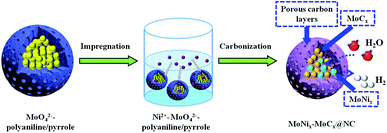
A triple synergistic effect from pitaya-like MoNix–MoCx hybrids encapsulated in N-doped C nanospheres for efficient hydrogen evolution†
Jing-Qi
Chi
a,
Jia-Hui
Lin
ab,
Jun-Feng
Qin
a,
Bin
Dong
 *ab,
Kai-Li
Yan
a,
Zi-Zhang
Liu
ab,
Xin-Yu
Zhang
ab,
Yong-Ming
Chai
*a and
Chen-Guang
Liu
a
*ab,
Kai-Li
Yan
a,
Zi-Zhang
Liu
ab,
Xin-Yu
Zhang
ab,
Yong-Ming
Chai
*a and
Chen-Guang
Liu
a
aState Key Laboratory of Heavy Oil Processing, China University of Petroleum (East China), Qingdao 266580, PR China. E-mail: dongbin@upc.edu.cn; ymchai@upc.edu.cn; Fax: +86-532-86981787; Tel: +86-532-86981376
bCollege of Science, China University of Petroleum (East China), Qingdao 266580, PR China
First published on 15th May 2018
Abstract
To enhance the intrinsic activity and the density of active sites of catalysts for the hydrogen evolution reaction (HER), a facile strategy of using an organic–inorganic precursor followed by carbonization is adopted to prepare ternary core–shell nanostructures composed of ultrafine hybrids of MoNi alloys and MoCx nanoparticles encapsulated in N-doped carbon nanospheres (MoNix–MoCx@NC). The well-defined pitaya-like nanostructures of MoNix–MoCx nanoparticles encapsulated by N-doped carbon nanospheres can be obtained with MoNix–MoCx hybrids as the core and ultrathin N-doped C layers as the shell. A triple synergistic effect has been achieved for the HER. The first synergistic effect from homogeneously dispersed MoNix alloys and MoCx nanoparticles can improve the intrinsic activity and conductivity of MoCx. The second synergistic effect from the MoNix–MoCx and NC shell can enhance the density of active sites and conductivity of MoNix–MoCx. The third synergistic effect from N-doped C can accelerate the charge transfer rate and improve close interaction between NC and MoNix–MoCx. The MoNix–MoCx@NC sample at an optimized low temperature of 700 °C exhibits excellent performance and long-term stability in both acidic and alkaline solution. It requires a lower overpotential of only 172 mV and 168 mV at 10 mA cm−2 in acidic and alkaline solution, respectively. This work provides a new approach to design multiple synergistic effects from excellent catalytic interface through an organic–inorganic hybrid method for efficient electrocatalysis.
Introduction
Hydrogen, as a clean, renewable, and high energy density fuel, has been considered as a promising alternative to carbon-based fuels.1–3 Water electrolysis offers an environmentally friendly method to produce highly pure hydrogen but needs efficient electrocatalysts to lower the overpotential, thereby accelerating the hydrogen evolution reaction (HER) rate and making the water splitting process more efficient.4–6 Pt or its alloys, as the state-of-the-art HER electrocatalysts, suffer from high cost and scarcity, which hampers their widespread utilization.7,8 Consequently, it is highly desirable to design and develop effective and durable non-Pt electrocatalysts for an efficient HER.9,10As we know, Mo-based compound materials such as MoNix,11,12 MoCx,13–15 MoSx (ref. 16–18) and MoPx (ref. 19 and 20) have been considered as an important series of compounds for an efficient HER because of their Pt-like electronic structures. Specifically, MoNix alloys are recognized as promising alternatives to reduce the energy barrier of the Volmer step for water splitting and accelerate the HER kinetics under alkaline conditions with Ni atoms acting as water dissociation centres and Mo atoms acting as adsorption centres.21,22 Therefore, the introduction of Ni into Mo can efficiently modify the electronic structures and thus optimize the Gibbs free energy of hydrogen adsorption (ΔGH*) on the metal surface.23 Moreover, MoCx (Mo2C or MoC) has aroused extensive interest as an efficient catalyst in both acidic and alkaline solution because of its similar electronic structure to Pt.24,25 Designing various nanostructures such as nanowires,26 nanosheets,27 and nanoparticles28 to expose rich active sites can largely improve the catalytic activities of MoCx. However, it is usually hard to gain well-defined MoCx nanostructures because they are usually obtained at extremely high pyrolysis temperature.29 So the inevitable sintering and agglomeration of MoCx nanostructures result in the obvious reduction of active sites under harsh conditions, thus leading to a loss of activity.30 Nanocrystalline MoCx is thereby anchored onto conductive carbon supports, such as carbon nanowires31,32 or graphene,33,34 to mitigate the aggregation of MoCx nanostructures. Very recently, an N-doped carbon matrix has been reported as an ideal support for encapsulating MoCx catalysts to accomplish an improved HER activity by tuning the electronic structures.35,36 However, it's still a great challenge to synthesize combined active MoNix and MoCx nanoparticles with well-dispersed nanostructures to avoid agglomeration and corrosion under harsh conditions and to expose rich active sites for enhanced HER performance.
Herein, we have rationally designed and synthesized pitaya-like hybrids of MoNix–MoCx encapsulated within N-doped carbon nanospheres as efficient HER electrocatalysts by employing organic–inorganic hybrids as the precursor for the first time. The entire synthesis process for preparing MoNix–MoCx@NC is schematically illustrated in Scheme 1. The present synthesis method depends on the strong interaction between Mo–Ni and polyaniline/pyrrole which ensures a confined carbonization reaction. Ultrathin N-doped carbon layers with rich mesopores formed at high carbonization temperature can effectively prevent the stacking and corrosion of the obtained highly active MoNix–MoCx phase and accomplish improved HER performance through tuning electronic structures. Benefiting from the unique NC encapsulation and the combined MoNix and MoCx nanoparticles encapsulated within in ultrathin NC, the as-prepared MoNix–MoCx@NC exhibits remarkable HER performance in both acidic and alkaline solution. Remarkably, the optimized MoNix–MoCx@NC at a relatively low carbonization temperature of 700 °C exhibits enhanced activity with a lower overpotential of only 172 mV and 168 mV at 10 mA cm−2 in acidic and alkaline media, respectively. The mechanism of enhanced performance for MoNix–MoCx@NC has been discussed.
Experimental section
Synthesis of Ni2+–MoO42−–polyaniline/pyrrole
The organic–inorganic hybrids of Ni2+–MoO42−–polyaniline/pyrrole precursors were prepared in the presence of a surfactant (Triton X-100) as the template. In detail, 2.02 g of (NH4)6Mo7O24·4H2O were dispersed in 50 mL deionized water, and then 0.08 g Triton X-100, 0.42 g aniline and 0.28 g pyrrole were added dropwise to the above solution with magnetic stirring for 1 h and ultrasonication for another 1 h to form a homogeneous solution. After that, 20 mL aqueous solution containing 1.95 g (NH4)2S2O8 precooled at 0 °C for 1 h was added to the above aqueous solution for the polymerization reaction for 12 h at 0 °C. Finally, the obtained sample was washed with ethanol and deionized water several times. Then the wet cross-linked MoO42−–polyaniline/pyrrole polymer was dispersed in 20 mL aqueous solution containing 0.5 g Ni(NO3)2 6H2O and then ultrasonicated for 0.5 h. Finally, the obtained uniform solution was maintained at 60 °C for 12 h to form homogeneous powder, which was named Ni2+–MoO42−–polyaniline/pyrrole.Synthesis of MoNix–MoCx@NC
To prepare the MoNix–MoCx@NC nanocomposite, 0.25 g of Ni2+–MoO42−–polyaniline/pyrrole was carbonized in a flow of Ar at 700 °C for 4 h with a heating rate of 5 °C min−1. After being separated by centrifugation and washed with deionized water and ethanol several times to remove inactive and unstable impurities, black MoNix–MoCx@NC nanohybrids were obtained, which were dried in a vacuum at 60 °C. To obtain some profound cognition of the relationships between the nanostructures and electrocatalytic activity of MoNix–MoCx@NC, MoNix–MoCx@NC (600 °C), MoNix–MoCx@NC (800 °C) and MoNix–MoCx@NC (900 °C) were prepared under identical conditions except that the carbonization temperature is 600 °C, 800 °C and 900 °C, respectively.Synthesis of NC, Ni@NC and MoCx@NC composites
The synthesis procedure of the NC composite is similar to that of MoNix–MoCx@NC without adding (NH4)6Mo7O24·4H2O and Ni(NO3)2·6H2O. The synthesis procedure of Ni@NC and MoCx@NC composites is very similar to that of MoNix–MoCx@NC without adding (NH4)6Mo7O24·4H2O or Ni(NO3)2·6H2O.Characterization
The XRD patterns were recorded on an X'Pert PRO MPD diffractometer equipped with Cu Kα radiation (λ = 1.54 Å). The Raman spectra of samples were obtained on a Renishaw inVia 2000 Raman spectrometer with excitation by an argon ion laser (514 nm). XPS was recorded by using an X-ray photoelectron (Thermo Fisher Scientific II spectrometer) using Al Kα radiation (hν = 1486.6 eV) and the C 1s peak at 284.6 eV as an internal standard. The surface morphologies of all the materials were examined by using a SEM (Hitachi S-4800) using an apparatus provided with EDX. The TEM and HRTEM images were recorded on a FEI Tecnai G2 apparatus operating at 200 kV. BET-specific surface areas and pore size distributions were determined on a tristar II Plus system at liquid N2 temperature.Electrochemical measurements
All electrochemical studies were performed using an electrochemical station (Gamry Reference 600) in a typical three-electrode set-up with a saturated calomel electrode (SCE) as the reference electrode and a graphite rod counter electrode. All of the potentials were corrected to the reversible hydrogen electrode (RHE) via the following calculations: ERHE = ESCE + 0.059 pH + 0.243. To prepare the working electrode: 4 mg of catalyst and 20 μL Nafion solution (5 wt%) were dispersed in 1 mL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v water/ethanol solution by sonicating for at least 30 min to form a homogeneous ink. Then 5 μL of suspensions were coated on a glassy carbon electrode (GCE) with a diameter of 4 mm and dried in air before measurement. The linear sweep voltammetry (LSV) curve towards the HER was recorded in 0.5 M H2SO4 and 1.0 M KOH with a scan rate of 5 mV s−1. Electrical impedance spectroscopy (EIS) measurements were carried out at a potential of −0.1 V vs. RHE from 105 to 0.1 Hz with an amplitude of 5 mV. To estimate the double-layer capacitances (Cdl), the electrocatalysts were cycled in the potential region of the non-faradaic region at various scan rates from 40 to 120 mV s−1. An accelerated stability test of the catalysts was carried out by CV from −0.4 to 0.1 V (vs. RHE) at 100 mV s−1 for 1000 cycles or chronoamperometry at −0.16 V (vs. RHE) for 10 h.
1 v/v water/ethanol solution by sonicating for at least 30 min to form a homogeneous ink. Then 5 μL of suspensions were coated on a glassy carbon electrode (GCE) with a diameter of 4 mm and dried in air before measurement. The linear sweep voltammetry (LSV) curve towards the HER was recorded in 0.5 M H2SO4 and 1.0 M KOH with a scan rate of 5 mV s−1. Electrical impedance spectroscopy (EIS) measurements were carried out at a potential of −0.1 V vs. RHE from 105 to 0.1 Hz with an amplitude of 5 mV. To estimate the double-layer capacitances (Cdl), the electrocatalysts were cycled in the potential region of the non-faradaic region at various scan rates from 40 to 120 mV s−1. An accelerated stability test of the catalysts was carried out by CV from −0.4 to 0.1 V (vs. RHE) at 100 mV s−1 for 1000 cycles or chronoamperometry at −0.16 V (vs. RHE) for 10 h.
Results and discussion
XRD is employed to study the crystal phase of NC, Ni@NC, MoCx@NC and MoNix–MoCx@NC (Fig. S1† and 1a). All the samples exhibiting a similar broad peak at 24.5° which corresponds to the (002) facets of the amorphous carbon matrix. The obvious diffraction peaks of Ni@NC at 44.6° (111), 51.9° (200) and 76.4° (220) reveal the formation of well-crystallized metallic Ni during the pyrolysis of the mixture of polyaniline/pyrrole and nickel salt. For the MoCx@NC, the pyrolysis of the organic–inorganic hybrids of polyaniline/pyrrole and molybdate leads to the formation of Mo2C encapsulated by NC, where the obvious diffraction peaks at 34.6°, 37.6, 39.6°, 52.2°, 61.8°, 69.6° and 74.7° belong to the (021), (200), (102), (221), (023), (321) and (223) facets of the electrochemical active phase of β-Mo2C (PDF no. 01-071-0242), respectively. Interestingly, when the polyaniline/pyrrole, nickel salt and molybdate coexist in the organic–inorganic hybrids, the diffraction peaks of MoNix–MoCx@NC can be assigned to the β-Mo2C (PDF no. 01-071-0242), MoC (PDF no. 03-065-6664), Mo0.84Ni0.16 (PDF no. 00-050-1270) and MoNi4 (PDF no. 00-003-1036) phases. It is worth noting that MoNix alloys as highly active species have been well-explored in recent studies and the presence of β-Mo2C indicates that the reaction between molybdate and polyaniline/pyrrole occurs during the carbonization process. In addition, the existence of β-Mo2C and MoC hybrids can efficiently tune the electron density of the carbon surface and optimize the hydrogen binding energy through the synergistic effect between the two species, thus enhancing the HER kinetics and the HER performance.37 The obtained MoNix–MoCx@NC is further characterized by Raman spectroscopy. As shown in Fig. 1b, the first peak located at 1350 cm−1, associated with the D mode, is related to the disorder induced in the carbon material. The second peak is centered at 1495 cm−1 and is ascribed to the amorphous sp2 phase. The G-mode centering at 1580 cm−1 provides information about the in-plane vibration of graphitic carbon atoms. The intensity ratio of ID/IG = (1.22) > 1 indicates that the carbon matrix is rather disordered with rich structural defects, which may improve the HER activity of the MoNix–MoCx@NC.38 The specific surface area of MoNix–MoCx@NC hybrids is calculated by measuring the N2 adsorption/desorption isotherm. Fig. 1c shows that MoNix–MoCx@NC has a large surface area of 136.2 m2 g−1 and that the mesoporous structure is centered at 3.64 nm, which is verified by the pore size distributions (Fig. 1d).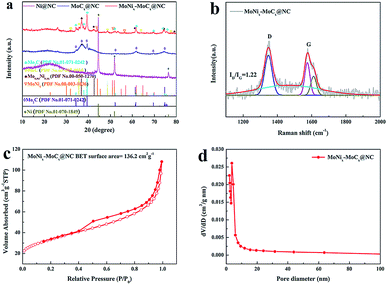 | ||
| Fig. 1 (a) XRD patterns of Ni@NC, MoCx@NC and MoNix–MoCx@NC. (b) Raman spectrum, (c) the N2 adsorption–desorption isotherm and (d) pore size distribution of MoNix–MoCx@NC. | ||
To further interpret the doped elements and electronic configurations, XPS measurements are conducted on MoNix–MoCx@NC. The XPS survey scan (Fig. 2a) indicates that the surface of MoNix–MoCx@NC is composed of Mo, Ni, C, N and O. Fig. 2b exhibits the high resolution spectrum of Mo 3d, which is deconvoluted into two doublets (Fig. 2b). The peaks at 228.4 and 231.7 eV can be ascribed to C-bound Mo in Mo2C,39,40 and the peaks at 232.7 and 235.8 eV are associated with the oxidation states of molybdenum (MoOx).41 The presence of superficial oxides seems to be unavoidable for Mo2C, due to their partial oxidation upon exposure to air. Fig. 2c shows the high resolution spectrum of Ni 2p. The Ni 2p3/2 region can be fitted with three components at 853.1, 854.8 and 860.4 eV corresponding to Ni, Ni2+ and the shakeup peaks, respectively, further confirming the existence of metallic Ni species.42,43 By comparing the Mo 3d spectrum of Mo2C@NC and the Ni 2p spectrum of Ni@NC (Fig. S2a and b†), it can be seen that Ni 2p spectrum shifts to a higher binding energy, while Mo 3d shifts to a lower binding energy, which may be ascribed to the electron transfer effect between Mo and Ni.43 The spectrum of C 1s is reported in Fig. 2d, and the peak at 284.4 eV is the typical peak of carbides, while those at 284.9 and 285.9 eV are assigned to C–C and C–N, respectively.44,45 The N 1s XPS spectrum (Fig. 2e and f) exhibits three types of nitrogen species including pyridinic-N (398.1 eV), pyrrolic-N (399.6 eV), and N–Mo (395.1 eV), which indicates that N is doped into the coated carbon matrix and carbides.46,47 The doping of N elements with large electronegativity into carbides and the carbon matrix can tune electronic structures effectively and thus accomplish an intrinsically enhanced HER activity. In summary, the observations of XPS results indicate the successful preparation of the MoNix–MoCx@NC hybrids.
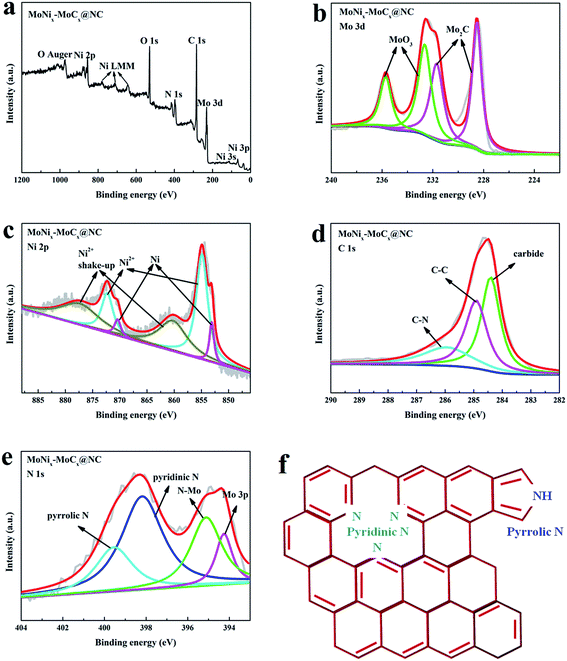 | ||
| Fig. 2 (a) XPS high-resolution scans of MoNix–MoCx@NC in (b) Mo 3d, (c) Ni 2p, (d) C 1s and (e) N 1s regions. | ||
The morphology of all the as-prepared samples is investigated by SEM. As shown in the typical SEM image (Fig. S3a†), the NC obtained at 700 °C is composed of unique nanospheres with relatively smooth surfaces with a diameter of ∼90 nm. After the introduction of nickel salt or molybdate into the polymer, the SEM images of Ni@NC and MoCx@NC show that these nanospheres preserve the intact spherical-like morphology with a size of ∼90 nm (Fig. S3b and c†). Fig. 3a shows that MoNix–MoCx@NC hybrids also exhibit an intact spherical morphology with a diameter of about ∼90 nm, which is consistent with that of Ni@NC and MoCx@NC, but the surfaces become rougher. The TEM image of MoNix–MoCx@NC in Fig. 3b reveals that a large number of nanoparticles with a size of only several nanometers encapsulated in a spherical shell can be clearly observed in every sphere. A closer examination of MoNix–MoCx@NC confirms that the shell in every nanosphere comprises ultrathin carbon layers and the MoNix and MoCx nanoparticles are tightly cross-linked with the carbon matrix (Fig. 3c). It can also be easily seen that the nanospheres exhibit porous nanostructures, as confirmed by the above-mentioned BET results, exhibiting a large surface area of 136.2 m2 g−1. The HRTEM image (Fig. 3d) displays that the lattice fringe spacings of 0.244 nm and 0.228 nm correspond to the (111) facets of Mo0.84Ni0.16 alloys and the (102) facets of β-Mo2C, respectively. Of special note, the intimate cross-linking of MoNix alloys and MoCx in the MoNix–MoCx@NC sample would effectively facilitate the electronic interaction, thus promoting electron transfer in the electrocatalytic process. These results indicate that carbonized MoNix–MoCx@NC exhibits a typical pitaya-like morphology with ultrathin carbon layers as peel and cross-linked MoNix and MoCx as seeds. The unique core–shell structures can effectively prevent the active sites from aggregation and excessive growth, exposing more active sites for the HER. To further explore the formation process of MoNix–MoCx@NC, we have also compared the morphology of NC, Ni@NC and MoCx@NC with that of MoNix–MoCx@NC. As shown in Fig. S4,† NC possesses a uniform hollow nanospherical morphology with the hollow core of 25 nm in diameter. The formation of hollow nanostructures can be ascribed to the confined interfacial copolymerization of pyrrole and aniline at the existence of Triton X-100 micelles.48 After the introduction of molybdate into the reaction system, molybdate diffuses into various positions of the polymer, forming strong interaction with the polymer and homogeneously dispersing throughout the whole nanospheres. After pyrolysis at high temperature, a unique pitaya-like morphology is formed with ultrathin carbon layers as peel and MoCx as seeds (Fig. 3e). As for Ni@NC, the introduction of nickel salt into polymer nanospheres has no obvious effect on the hollow nanostructures and metallic Ni is homogeneously encapsulated by carbon layers, indicating the stable properties of the carbon framework (Fig. 3f). So it can be easily speculated that the added molybdate that promotes the formation of the pitaya-like morphology and nickel salt have a confined reaction within the carbon layers.
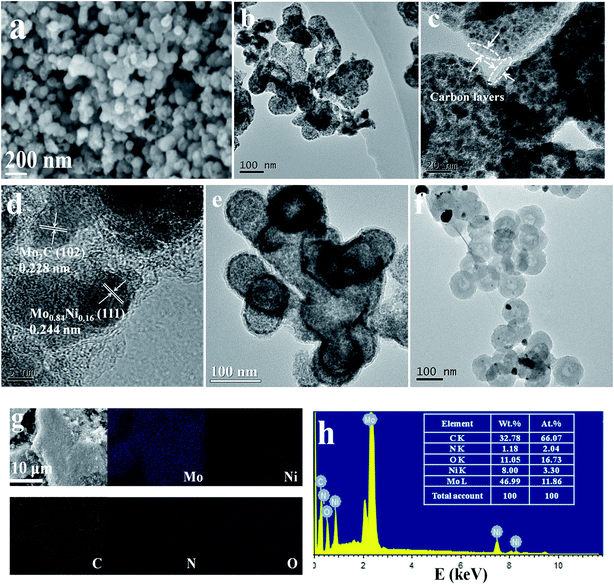 | ||
| Fig. 3 (a) SEM image, (b, c) TEM images and (d) HRTEM image of MoNix–MoCx@NC. (e) TEM image of MoCx@NC. (f) TEM image of Ni@NC. (g) SEM mapping and (h) EDX of MoNix–MoCx@NC. | ||
Elemental mapping is conducted to investigate the elemental distribution of the Ni@NC, MoCx@NC and MoNix–MoCx@NC. It can be seen from Fig. S5a and b† that Ni, C, N and O elements for Ni@NC and Mo, C, N and O for MoCx@NC are homogeneously dispersed on the surface of the samples, respectively. The elemental mapping of MoNix–MoCx@NC exhibits that Mo, Ni, C, N and O elements are evenly distributed over the whole carbon matrix (Fig. 3g). The EDX spectrum (Fig. 3h) also demonstrates that the MoNix–MoCx@NC is composed of Mo, Ni, C, N and O elements, and Mo and Ni elements account for an atomic percentage of 11.86% and 3.30%, respectively. The EDX spectra (Fig. S6a and b†) of Ni@NC and MoCx@NC are also displayed to clearly show the composites of the samples.
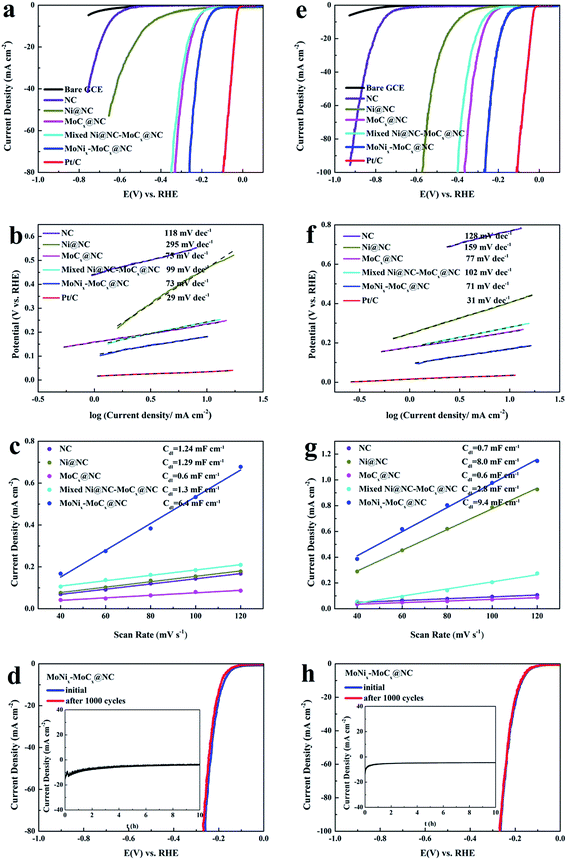
The electrocatalytic HER activities of MoNix–MoCx@NC in 0.5 M H2SO4 is tested. For comparison, the HER activities of commercial 20 wt% Pt/C, a bare GCE, NC, Ni@NC, MoCx@NC and mixed Ni@NC-MoCx@NC are also investigated. In the polarization curves (Fig. 4a), the bare GCE has no noticeable HER activity, while the Pt/C exhibits superior HER activity with a nearly zero onset overpotential. The MoNix–MoCx@NC also presents better performance with a small overpotential of 172 mV to drive a current density of 10 mA cm−2 in 0.5 M H2SO4, which is lower than those of Ni@NC (462 mV), MoCx@NC (233 mV) and mixed Ni@NC–MoCx@NC (241 mV). Such a low overpotential compares favorably to many of the currently reported molybdenum carbide-based electrocatalysts for the HER in acidic media (Table S1†). This result implies that a synergistic effect exists between MoNix and MoCx. The Ni with larger electronegativity could easily obtain electrons from the surrounding Mo atoms, resulting in a more positive charge of Mo atoms. This synergistic effect that Ni acts as an electron acceptor and MoCx acts as an electron donor could decrease ΔGH* and in turn promote the release of H2. So the incorporation of Ni into the MoCx@NC can not only optimize the ΔGH* but also improve the conductivity of MoCx@NC, leading to an enhanced HER activity.14,49
In addition, the Tafel slope determined from the LSV via the Tafel equation (η = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j + a, where η is the overpotential, b is the Tafel slope and j is the current density) is an inherent property of the catalyst, which is used to elucidate the possible electrocatalytic HER kinetics. As shown in Fig. 4b, the Tafel slope for MoNix–MoCx@NC is 73 mV dec−1, suggesting that hydrogen evolution on MoNix–MoCx@NC should obey the Volmer–Heyrovsky mechanism. The Tafel slope of MoNix–MoCx@NC is significantly lower than those of the Ni@NC (295 mV dec−1), MoCx@NC (75 mV dec−1) and Mixed Ni@NC–MoCx@NC (99 mV dec−1), demonstrating the faster HER kinetics of the MoNix–MoCx@NC. EIS data of all the samples are also obtained to gain a further insight into the HER kinetics of the catalysts (Fig. S7†). By simulating an equivalent circuit as shown in the inset of Fig. S7a,† the charge transfer resistance (Rct) of all samples in Table S2† shows that MoNix–MoCx@NC has a much smaller Rct value of 47.38 Ω compared to those of Ni@NC (2612 Ω), MoCx@NC (86.89 Ω) and mixed Ni@NC–MoCx@NC (91.37 Ω), suggesting a faster charge transfer rate and enhanced HER performance of MoNix–MoCx@NC. The lowest impedance exhibited by the hybrids of MoNix–MoCx@NC may be ascribed to the adulteration of NC and MoNix alloys largely improving the charge transfer rate. Moreover, the electrochemical surface area (ECSA) of all the samples can be obtained by calculating the double-layer capacitance (Cdl) values. Typical CV results of MoNix–MoCx@NC with various scan rates from 40 to 120 mV s−1 are shown in Fig. S8a.† The measured Cdl for MoNix–MoCx@NC is calculated to be 6.4 mF cm−2, larger than those of NC (1.24 mF cm−2), Ni@NC (1.29 mF cm−2), MoCx@NC (0.6 mF cm−2) and Mixed Ni@NC–MoCx@NC (1.3 mF cm−2), revealing the higher surface area and more exposed active sites of MoNix–MoCx@NC (Fig. 4c).
j + a, where η is the overpotential, b is the Tafel slope and j is the current density) is an inherent property of the catalyst, which is used to elucidate the possible electrocatalytic HER kinetics. As shown in Fig. 4b, the Tafel slope for MoNix–MoCx@NC is 73 mV dec−1, suggesting that hydrogen evolution on MoNix–MoCx@NC should obey the Volmer–Heyrovsky mechanism. The Tafel slope of MoNix–MoCx@NC is significantly lower than those of the Ni@NC (295 mV dec−1), MoCx@NC (75 mV dec−1) and Mixed Ni@NC–MoCx@NC (99 mV dec−1), demonstrating the faster HER kinetics of the MoNix–MoCx@NC. EIS data of all the samples are also obtained to gain a further insight into the HER kinetics of the catalysts (Fig. S7†). By simulating an equivalent circuit as shown in the inset of Fig. S7a,† the charge transfer resistance (Rct) of all samples in Table S2† shows that MoNix–MoCx@NC has a much smaller Rct value of 47.38 Ω compared to those of Ni@NC (2612 Ω), MoCx@NC (86.89 Ω) and mixed Ni@NC–MoCx@NC (91.37 Ω), suggesting a faster charge transfer rate and enhanced HER performance of MoNix–MoCx@NC. The lowest impedance exhibited by the hybrids of MoNix–MoCx@NC may be ascribed to the adulteration of NC and MoNix alloys largely improving the charge transfer rate. Moreover, the electrochemical surface area (ECSA) of all the samples can be obtained by calculating the double-layer capacitance (Cdl) values. Typical CV results of MoNix–MoCx@NC with various scan rates from 40 to 120 mV s−1 are shown in Fig. S8a.† The measured Cdl for MoNix–MoCx@NC is calculated to be 6.4 mF cm−2, larger than those of NC (1.24 mF cm−2), Ni@NC (1.29 mF cm−2), MoCx@NC (0.6 mF cm−2) and Mixed Ni@NC–MoCx@NC (1.3 mF cm−2), revealing the higher surface area and more exposed active sites of MoNix–MoCx@NC (Fig. 4c).
The durability of the MoNix–MoCx@NC catalyst is also investigated to evaluate the HER performance of the catalyst (Fig. 4d). After continuous CV for 1000 cycles at a scan rate of 100 mV s−1, MoNix–MoCx@NC exhibits almost the same polarization curve as the initial plot with only negligible current density degradation. In addition, in the i–t test presented in the inset of Fig. 4d, the current density of MoNix–MoCx@NC remains nearly unchanged even after 10 h. Moreover, the structure of MoNix–MoCx@NC after the 1000 CV test in 0.5 M H2SO4 is investigated by TEM (Fig. S9a†). As expected, MoNix–MoCx@NC maintains an intact pitaya-like morphology without obvious aggregation, further indicating the stable structure of the MoNix–MoCx@NC nanospheres. The results demonstrate that the MoNix–MoCx@NC is an efficient and stable catalyst towards the HER. The excellent stability of MoNix–MoCx@NC may be ascribed to the N-doped carbon layer encapsulation that can effectively prevent the corrosion of active sites in harsh acidic media.
Importantly, active and durable electrocatalysts which can operate in a water-alkali environment is also highly demanded.50 In this regard, the HER performance of the MoNix–MoCx@NC hybrids in 1.0 M KOH is investigated. As expected, the obtained MoNix–MoCx@NC also exhibits good catalytic performance in 1.0 M KOH, affording a current density of 10 mA cm−2 at an overpotential of only 168 mV, which is much lower than those of the contrastive samples (Fig. 4e). It is worth noting that such a high catalytic activity in basic media exceeds those of many recently reported molybdenum carbide-based HER electrocatalysts (Table S1†). Moreover, MoNix–MoCx@NC shows a lower Tafel slope of 71 mV per decade, lower than those of the other contrastive catalysts [NC (128 mV per decade), Ni@NC (159 mV per decade), MoCx@NC (159 mV per decade) and mixed Ni@NC–MoCx@NC (159 mV per decade, Fig. 4f)]. It could also be seen that MoNix–MoCx@NC exhibits a lower Rct of 19.23 Ω and a larger Cdl of 9.4 mF cm−2 (Fig. 4g, S7b, and Table S2†). Stability tests by CV or i–t plots both demonstrate the high durability of MoNix–MoCx@NC in basic solution (Fig. 4h). Moreover, the TEM image after the 1000 CV test in 1 M KOH also confirms the stable pitaya-like structure of MoNix–MoCx@NC (Fig. S9b†). To reveal the protection effect of N-doped carbon layers, we have compared the stability of bulk Mo2C with that of MoNix–MoCx@NC. It can be seen from Fig. S10a and b† that bulk Mo2C experiences severe current degradation after continuous 1000 CV cycles in both acidic and alkaline media, so NC layers play a critical role in protecting active sites from corrosion under harsh conditions. Overall, electrochemical measurements in acidic and basic solution both demonstrate the remarkable HER electrocatalytic activity and high stability of MoNix–MoCx@NC.
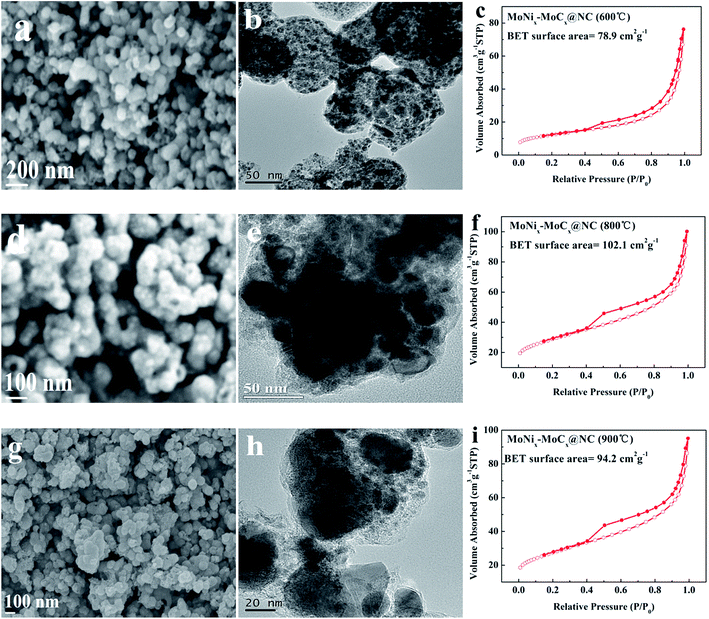
To reveal the relationship between the nanostructures and the activity of the MoNix–MoCx@NC hybrids, a series of relevant samples are prepared by varying the carbonization temperature, and their properties are examined. Fig. S11–S13† and 5 exhibit the XRD, Raman, SEM, TEM, EDX and BET results of MoNix–MoCx@NC obtained at various carbonization temperatures. It can be seen from Fig. S11† that the patterns for the MoNix–MoCx@NC series samples are all composed of hybrids of β-Mo2C (PDF no. 01-071-0242), MoC (PDF no. 03-065-6664), Mo0.84Ni0.16 (PDF no. 00-050-1270) and MoNi4 (PDF no. 00-003-1036) phases but with different peak intensities. So there exists an optimum ratio of MoNix/MoCx for the MoNix–MoCx@NC series samples to serve as efficient catalysts for the HER. The Raman results show that MoNix–MoCx@NC (700 °C) has a larger value of ID/IG than other samples, indicating that the carbon matrix is more disordered with richer structural defects, which may be beneficial for enhanced HER activity (Fig. S12a–c†). The SEM and TEM images of MoNix–MoCx@NC (600 °C) in Fig. 5a and b show that MoNix and MoCx nanoparticles are encapsulated by carbon nanospheres with a typical pitaya-like morphology and uniformly dispersed in the carbon matrix. MoNix–MoCx@NC (600 °C) has a specific surface area of 78.9 m2 g−1 (Fig. 5c), smaller than that of MoNix–MoCx@NC (700 °C), possibly because thick NC layers are produced without rich nanopores and MoNix–MoCx active sites are not well exposed when the carbonization reaction occurs at low temperature. Furthermore, Fig. 5d–i exhibit SEM, TEM and BET results of MoNix–MoCx@NC obtained at a carbonization temperature of 800 °C and 900 °C, respectively. As the carbonization temperature increases to 800 °C and 900 °C, the pitaya-like morphology is slightly destroyed, and the size shrinks to ∼80 nm and the nanoparticles obviously tend to aggregate. This phenomenon is attributed to the decreased BET surface area at such a high carbonization temperature. The XRD results show that the ratio of MoNix/MoCx also changes with the increase of temperature, which may be responsible for the different HER performance of MoNix–MoCx@NC obtained at various temperatures (Fig. S11†). EDX measurements are also conducted to compare the atomic percentages of N element in the MoNix–MoCx@NC series samples, because N doping with an increased disorder is beneficial to an enhanced activity and the existence of pyridinic N is beneficial for improving the conductivity of catalysts.51,52 EDX spectra in Fig. S13† show that the amount of N element decreases with an increased carbonization temperature. So an appropriate carbonization temperature is of vital importance for the preparation of element-doped MoCx-based materials for the HER.
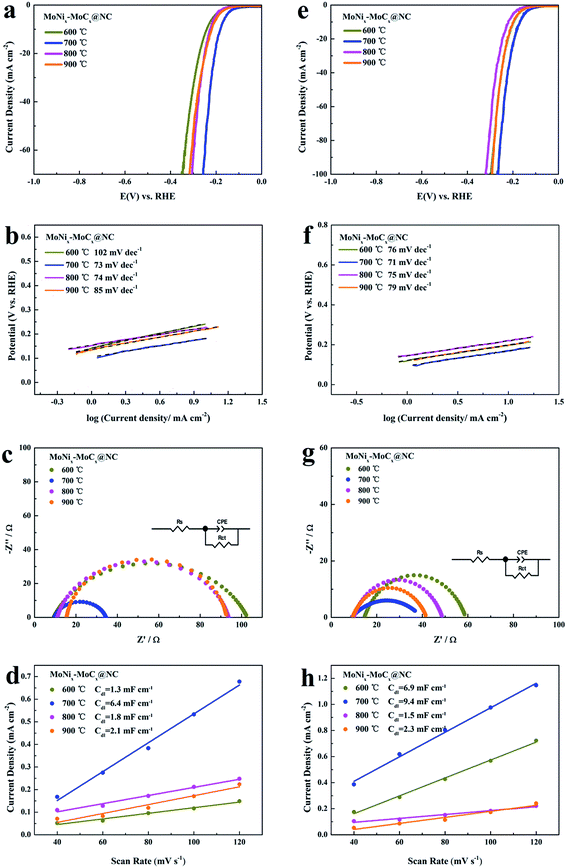
The HER performance of the pitaya-like MoNix–MoCx@NC series samples is evaluated under both acidic and alkaline conditions. The overpotentials of MoNix–MoCx@NC obtained at 600 °C, 800 °C and 900 °C in 0.5 M H2SO4 reaching a current density of 10 mA cm−2 are 237, 217 and 210 mV, respectively, and the Tafel slopes are 102, 74 and 85 mV dec−1, respectively (Fig. 6a and b), indicating their inferior catalytic performance to MoNix–MoCx@NC (700 °C). Fig. 6c–d and Table S3† show that MoNix–MoCx@NC (600 °C), MoNix–MoCx@NC (800 °C) and MoNix–MoCx@NC (900 °C) exhibit a larger charge transfer rate and smaller Cdl than MoNix–MoCx@NC (700 °C). Fig. 6e–h exhibit the HER activity of the MoNix–MoCx@NC series samples in 1.0 M KOH. The HER performance is in good agreement with the results obtained in acidic solution. MoNix–MoCx@NC (700 °C) still exhibits excellent activity with a lower overpotential, smaller Tafel slope, lower charge transfer resistance and larger Cdl in 1.0 M KOH, which is superior to the other contrastive samples. Among these catalysts, the MoNix–MoCx@NC (700 °C) proves to be a robust efficient catalyst in both acidic and alkaline solution, possibly because MoNix–MoCx@NC (700 °C) integrates the advantage of the optimal ratio of MoNix/MoCx, rich defects, a large BET surface area and a high percentage of N element. Therefore, the selection of the carbonization temperature is essential to form highly active catalysts for the HER.
Based on the above experimental data, the remarkable HER performance of MoNix–MoCx@NC in both acidic and alkaline media can be attributed to the following reasons: (1) Ni2+–MoO42−–polyaniline/pyrrole precursors prevent excessive growth and severe stacking of Ni and Mo2C and possess a porous structure during the carburization process, benefitting the well dispersion of Ni and Mo2C coated with NC. (2) The large surface area (136.2 m2 g−1) of the MoNix–MoCx@NC is beneficial for exposing rich active sites and accelerating mass transfer among catalysts and electrolytes. (3) Because C atoms adjacent to the N atoms are the most active sites in MoNix–MoCx@NC, the Ni, Mo2C and N dopants synergistically co-activate adjacent C atoms in the carbon materials, leading to the variation in the electronic structures of the catalysts and making MoNix–MoCx@NC an efficient catalyst towards the HER.
Conclusions
In summary, well-defined pitaya-like N-doped hybrids of MoNix–MoCx@C have been developed as an excellent HER electrocatalyst via Ni ion doped organic–inorganic polymerization followed by a carbonization process. The existence of Ni ions and MoO42− in the polymer network promotes the formation of pitaya-like Mo2C@NC nanospheres, and MoNix and MoCx nanocrystals are well-dispersed in the ultrathin NC layers, which may provide a triple synergistic effect for improving the activity and conductivity of MoCx. The conductive NC shell encapsulation not only protects the active sites from agglomeration and corrosion under harsh conditions but also leads to the variation in the electronic structures of the catalysts. The strategy for synthesizing unique core–shell nanostructures may open up new opportunities to design multiple synergistic effects from excellent catalytic interface for an efficient HER.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the Shandong Provincial Natural Science Foundation (ZR2017MB059), the National Natural Science Foundation of China (21776314) and the Fundamental Research Funds for the Central Universities (18CX05016A).References
- X. X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- Y. Shi and B. Zhang, Chem. Soc. Rev., 2016, 45, 1529–1541 RSC.
- S. Wang, J. Wang, M. Zhu, X. Bao, B. Xiao, D. Su, H. Li and Y. Wang, J. Am. Chem. Soc., 2015, 137, 15753–15759 CrossRef PubMed.
- X. Shang, K. L. Yan, S. S. Lu, B. Dong, W. K. Gao, J. Q. Chi, Z. Z. Liu, Y. M. Chai and C. G. Liu, J. Power Sources, 2017, 363, 44–53 CrossRef.
- Y. Chen, G. Yu, W. Chen, Y. Liu, G. D. Li, P. Zhu, Q. Tao, Q. Li, J. Liu, X. Shen, H. Li, X. Huang, D. Wang, T. Asefa and X. X. Zou, J. Am. Chem. Soc., 2017, 139, 12370–12373 CrossRef PubMed.
- B. Dong, X. Zhao, G. Q. Han, X. Li, X. Shang, Y. R. Liu, W. H. Hu, Y. M. Chai, H. Zhao and C. G. Liu, J. Mater. Chem. A, 2016, 4, 13499–13508 Search PubMed.
- J. Wang, Z. Wei, S. Mao, H. Li and Y. Wang, Energy Environ. Sci., 2018, 11, 800–806 Search PubMed.
- X. Shang, K. L. Yan, Y. Rao, B. Dong, J. Q. Chi, Y. R. Liu, X. Li, Y. M. Chai and C. G. Liu, Nanoscale, 2017, 9, 12353–12363 RSC.
- G. Han, Y. H. Jin, R. A. Burgess, N. E. Dickenson, X. M. Cao and Y. Sun, J. Am. Chem. Soc., 2017, 139, 15584–15587 CrossRef PubMed.
- J. Q. Chi, K. L. Yan, Z. Xiao, B. Dong, X. Shang, W. K. Gao, X. Li, Y. M. Chai and C. G. Liu, Int. J. Hydrogen Energy, 2017, 42, 20599–20607 CrossRef.
- J. Zhang, T. Wang, P. Liu, Z. Liao, S. Liu, X. Zhuang, M. Chen, E. Zschech and X. Feng, Nat. Commun., 2017, 8, 15437 CrossRef PubMed.
- Y. Jin, X. Yue, C. Shu, S. Huang and P. Shen, J. Mater. Chem. A, 2017, 5, 2508–2513 Search PubMed.
- W. J. Zhou, J. Jia, J. Lu, L. Yang, D. Hou, G. Li and S. Chen, Nano Energy, 2016, 28, 29–43 CrossRef.
- Y. Liu, G. Yu, G. D. Li, Y. Sun, T. Asefa, W. Chen and X. X. Zou, Angew. Chem., Int. Ed., 2015, 54, 10752–10757 CrossRef PubMed.
- H. Lin, N. Liu, Z. Shi, Y. Guo, Y. Tang and Q. Gao, Adv. Funct. Mater., 2016, 26, 5590–5598 CrossRef.
- J. Chi, X. Shang, S. Lu, B. Dong, Z. Liu, K. Yan, W. Gao, Y. Chai and C. Liu, Carbon, 2017, 124, 555–564 CrossRef.
- K. L. Yan, J. F. Qin, Z. Z. Liu, B. Dong, J. Q. Chi, W. K. Gao, J. H. Lin, Y. M. Chai and C. G. Liu, Chem. Eng. J., 2018, 334, 922–931 CrossRef.
- X. Shang, W. H. Hu, X. Li, B. Dong, Y. R. Liu, G. Q. Han, Y. M. Chai and C. G. Liu, Electrochim. Acta, 2017, 224, 25–31 CrossRef.
- Q. Yue, Y. Wan, Z. Sun, X. Wu, Y. Yuan and P. Du, J. Mater. Chem. A, 2015, 3, 16941–16947 Search PubMed.
- Z. C. Xing, Q. Liu, A. M. Asiri and X. P. Sun, Adv. Mater., 2014, 26, 5702–5707 CrossRef PubMed.
- B. Hinnemann, P. Moses, J. Bonde, K. Jørgensen, J. Nielsen, S. Horch, I. Chorkendorff and J. Nørskov, J. Am. Chem. Soc., 2005, 127, 5308–5309 CrossRef PubMed.
- P. Tran, T. Tran, M. Orio, S. Torelli, Q. Truong, K. Nayuki, Y. Sasaki, S. Chiam, R. Yi, I. Honma, J. Barber and V. Artero, Nat. Mater., 2016, 15, 640–646 CrossRef PubMed.
- X. Wang, R. Su, H. Aslan, J. Kibsgaard, S. Wendt, L. Meng, M. Dong, Y. Huang and F. Besenbacher, Nano Energy, 2015, 12, 9–18 CrossRef.
- Y. Chen, Y. Zhang, W. Jiang, X. Zhang, Z. Dai, L. Wan and J. Hu, ACS Nano, 2016, 10, 8851–8860 CrossRef PubMed.
- C. Wan, Y. N. Regmi and B. M. Leonard, Angew. Chem., Int. Ed., 2014, 53, 6407–6410 CrossRef PubMed.
- Z. Shi, K. Nie, Z. Shao, B. Gao, H. Lin, H. Zhang, B. Liu, Y. Wang, Y. Zhang, X. Sun, X. Cao, P. Hu, Q. Gao and Y. Tang, Energy Environ. Sci., 2017, 10, 1262–1271 Search PubMed.
- H. Ang, H. T. Tan, Z. M. Luo, Y. Zhang, Y. Y. Guo, G. Guo, H. Zhang and Q. Yan, Small, 2015, 11, 6278–6284 CrossRef PubMed.
- R. Ma, Y. Zhou, Y. Chen, P. Li, Q. Liu and J. Wang, Angew. Chem., Int. Ed., 2015, 54, 14723–14727 CrossRef PubMed.
- Y. Zhao, K. Kamiya, K. Hashimoto and S. Nakanishi, J. Am. Chem. Soc., 2015, 137, 110–113 CrossRef PubMed.
- Z. H. Pu, M. Wang, Z. K. Kou, I. S. Amiinu and S. C. Mu, Chem. Commun., 2016, 52, 12753–12756 RSC.
- K. Zhang, Y. Zhao, D. Fu and Y. Chen, J. Mater. Chem. A, 2015, 3, 5783–5788 Search PubMed.
- D. H. Youn, S. Han, J. Y. Kim, J. Y. Kim, H. Park, S. H. Choi and J. S. Lee, ACS Nano, 2014, 8, 5164–5173 CrossRef PubMed.
- J. S. Li, Y. Wang, C. H. Liu, S. L. Li, Y. G. Wang, L. Z. Dong, Z. H. Dai, Y. F. Li and Y. Q. Lan, Nat. Commun., 2016, 7, 11204 CrossRef PubMed.
- L. Huo, B. Liu, G. Zhang and J. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 18107–18118 Search PubMed.
- Y. Huang, Q. Gong, X. Song, K. Feng, K. Nie, F. Zhao, Y. Wang, M. Zeng, J. Zhong and Y. Li, ACS Nano, 2016, 10, 11337–11343 CrossRef PubMed.
- J. Chi, W. Gao, J. Lin, B. Dong, J. Qin, Z. Liu, B. Liu, Y. Chai and C. Liu, J. Catal., 2018, 360, 9–19 CrossRef.
- H. Lin, Z. Shi, S. He, X. Yu, S. Wang, Q. Gao and Y. Tang, Chem. Sci., 2016, 7, 3399–3405 RSC.
- I. Abidat, C. Morais, S. Pronier, N. Guignard, J. Comparot, C. Canaff, T. Napporn, A. Habrioux, A.-S. Mamede, J.-F. Lamonier and K. B. Kokoh, Carbon, 2017, 111, 849–858 CrossRef.
- Y. J. Tang, M. R. Gao, C. H. Liu, S. L. Li, H. L. Jiang, Y. Q. Lan, M. Han and S. H. Yu, Angew. Chem., Int. Ed., 2015, 54, 12928–12932 CrossRef PubMed.
- W. Cui, N. Cheng, Q. Liu, C. Ge, A. M. Asiri and X. Sun, ACS Catal., 2014, 4, 2658–2661 CrossRef.
- M. Xiang, D. Li, W. Li, B. Zhong and Y. Sun, Catal. Commun., 2007, 8, 513–518 CrossRef.
- X. Xu, F. Nosheen and X. Wang, Chem. Mater., 2016, 28, 6313–6320 CrossRef.
- Y. Zhang, Y. Wang, S. Jia, H. Xu, J. Zang, J. Lu and X. Xu, Electrochim. Acta, 2016, 222, 747–754 CrossRef.
- X. Li, Y. Fang, X. Lin, M. Tian, X. An, Y. Fu, R. Li, J. Jin and J. Ma, J. Mater. Chem. A, 2015, 3, 17392–17402 Search PubMed.
- A. Bhaskar, M. Deepa and T. Narasinga Rao, ACS Appl. Mater. Interfaces, 2013, 5, 2555–2566 Search PubMed.
- H. Yan, C. Tian, L. Wang, A. Wu, M. Meng, L. Zhao and H. Fu, Angew. Chem., Int. Ed., 2015, 54, 6325–6329 CrossRef PubMed.
- B. Cao, G. M. Veith, J. C. Neuefeind, R. R. Adzic and P. G. Khalifah, J. Am. Chem. Soc., 2013, 135, 19186–19192 CrossRef PubMed.
- C. Q. Zhou, J. Han, G. P. Song and R. Guo, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3563–3572 CrossRef.
- K. Xiong, L. Li, L. Zhang, W. Ding, L. Peng, Y. Wang, S. Chen, S. Tan and Z. Wei, J. Mater. Chem. A, 2015, 3, 1863–1867 Search PubMed.
- L. L. Ji, J. Y. Wang, L. X. Guo and Z. F. Chen, J. Mater. Chem. A, 2017, 5, 5178–5186 Search PubMed.
- H. B. Yang, J. W. Miao, S. F. Hung, J. Z. Chen, H. B. Tao, X. Z. Wang, L. P. Zhang, R. Chen, J. J. Gao, H. M. Chen, L. M. Dai and B. Liu, Sci. Adv., 2016, 2, e1501122 Search PubMed.
- J. D. Wiggins-Camacho and K. J. Stevenson, J. Phys. Chem. C, 2009, 113, 19082–19090 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8se00135a |
| This journal is © The Royal Society of Chemistry 2018 |