K. Noel, S. N. Habisreutinger, A. Petrozza and H. J. Snaith, Adv. Energy Mater., 2015, 5, 1500963 CrossRef
.
- N.-G. Park, M. Grätzel, T. Miyasaka, K. Zhu and K. Emery, Nat. Energy, 2016, 1, 16152 CrossRef
.
- G. Grancini, C. Roldán-Carmona, I. Zimmermann, E. Mosconi, X. Lee, D. Martineau, S. Narbey, F. Oswald, F. De Angelis, M. Graetzel and M. K. Nazeeruddin, Nat. Commun., 2017, 8, 15684 CrossRef PubMed
.
- J. A. Christians, P. Schulz, J. S. Tinkham, T. H. Schloemer, S. P. Harvey, B. J. Tremolet de Villers, A. Sellinger, J. J. Berry and J. M. Luther, Nat. Energy, 2018, 3, 68–74 CrossRef
.
- F. Zhang, S. Wang, X. Li and Y. Xiao, Curr. Nanosci., 2016, 12, 137–156 CrossRef
.
- J. W. Lee, D. J. Seol, A. N. Cho and N. G. Park, Adv. Mater., 2014, 26, 4991–4998 CrossRef PubMed
.
- J. H. Noh, S. H. Im, J. H. Heo, T. N. Mandal and S. Il Seok, Nano Lett., 2013, 13, 1764–1769 CrossRef PubMed
.
- N. Pellet, P. Gao, G. Gregori, T. Y. Yang, M. K. Nazeeruddin, J. Maier and M. Grätzel, Angew. Chem., Int. Ed., 2014, 53, 3151–3157 CrossRef PubMed
.
- F. Giordano, A. Abate, J. P. Correa Baena, M. Saliba, T. Matsui, S. H. Im, S. M. Zakeeruddin, M. K. Nazeeruddin, A. Hagfeldt and M. Graetzel, Nat. Commun., 2016, 7, 10379 CrossRef PubMed
.
- M. Saliba, T. Matsui, J.-Y. Seo, K. Domanski, J.-P. Correa-Baena, M. K. Nazeeruddin, S. M. Zakeeruddin, W. Tress, A. Abate, A. Hagfeldt and M. Grätzel, Energy Environ. Sci., 2016, 9, 1989–1997 Search PubMed
.
- H. Cho, S.-H. Jeong, M.-H. Park, Y.-H. Kim, C. Wolf, C.-L. Lee, J. H. Heo, A. Sadhanala, N. Myoung, S. Yoo, S. H. Im, R. H. Friend and T.-W. Lee, Science, 2015, 350, 1222–1225 CrossRef PubMed
.
- D. Bi, W. Tress, M. I. Dar, P. Gao, J. Luo, C. Renevier, K. Schenk, A. Abate, F. Giordano, J.-P. Correa Baena, J.-D. Decoppet, S. M. Zakeeruddin, M. K. Nazeeruddin, M. Grätzel and A. Hagfeldt, Sci. Adv., 2016, 2, e1501170 Search PubMed
.
- S. Paek, P. Schouwink, E. N. Athanasopoulou, K. T. Cho, G. Grancini, Y. Lee, Y. Zhang, F. Stellacci, M. K. Nazeeruddin and P. Gao, Chem. Mater., 2017, 29, 3490–3498 CrossRef
.
- M. Konstantakou, D. Perganti, P. Falaras and T. Stergiopoulos, Crystals, 2017, 7, 291 CrossRef
.
- Y. Li, J. Wang, Y. Yuan, X. Dong and P. Wang, Sustainable Energy Fuels, 2017, 1, 1041–1048 Search PubMed
.
- X. Zheng, B. Chen, C. Wu and S. Priya, Nano Energy, 2015, 17, 269–278 CrossRef
.
- W. Shockley and H. J. Queisser, J. Appl. Phys., 1961, 32, 510–519 CrossRef
.
- C. C. Stoumpos, C. D. Malliakas and M. G. Kanatzidis, Inorg. Chem., 2013, 52, 9019–9038 CrossRef PubMed
.
- M. Kulbak, D. Cahen and G. Hodes, J. Phys. Chem. Lett., 2015, 6, 2452–2456 CrossRef PubMed
.
- F. Fabregat-Santiago, M. Kulbak, A. Zohar, M. Vallés-Pelarda, G. Hodes, D. Cahen and I. Mora-Seró, ACS Energy Lett., 2017, 2, 2007–2013 CrossRef
.
- C. K. Møller, Nature, 1958, 182, 1436 CrossRef
.
- J.-W. Lee, D.-H. Kim, H.-S. Kim, S.-W. Seo, S. M. Cho and N.-G. Park, Adv. Energy Mater., 2015, 5, 1501310 CrossRef
.
-
G. B. Haxel, J. B. Hedrick, G. J. Orris, P. H. Stauffer and J. W. Hendley II, Rare earth elements: critical resources for high technology, 2002 Search PubMed
.
- N. Espinosa, L. Serrano-Luján, A. Urbina and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2015, 137, 303–310 CrossRef
.
- J. Gong, S. B. Darling and F. You, Energy Environ. Sci., 2015, 8, 1953–1968 Search PubMed
.
- J. Zhang, X. Gao, Y. Deng, B. Li and C. Yuan, ChemSusChem, 2015, 8, 3882–3891 CrossRef PubMed
.
- L. Serrano-Lujan, N. Espinosa, T. T. Larsen-Olsen, J. Abad, A. Urbina and F. C. Krebs, Adv. Energy Mater., 2015, 5, 1501119 CrossRef
.
- I. Celik, Z. Song, A. J. Cimaroli, Y. Yan, M. J. Heben and D. Apul, Sol. Energy Mater. Sol. Cells, 2016, 156, 157–169 CrossRef
.
- M. Monteiro Lunardi, A. Wing Yi Ho-Baillie, J. P. Alvarez-Gaitan, S. Moore and R. Corkish, Prog. Photovoltaics, 2017, 25, 679–695 Search PubMed
.
- J.-A. Alberola-Borràs, R. Vidal, E. J. Juárez-Pérez, E. Mas-Marzá, A. Guerrero and I. Mora-Seró, Sol. Energy Mater. Sol. Cells, 2018, 179, 169–177 CrossRef
.
- L. Huang, Z. Hu, J. Xu, X. Sun, Y. Du, J. Ni, H. Cai, J. Li and J. Zhang, Sol. Energy Mater. Sol. Cells, 2016, 152, 118–124 CrossRef
.
- B. J. Kim, D. H. Kim, S. L. Kwon, S. Y. Park, Z. Li, K. Zhu and H. S. Jung, Nat. Commun., 2016, 7, 11735 CrossRef PubMed
.
- J. Zhang, X. Gao, Y. Deng, Y. Zha and C. Yuan, Sol. Energy Mater. Sol. Cells, 2017, 166, 9–17 CrossRef
.
- T. Ibn-Mohammed, S. C. L. Koh, I. M. Reaney, A. Acquaye, G. Schileo, K. B. Mustapha and R. Greenough, Renewable Sustainable Energy Rev., 2017, 80, 1321–1344 CrossRef
.
- J. H. Noh, N. J. Jeon, Y. C. Choi, M. K. Nazeeruddin, M. Grätzel and S. Il Seok, J. Mater. Chem. A, 2013, 1, 11842 Search PubMed
.
-
F. Consoli, Guidelines for Life-cycle Assessment: a Code of Practice, Society of Environmental Toxicology and Chemistry, Brussels, Belgium, 1993 Search PubMed
.
- Environ. Sci. Pollut. Res., 1994, 1, 55 Search PubMed.
- Y. Jiang, M. R. Leyden, L. Qiu, S. Wang, L. K. Ono, Z. Wu, E. J. Juarez-Perez and Y. Qi, Adv. Funct. Mater., 2018, 28, 1703835 CrossRef
.
- R. Frischknecht, N. Jungbluth, H. J. Althaus, G. Doka, R. Dones, T. Heck, S. Hellweg, R. Hischier, T. Nemecek, G. Rebitzer and M. Spielmann, Int. J. Life Cycle Assess., 2005, 10, 3–9 CrossRef
.
- F. Eloy, R. Lenaers and C. Moussebois, Helv. Chim. Acta, 1962, 45, 437–441 CrossRef
.
- W. Lossen and P. Schifferdecker, Justus Liebigs Ann. Chem. Pharm., 1873, 166, 295–320 CrossRef
.
-
P. M. Brown, M. C. Northrup and B. F. Bakke, US Pat., 6436879B1, 2002, pp. 1–12.
.
-
H. de Bruijn, R. van Duin and M. A. J. Huijbregts, Handbook on Life Cycle Assessment, Springer Netherlands, Dordrecht, 2002, vol. 7 Search PubMed
.
- M. Gorrée, J. B. Guinée, G. Huppes and L. van Oers, Int. J. Life Cycle Assess., 2002, 7, 158–166 CrossRef
.
-
J. Emsley, Nature's Building Blocks: an A-Z Guide to the Elements, Oxford University Press, Oxford, 2001 Search PubMed
.
-
U.S. Geological Survey, Mineral Commodities Summaries 2017, Reston, Virginia, 2017 Search PubMed
.
- O. Jolliet, M. Margni, R. Charles, S. Humbert, J. Payet, G. Rebitzer and R. Rosenbaum, Int. J. Life Cycle Assess., 2003, 8, 324–330 CrossRef
.
- R. Frischknecht, N. Jungbluth, H.-J. Althaus, G. Doka, R. Dones, T. Heck, S. Hellweg, R. Hischier, T. Nemecek, G. Rebitzer and M. Spielmann, Int. J. Life Cycle Assess., 2004, 10, 3–9 CrossRef
.
- R. K. Rosenbaum, T. M. Bachmann, L. S. Gold, M. A. J. Huijbregts, O. Jolliet, R. Juraske, A. Koehler, H. F. Larsen, M. MacLeod, M. Margni, T. E. McKone, J. Payet, M. Schuhmacher, D. van de Meent and M. Z. Hauschild, Int. J. Life Cycle Assess., 2008, 13, 532–546 CrossRef
.
- Pré Sustainability, 2016.
- M. A. Green, Y. Hishikawa, E. D. Dunlop, D. H. Levi, J. Hohl-Ebinger and A. W. Y. Ho-Baillie, Prog. Photovoltaics, 2018, 26, 3–12 Search PubMed
.
- National Renewable Energy Laboratory, 2016.
- X. Zhang, X. Ren, B. Liu, R. Munir, X. Zhu, D. Yang, J. Li, Y. Liu, D.-M. Smilgies, R. Li, Z. Yang, T. Niu, X. Wang, A. Amassian, K. Zhao and S. Liu, Energy Environ. Sci., 2017, 10, 2095–2102 Search PubMed
.
- C. Roldán-Carmona, P. Gratia, I. Zimmermann, G. Grancini, P. Gao, M. Graetzel and M. K. Nazeeruddin, Energy Environ. Sci., 2015, 8, 3550–3556 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Inventory data, impact scores, further analyses, and abiotic depletion characterisation factor update. See DOI: 10.1039/c8se00053k |
| This journal is © The Royal Society of Chemistry 2018 |