Optimal synthetic conditions for a novel and high performance Ni-rich cathode material of LiNi0.68Co0.10Mn0.22O2†
Xing
Li
 *a,
Kangjia
Zhang
a,
Siyuan
Wang
a,
Mingshan
Wang
*a,
Fei
Jiang
a,
Yang
Liu
a,
Yun
Huang
a and
Jianming
Zheng
*b
*a,
Kangjia
Zhang
a,
Siyuan
Wang
a,
Mingshan
Wang
*a,
Fei
Jiang
a,
Yang
Liu
a,
Yun
Huang
a and
Jianming
Zheng
*b
aThe Center of New Energy Materials and Technology, Southwest Petroleum University, Xindu Road 8, Chengdu, Sichuan 610500, China. E-mail: lixing@swpu.edu.cn; wangmingshan@swpu.edu.cn
bResearch Institute (RI), NingDe Amperex Technology Limited, Ningde, Fujian 352100, China. E-mail: ZhengJM@ATLBattery.com
First published on 1st June 2018
Abstract
Layered Ni-rich oxides (LiNixCoyMnzO2) are considered as the most promising cathode materials for lithium ion batteries because of their high discharge capacity, high Li+ ion deintercalation/intercalation potential, and low cobalt content. However, because of the similar ionic radius of Li+ (0.76 Å) and Ni2+ (0.69 Å), the Ni-rich cathodes often suffer from poor cycling stability because of the serious cation mixing, and the poor interfacial/structural stability during the electrochemical process. In this work, the effects of sintering temperature, sintering time and excess lithium amount on the structure, morphology and electrochemical performance of a novel spherical high Ni-rich cathode material LiNi0.68Co0.10Mn0.22O2 cathode are systematically investigated. The results indicate that a sintering temperature of 780 °C with a sintering time of 16 h and an excess lithium amount of 5 wt% could achieve a more stable and lower cation mixing degree LiNi0.68Co0.10Mn0.22O2 cathode. It delivers a reversible discharge capacity as high as 197.4 mA h g−1 at C/10, and exhibits a capacity retention of 95.9%, 90.2% and 83.5% at C/3, 1C and 3C after 200 cycles at cut-off voltages of 2.7–4.4 V, respectively. These results demonstrate that the optimized LiNi0.68Co0.10Mn0.22O2 is a promising cathode material for high energy density lithium ion batteries.
1. Introduction
With the rapid development of consumer electronic devices and pure electric vehicles (EVs),1 the high volumetric and gravimetric energy densities of lithium-ion batteries (LIBs) are urgently needed to be increased, so as to boost the service time per charge.2–5 High energy density LIBs certainly pose a strong demand for high energy density electrode active materials.6,7 Ni-rich layered oxides (LiNixCoyMnzO2, NCM) have been regarded as the most promising cathode candidates for constructing high energy density LIBs because of their high reversible capacity, high Li+ ion intercalation/deintercalation potential, low cobalt content and thus low production cost.8–12 Actually, since Yabuuchi and Ohziku reported the high performance of the layered LiNi1/3Co1/3Mn1/3O2 cathode material in 2003,13 a series of Ni-rich layered cathode materials, such as LiNi0.4Co0.2Mn0.4O2,14–16 LiNi0.5Co0.2Mn0.3O2,17–19 LiNi0.6Co0.2Mn0.2O2,20–22 and LiNi0.8Co0.1Mn0.1O2 (ref. 21, 23 and 24), have been well developed. Previous research demonstrates that increasing the Ni content in the compounds is favorable to improve the charge/discharge capacity of Ni-rich layered cathode materials because of the presence of multivalent states of Ni during the electrochemical process.25 In fact, during the Li+ extraction/intercalation, there are two chemical transformations of Ni from Ni2+ to Ni3+ and then to Ni4+, and their reversible processes, which could deliver more charge/discharge capacity. Of course, the transition between Co3+ ↔ Co4+ also contributes to the capacity, while Mn maintains the Mn4+ state to keep the structural stability of the cathode material. Unfortunately, the high Ni-rich layered cathode materials LiNixCoyMnzO2 (x > 0.6) often suffer from serious cation mixing and a thermodynamically unstable surface microstructure because of the similar ionic radius of Li+ (0.76 Å) and Ni2+ (0.69 Å),26–29 which may result in undesired phase transformation and hence poor cycling stability. For example, for LiNi0.8Co0.1Mn0.1O2, though it exhibits a reversible discharge specific capacity as high as 200 mA h g−1,30,31 it experiences serious capacity degradation and poor cycling stability.32–35 A similar phenomenon can also be found in the LiNi0.85Co0.075Mn0.075O2 cathode material with higher Ni content.36 These results further indicate that though increasing the Ni content is favorable to improve the specific discharge capacity, the capacity retention, the safety characteristics and the phase stability would sharply deteriorate especially when the content of Ni ≥ 0.8.37–39For NCM cathode materials, the high Ni-rich content could contribute to a high capacity, while Co is useful for the rate performance and processing ability, and Mn is helpful to maintain the structural stability of the α-NaFeO2 phase.40,41 It seems that there is an optimal value for the content of Ni, Co and Mn in the high capacity Ni-rich LiNixCoyMnzO2 (x > 0.6) cathode materials. In this work, we designed and synthesized a novel spherical high Ni-rich cathode material LiNi0.68Co0.10Mn0.22O2 through a co-precipitation approach. We kept its cobalt content in line with LiNi0.8Co0.1Mn0.1O2, and adopt the ratio of Ni and Mn as 0.68 and 0.22, to balance the energy density and thermal stability of the Ni-rich cathode material. The effects of sintering temperature, sintering time and excess lithium amount on the structure, morphology and electrochemical performance of the as-prepared LiNi0.68Co0.10Mn0.22O2 cathode were systematically investigated. It was found that the LiNi0.68Co0.10Mn0.22O2 cathode prepared under the optimized conditions could deliver a reversible capacity of 197.4 mA h g−1 (almost as high as 200 mA h g−1 of LiNi0.8Co0.1Mn0.1O2). Moreover, it also presents more excellent cycling stability and rate capability. The fundamental mechanisms underlying the superior electrochemical performance were also investigated and discussed in detail in this work.
2. Experimental
2.1 Materials synthesis
A spherical Ni0.68Co0.10Mn0.22(OH)2 compound was synthesized by a co-precipitation method.33 The experimental process is briefly introduced here: first, a mixed aqueous solution with a concentration of 2.0 mol L−1 consisting of NiSO4, CoSO4, and MnSO4 (Ni2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co2+
Co2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn2+ = 0.68
Mn2+ = 0.68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.10
0.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.22, molar ratio) was prepared in a container. Then, NH3·H2O (10 mol L−1) and NaOH (4 mol L−1) solutions were added dropwise into the as prepared mixed solution. This process was performed in a N2 atmosphere and the solution was kept under pH = 11.5 with vigorous stirring. Finally, the spherical Ni0.68Co0.10Mn0.22(OH)2 precursor was obtained via filtering and thoroughly washing with deionized water.
0.22, molar ratio) was prepared in a container. Then, NH3·H2O (10 mol L−1) and NaOH (4 mol L−1) solutions were added dropwise into the as prepared mixed solution. This process was performed in a N2 atmosphere and the solution was kept under pH = 11.5 with vigorous stirring. Finally, the spherical Ni0.68Co0.10Mn0.22(OH)2 precursor was obtained via filtering and thoroughly washing with deionized water.
The Ni0.68Co0.10Mn0.22(OH)2 precursor was then mixed with LiOH, and pre-calcined at 500 °C for 5 h in an air atmosphere. After intermediate grinding, it was further calcined at higher temperature for 12–20 hours under an oxygen atmosphere to obtain the final Ni-rich LiNi0.68Co0.10Mn0.22O2 product. Different excess lithium amounts, calcination temperatures and calcination times were systematically investigated to obtain the optimum synthetic conditions of the LiNi0.68Co0.10Mn0.22O2 cathode.
2.2 Materials characterization
The crystal structures of the samples were identified by powder X-ray diffraction (X-Pert PRO MPD PANalytical B.V. Netherlands) using Cu-Kα (wavelength 0.15046 nm) as radiation in the 2θ range from 10° to 80° with a step size of 0.04° and a step time of 5 s. The morphologies of the as prepared samples were observed by scanning electron microscopy (SEM, ZEISS-EVO-MA15 Germany). The particle size distribution of the samples was analyzed using a laser particle size analyzer (Master sizer 2000, Malvin instruments company British).2.3 Electrochemical measurements
The active material (LiNi0.68Co0.10Mn0.22O2), acetylene black and polyvinylidene difluoride (PVDF) binder were well mixed in a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in N-methyl-2-pyrrolidinone (NMP) to form a slurry, which then was pasted on aluminum foil, followed by drying at 90 °C for 16 h in a vacuum oven. The dried electrode was then cut into disks with a diameter of 14 mm and used as the cathode. The separator was Celgard 2400, the electrolyte was 1.0 M LiPF6/EC-DEC-DMC, and the anode was lithium foil. The CR2032 coin cell constructed with the as prepared cathode, the separator, the electrolyte and the lithium foil anode was employed to measure the electrochemical performance of LiNi0.68Co0.10Mn0.22O2. The CR2032 coin cells were assembled in an argon filled glovebox with the H2O/O2 content less than 0.1 ppm, and were tested on a BTS-5V20mA cell testing instrument (NEWARE Electronic Co., Ltd) at different current rates of C/10, C/3, 1C, 2C, 3C, 5C, 10C and 20C (1C = 200 mA g−1) in the voltage range of 2.7–4.4 V and at an environmental temperature of 30 °C. Cyclic voltammetry (CV) curves were recorded between 2.7 and 4.4 V with a scan rate of 0.1 mV s−1 and electrochemical impedance spectroscopy was performed from 100 kHz to 10 mHz with a potential amplitude of 5 mV using an electrochemical workstation of CHI 660D.
1 in N-methyl-2-pyrrolidinone (NMP) to form a slurry, which then was pasted on aluminum foil, followed by drying at 90 °C for 16 h in a vacuum oven. The dried electrode was then cut into disks with a diameter of 14 mm and used as the cathode. The separator was Celgard 2400, the electrolyte was 1.0 M LiPF6/EC-DEC-DMC, and the anode was lithium foil. The CR2032 coin cell constructed with the as prepared cathode, the separator, the electrolyte and the lithium foil anode was employed to measure the electrochemical performance of LiNi0.68Co0.10Mn0.22O2. The CR2032 coin cells were assembled in an argon filled glovebox with the H2O/O2 content less than 0.1 ppm, and were tested on a BTS-5V20mA cell testing instrument (NEWARE Electronic Co., Ltd) at different current rates of C/10, C/3, 1C, 2C, 3C, 5C, 10C and 20C (1C = 200 mA g−1) in the voltage range of 2.7–4.4 V and at an environmental temperature of 30 °C. Cyclic voltammetry (CV) curves were recorded between 2.7 and 4.4 V with a scan rate of 0.1 mV s−1 and electrochemical impedance spectroscopy was performed from 100 kHz to 10 mHz with a potential amplitude of 5 mV using an electrochemical workstation of CHI 660D.
3. Results and discussion
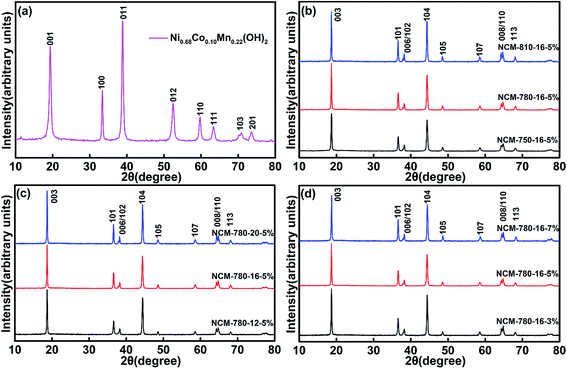
Fig. 1a shows the XRD pattern of the spherical Ni0.68Co0.10Mn0.22(OH)2 precursor, which demonstrates a typical M(OH)2 (M = metal ions) layered structure belonging to the space group p![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1. Fig. 1b presents the XRD patterns of the LiNi0.68Co0.10Mn0.22O2 samples prepared at different calcination temperatures (750 °C, 780 °C and 810 °C) with a fixed calcination time of 16 h and an excess lithium (Li) amount of 5%. The corresponding samples are labeled as NCM-750-16-5%, NCM-780-16-5% and NCM-810-16-5% in Fig. 1b. All the diffraction peaks presented in Fig. 1b could be indexed to the layered hexagonal structure of α-NaFeO2 with the space group R
m1. Fig. 1b presents the XRD patterns of the LiNi0.68Co0.10Mn0.22O2 samples prepared at different calcination temperatures (750 °C, 780 °C and 810 °C) with a fixed calcination time of 16 h and an excess lithium (Li) amount of 5%. The corresponding samples are labeled as NCM-750-16-5%, NCM-780-16-5% and NCM-810-16-5% in Fig. 1b. All the diffraction peaks presented in Fig. 1b could be indexed to the layered hexagonal structure of α-NaFeO2 with the space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. It is well established that the distinct splitting peaks of (006)/(102) and (108)/(110) indicate a well-ordered layered structure.38,42 The ratio of I(003)/I(104) reflects the cation mixing degree, and a low ratio of I(003)/I(104) less than 1.2 generally demonstrates a high degree of cation mixing.43,44 As shown in Table S1,† the sample of NCM-780-16-5% exhibits an I(003)/I(104) ratio of 1.339, which is much higher than 1.318 for NCM-810-16-5%, and 1.206 for NCM-750-16-5%. These results indicate that 780 °C is the optimized calcination temperature to achieve the smallest cation mixing degree. For the calcination temperature of 750 °C, it probably cannot provide enough thermodynamics energy to promote a well formed layered crystal structure, thus causing some Ni2+ to enter the Li+ sites and apparent cation mixing. Meanwhile, for the calcination temperature of 810 °C, the larger cation mixing degree might be ascribed to the lattice oxygen loss at the higher calcination temperature, hence leading to oxygen vacancies and a slight increase of Ni2+ in the crystal lattice, which is susceptible to the Li+/Ni2+ mixing.45–48
m. It is well established that the distinct splitting peaks of (006)/(102) and (108)/(110) indicate a well-ordered layered structure.38,42 The ratio of I(003)/I(104) reflects the cation mixing degree, and a low ratio of I(003)/I(104) less than 1.2 generally demonstrates a high degree of cation mixing.43,44 As shown in Table S1,† the sample of NCM-780-16-5% exhibits an I(003)/I(104) ratio of 1.339, which is much higher than 1.318 for NCM-810-16-5%, and 1.206 for NCM-750-16-5%. These results indicate that 780 °C is the optimized calcination temperature to achieve the smallest cation mixing degree. For the calcination temperature of 750 °C, it probably cannot provide enough thermodynamics energy to promote a well formed layered crystal structure, thus causing some Ni2+ to enter the Li+ sites and apparent cation mixing. Meanwhile, for the calcination temperature of 810 °C, the larger cation mixing degree might be ascribed to the lattice oxygen loss at the higher calcination temperature, hence leading to oxygen vacancies and a slight increase of Ni2+ in the crystal lattice, which is susceptible to the Li+/Ni2+ mixing.45–48
Fig. 1c exhibits the XRD patterns of LiNi0.68Co0.10Mn0.22O2 samples prepared under different calcination times (12 h, 16 h and 20 h) with a fixed calcination temperature of 780 °C and an excess lithium amount of 5%. The corresponding samples are labeled as NCM-780-12-5%, NCM-780-16-5% and NCM-780-20-5% in Fig. 1c. All the diffraction peaks shown in Fig. 1c could be indexed to the layered hexagonal structure of α-NaFeO2 with the space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. As shown in Table S2,† the sample of NCM-780-20-5% shows the largest I(003)/I(104) ratio of 1.346 and the sample of NCM-780-16-5% shows a similar ratio of 1.339, while the sample of NCM-780-12-5% presents the smallest ratio of 1.212. These results indicate that at the optimal calcination temperature, prolonging the calcination time is favorable for reducing the cation mixing, which might be attributed to the fact that a longer calcination time could achieve better crystallinity with well-defined layered characteristics. It is worth noting that the optimized calcination time is considered as 16 h based on the layered structure property and from the production cost point of view.
m. As shown in Table S2,† the sample of NCM-780-20-5% shows the largest I(003)/I(104) ratio of 1.346 and the sample of NCM-780-16-5% shows a similar ratio of 1.339, while the sample of NCM-780-12-5% presents the smallest ratio of 1.212. These results indicate that at the optimal calcination temperature, prolonging the calcination time is favorable for reducing the cation mixing, which might be attributed to the fact that a longer calcination time could achieve better crystallinity with well-defined layered characteristics. It is worth noting that the optimized calcination time is considered as 16 h based on the layered structure property and from the production cost point of view.
Fig. 1d presents the XRD patterns of LiNi0.68Co0.10Mn0.22O2 samples prepared with different excess Li amounts (3%, 5%, and 7%, molar percent) with a fixed calcination temperature of 780 °C and calcination time of 16 h. The corresponding samples are labeled as NCM-780-16-3%, NCM-780-16-5% and NCM-780-16-7% in Fig. 1d. All the diffraction peaks presented in Fig. 1d also could be indexed to the layered hexagonal structure of α-NaFeO2 with the space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. As shown in Table S3,† the sample of NCM-780-16-5% exhibits the largest I(003)/I(104) ratio of 1.339, while the samples of NCM-780-16-3% and NCM-780-16-7% show a relatively lower ratio of 1.151 and 1.247, respectively. A lower excess Li amount could not compensate for the Li+ vacancies arising from the high calcination temperature, while more excess Li amount might result in some Li entering the Ni2+ sites. Therefore, the optimal excess Li amount is determined to be 5%.
m. As shown in Table S3,† the sample of NCM-780-16-5% exhibits the largest I(003)/I(104) ratio of 1.339, while the samples of NCM-780-16-3% and NCM-780-16-7% show a relatively lower ratio of 1.151 and 1.247, respectively. A lower excess Li amount could not compensate for the Li+ vacancies arising from the high calcination temperature, while more excess Li amount might result in some Li entering the Ni2+ sites. Therefore, the optimal excess Li amount is determined to be 5%.
These results demonstrate that the optimized synthetic conditions for the LiNi0.68Co0.10Mn0.22O2 cathode are calcination at 780 °C under an oxygen atmosphere for 16 h with an excess Li amount of 5%, which could yield a more ordered hexagonal layered structure product with minimum cation mixing.
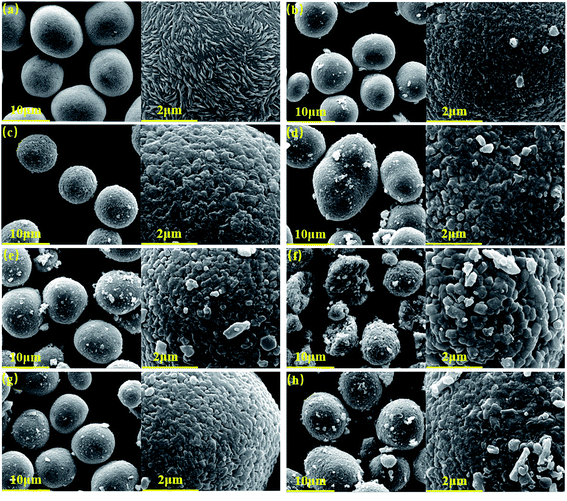
Fig. 2a presents the SEM images of the as prepared Ni0.68Co0.10Mn0.22(OH)2 precursor. The precursor exhibits a spherical micromorphology with an average particle size of ∼10 μm. Moreover, it is observed that the large spherical secondary particles are composed of nanosized rod-like particulates at higher magnification. Fig. 2b–d show the SEM images of the NCM-750-16-5%, NCM-780-16-5% and NCM-810-16-5%, respectively. With the increase of the calcination temperature, the spherical particle size grows and finally turns into an ellipsoid at a calcination temperature of 810 °C. Moreover, it can be observed that the optimized calcination temperature of 780 °C could achieve a more uniform and clearer surface of the as prepared LiNi0.68Co0.10Mn0.22O2. The white dots adhering to the as prepared products (Fig. 2b and d) are believed to be LiOH/Li2CO3 arising from the excess Li source, which is harmful to the electrochemical performance.49
Fig. 2e and f show the SEM images of NCM-780-12-5% and NCM-780-20-5%, respectively. In particular, from Fig. 2f, it can be found out that the too long calcination time could result in the increase of primary particle size and the crack of the secondary particle, which is not favorable for fast lithium ion diffusion, thus resulting in compromised electrochemical performance. Fig. 2g and h are the SEM images of the NCM-780-16-3% and NCM-780-16-7%, respectively. It seems that there are more nanosized particles residing on the surface of the LiNi0.68Co0.10Mn0.22O2 product, which indicates that the more excess Li amount could result in more LiOH/Li2CO3 residue on the surface of the as prepared product as shown in Fig. 2h.
The particle size distributions of the LiNi0.68Co0.10Mn0.22O2 samples synthesized under different conditions are shown in Fig. 3, where it can be observed that NCM-780-16-5% with the optimized synthetic conditions exhibits the most narrow particle size distribution. In fact, the narrow particle size distribution is favorable for the tap density, and the NCM-780-16-5% presents a tap density as high as 3.0 g cm−3. Moreover, Fig. 3 also demonstrates that the lower (750 °C)/higher (810 °C) calcination temperature, the shorter (12 h)/longer (20 h) calcination time and the less (3%)/more (7%) excess lithium amount are not favorable to get the narrow particle size distribution.
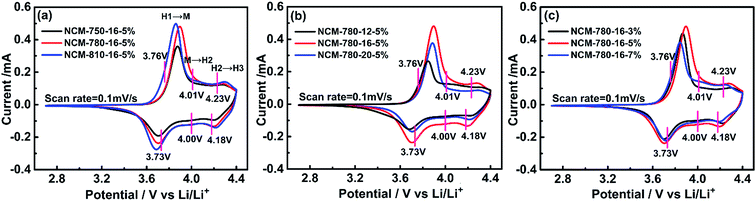
The electrochemical reaction kinetics of the as prepared cathode electrodes were investigated through recording the first cycle deintercalation/intercalation process at a scan rate of 0.1 mV s−1 between 2.7 and 0.4.4 V by cyclic voltammetry. As presented in Fig. 3, there are three distinct redox peaks located at 3.76/3.73 V, 4.01/4.00 V and 4.23/4.18 V, which correspond to the multiphase transitions of hexagonal to monoclinic (H1 → M), monoclinic to hexagonal (M → H2), and hexagonal to hexagonal (H2 → H3).50 This demonstrates that the Li+ deintercalation/intercalation mechanism for the as prepared LiNi0.68Co0.10Mn0.22O2 is similar to that of the reported LiNi0.8Co0.1Mn0.1O2 cathode.38 However, the redox peak at ∼3.6 V is not found for the LiNi0.68Co0.10Mn0.22O2 cathode as compared with the high Ni-rich LiNixCoyMnzO2 (x > 0.8), which demonstrates that the undesired first-order phase transition would not happen during the electrochemical cycling.50 Moreover, there is no cathodic peak near 3 V indicating that there is also no reduction of Mn4+/Mn3+ for LiNi0.68Co0.10Mn0.22O2.28 The potential differences for the first cycle of the cathode electrodes are listed in Table 1, which shows that NCM-780-16-5% exhibits the smallest polarization among the electrodes. Moreover, the redox current for the NCM-780-16-5% is almost the strongest, which further demonstrates that it has a better ordered hexagonal layered structure.51 These results stress that the electrochemical reaction kinetics for the as prepared LiNi0.68Co0.10Mn0.22O2 is similar to that of the previously reported Ni-rich cathode material, and the enhanced layered structure obtained under the optimized synthesis conditions is beneficial for enhancing the electrochemical performance of LiNi0.68Co0.10Mn0.22O2.
| Samples | Anodic peak [V] | Cathodic peak [V] | Potential difference [V] |
|---|---|---|---|
| NCM-750-16-5% | 3.878 | 3.692 | 0.187 |
| NCM-780-16-5% | 3.882 | 3.715 | 0.167 |
| NCM-810-16-5% | 3.866 | 3.683 | 0.183 |
| NCM-780-12-5% | 3.851 | 3.669 | 0.182 |
| NCM-780-20-5% | 3.879 | 3.702 | 0.177 |
| NCM-780-16-3% | 3.870 | 3.692 | 0.178 |
| NCM-780-16-7% | 3.872 | 3.690 | 0.182 |
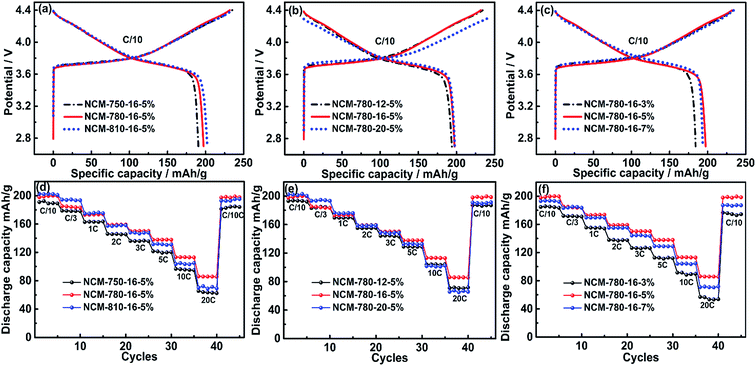
The initial charge/discharge profiles at C/10 of the LiNi0.68Co0.10Mn0.22O2 cathode materials synthesized under different conditions are compared in Fig. 4a–c. The initial discharge specific capacities of NCM-750-16-5%, NCM-780-16-5% and NCM-810-16-5% as shown in Fig. 4a are 190.6, 197.4 and 202.2 mA h g−1, respectively, which demonstrates that the high calcination temperature is favorable for achieving a high capacity, which could be attributed to the better material crystallinity obtained at high temperatures. Fig. 4b shows that the initial discharge capacities of NCM-780-12-5%, NCM-780-16-5% and NCM-780-20-5% are 193.8, 197.4 and 197.7 mA h g−1, respectively, which demonstrates that the 16 h calcination time is enough to achieve high discharge capacity, while further prolonging the calcination time shows a very limited effect on the charge/discharge capacity. Fig. 4c shows that the initial discharge capacities of the NCM-780-16-3% NCM-780-16-5% and NCM-780-16-7% are 184.2, 197.4 and 193.7 mA h g−1, respectively, which demonstrates that 5% of excess Li amount is the optimal. The initial coulombic efficiency (CE) of the LiNi0.68Co0.10Mn0.22O2 cathode material is presented in Table S4,† where it can be observed that NCM-780-16-5% exhibits the almost highest initial CE of 85.1%, while the other samples with the lower calcination temperature, longer calcination time and more excess Li amount present smaller initial CE. These results further demonstrate that the synthetic conditions of calcination temperature, calcination time, and Li excess amount are important factors affecting the electrochemical performances. Although NCM-810-16-5% exhibits the largest initial discharge capacity and the highest CE, its cycling stability is not the best which will be discussed in the following section. The possible reason could be the higher Li/Ni cation mixing in LiNi0.68Co0.10Mn0.22O2 particles at high calcination temperature as found in the XRD data (Fig. 2d).
Fig. 4d–f exhibit the rate capabilities of the LiNi0.68Co0.10Mn0.22O2 cathode materials synthesized under different conditions. As shown in Fig. 5d, although NCM-780-16-5% presents a little lower discharge capacity at a rate of C/10 and C/3, it shows an obviously higher discharge capacity of 159.0, 150.2, 137.8, 113.1 and 85.8 mA h g−1 at a higher rate of 3C, 5C, 10C and 20C, respectively, than 157.7, 146.4, 132.0, 103.1 and 71.3 mA h g−1 of NCM-810-16-5% at the same current rates, which indicates that the optimized temperature of 780 °C could achieve superior rate capabilities. Fig. 5e also shows that NCM-780-16-5% exhibits better rate capabilities than NCM-780-12-5% and NCM-780-20-5% (poorest), which demonstrates that the too long calcination time is not favorable for the electrochemical performance. Moreover, as shown in Fig. 5f, it can be observed that the optimized excess Li amount of 5% could achieve the best rate capability. These results indicate that the optimized synthesized conditions of 780 °C calcination temperature with 16 h calcination time and 5% excess Li amount are favorable for the rate capability of LiNi0.68Co0.10Mn0.22O2, which should be attributed to the improved layered structure with minimized cation mixing, and probably the superior surface microstructure with reduced LiOH/Li2CO3 species.
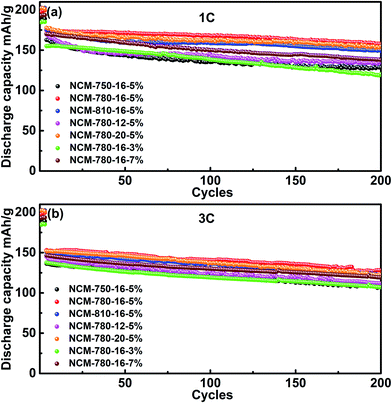
Fig. 5 presents the cycling stabilities of the LiNi0.68Co0.10Mn0.22O2 cathode materials synthesized under different conditions at a rate of 1C and 3C, respectively. It can be observed that NCM-780-16-5% exhibits the best cycling stability among the specimens at different current rates. For example, at a rate of 1C, it delivers an initial discharge capacity of 174.6 mA h g−1, and retains a discharge capacity of 157.5 mA h g−1 after 200 cycles, corresponding to a capacity retention of 90.2%. Similarly, at a rate of 3C, it delivers an initial discharge capacity of 153.0 mA h g−1, and retains 83.5% of its capacity after 200 cycles (127.7 mA h g−1). In fact, the LiNi0.68Co0.10Mn0.22O2 cathode material synthesized under the optimized conditions could exhibit a more excellent cycling stability when tested at a low current rate of C/3 as shown in Fig. S2.† At C/3, an initial discharge capacity of 184.3 mA h g−1 was obtained and 95.9% of this initial capacity can be retained after 200 cycles (176.7 mA h g−1). By contrast, the LiNi0.68Co0.10Mn0.22O2 cathode materials synthesized at the lower (750 °C)/higher (810 °C) calcination temperature, the shorter (12 h)/longer (20 h) calcination time or the less (3%)/more (7%) excess lithium amount show a relative poorer cycling stability. These results validate that LiNi0.68Co0.10Mn0.22O2 prepared under the optimized synthesized conditions (780 °C, 16 h, and 5% Li excess) is a promising cathode material for high energy density lithium ion batteries.
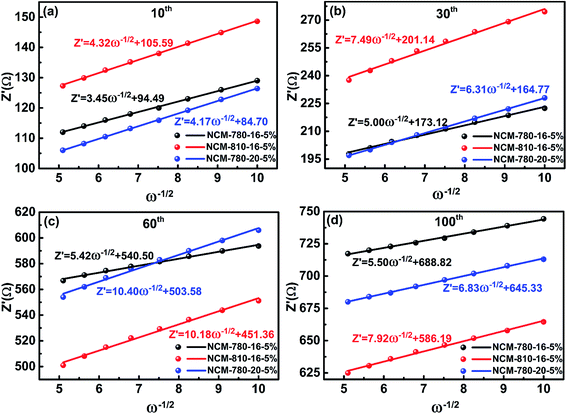
To get further insight into the fundamental mechanism for the optimal synthetic conditions to enhance the electrochemical performance, the EIS technique was carried out to study the interfacial electrochemistry and reaction kinetics of the LiNi0.68Co0.10Mn0.22O2 cathode materials.52Fig. 6 presents the EIS of three specimens of NCM-780-16-5%, NCM-810-16-5% and NCM-780-20-5% with similar cycling stabilities (as shown in Fig. 5) at the 10th, 30th, 60th and 100th cycles. It is found that all the impedance spectra show a semicircle in the high frequency region representing the surface film impedance (Rsf, so-called CEI layer); the semicircle located in the high-to-medium frequency region represents the charge transfer impedance (Rct), and an oblique line located in the low frequency region represents the Warburg impedance (W).53 Moreover, the intercept at high frequency with the real axis mainly corresponds to the electrolyte resistance (Re). The fitted results of the impedance spectra using the equivalent circuit as the inset in Fig. 6 are listed in Table 2. As expected, the total resistance (Re + Rsf + Rct) for NCM-780-16-5% is obviously smaller than those of NCM-810-16-5% and NCM-780-20-%. Moreover, the increases of Rsf and Rct for NCM-780-16-5% upon cycling, such as after 30, 50 and 100 cycles, are also smaller than those for NCM-810-16-5% and NCM-780-20-%. These further demonstrate that the optimal synthetic conditions could achieve a more robust LiNi0.68Co0.10Mn0.22O2 with low cation mixing degree, which is much more stable with electrolyte, thus mitigating the side reactions and structural transformation upon cycling. However, a higher calcination temperature of 810 °C (NCM-810-16-5%) or a longer calcination time of 20 h (NCM-780-20-5%) might result in more oxygen vacancies and increased Li/Ni cation mixing, which could result in an unstable surface microstructure and irreversible structural changes upon cycling. For the excess lithium amount, actually, it is easy to find out the mechanism that the higher amount of excess lithium would result in more serious Li/Ni mixing and the lower amount of excess lithium would not compensate for the lithium evaporation during the high temperature synthetic process.
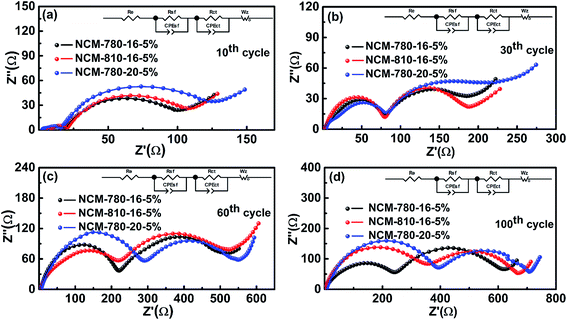 | ||
| Fig. 6 Nyquist plots of the NCM-780-16-5%, NCM-810-16-5% and NCM-780-20-5% electrodes after the (a) 10th, (b) 30th, (c) 60th, and (d) 100th cycles, respectively. | ||
| Cycle | NCM-780-16-5% | NCM-810-16-5% | NCM-780-20-5% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R e/ohm | R sf/ohm | R ct/ohm | R e/ohm | R sf/ohm | R ct/ohm | R e/ohm | R sf/ohm | R ct/ohm | |
| 10th | 5.3 | 16.2 | 70.8 | 5.3 | 17.7 | 74.3 | 1.9 | 16.2 | 94.8 |
| 30th | 2.3 | 85.7 | 76.3 | 3.0 | 76.4 | 81.9 | 2.6 | 85.4 | 96.9 |
| 60th | 3.1 | 202.5 | 200.5 | 4.1 | 204.6 | 345.5 | 5.5 | 297.1 | 221.1 |
| 100th | 3.9 | 235.1 | 259.4 | 2.8 | 327.8 | 355.5 | 6.5 | 397.2 | 267.5 |
The lithium ion diffusion coefficient (DLi+) of NCM-780-16-5%, NCM-810-16-5% and NCM-780-20-5% at the 10th, 30th, 60th and 100th cycle can be calculated from the Warburg impedance coefficient (σw) using eqn (1) and (2).54–57
| Zre = (Rsf + Rct + σwω−1/2) | (1) |
| DLi+ = R2T2/(2A2n4F4Cσw2) | (2) |
4. Conclusions
The effects of the calcination temperature, calcination time and excess Li amount on the electrochemical performance of the Ni-rich LiNi0.68Co0.10Mn0.22O2 cathode material have been systematically investigated. It was found that a calcination temperature of 780 °C with a calcination time of 16 h and an excess Li amount of 5% are the optimal synthetic conditions. LiNi0.68Co0.10Mn0.22O2 prepared under the optimal conditions presents a reversible discharge capacity of 197.4 mA h g−1, and retains up to 95.9% of this initial capacity after 200 cycles when cycled at C/3. This superior electrochemical performance is ascribed to the better layered structure property with minimal Li/Ni cation mixing, which is beneficial for improving the interfacial and structural stability of LiNi0.68Co0.10Mn0.22O2 during long-term cycling. This work highlights that the synthetic conditions are critically important for preparing high performance Ni-rich NCM cathode materials while developing high energy density lithium ion batteries.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was carried out with financial support from the National Natural Science Foundation of China (grant no. 51474196 and 51502250), the Science & Technology Department of Sichuan Province (grant no. 2017JQ0044), and the Southwest Petroleum University (grant no. 2015CXTD04 and X151517KCL50).References
- X. Li, Y. Tang, J. Song, W. Yang, M. Wang, C. Zhu, W. Zhao, J. Zheng and Y. Lin, Carbon, 2017, 129, 236–244 CrossRef.
- M. M. Thackeray, C. Wolverton and E. D. Isaacs, Energy Environ. Sci., 2012, 5, 7854–7863 Search PubMed.
- J. Yuan, J. Wen, J. Zhang, D. Chen and D. Zhang, Electrochim. Acta, 2017, 230, 116–122 CrossRef.
- P. Yue, Z. Wang, X. Li, X. Xiong, J. Wang, X. Wu and H. Guo, Electrochim. Acta, 2013, 95, 112–118 CrossRef.
- J. Kang, H. Q. Pham, D. H. Kang, H. Y. Park and S. W. Song, J. Alloys Compd., 2016, 657, 464–471 CrossRef.
- M. S. Wang, Z. Q. Wang, Z. Chen, Z. L. Yang, Z. L. Tang, H. Y. Luo, Y. Huang, X. Li and W. Xu, Chem. Eng. J., 2018, 334, 162–171 CrossRef.
- M. S. Wang, Z. Q. Wang, R. Jia, Z. L. Yang, Y. Yang, F. Y. Zhu, Y. Huang and X. Li, J. Electroanal. Chem., 2018, 815, 30–39 CrossRef.
- M. V. Reddy, G. V. S. Rao and B. V. R. Chowdari, J. Phys. Chem. C, 2007, 111, 11712–11720 Search PubMed.
- B. Huang, X. Li, Z. Wang, H. Guo, L. Shen and J. Wang, J. Power Sources, 2014, 252, 200–207 CrossRef.
- K. J. Park, B. B. Lim, M. H. Choi, H. G. Jung, Y. K. Sun, M. Haro, N. Vicente, J. Bisquert and G. Garcia-Belmonte, J. Mater. Chem. A, 2015, 3, 22183–22190 Search PubMed.
- J. Duan, G. Hu, Y. Cao, C. Tan, C. Wu, K. Du and Z. Peng, J. Power Sources, 2016, 326, 322–330 CrossRef.
- Y. Cho, P. Oh and J. Cho, Nano Lett., 2013, 13, 1145–1152 CrossRef PubMed.
- N. Yabuuchi and T. Ohzuku, J. Power Sources, 2003, 119–121, 171–174 CrossRef.
- Z. Chen, D. L. Chao, J. L. Liu, M. Copley, J. Y. Lin, Z. X. Shen, G. T. Kim and S. Passerini, J. Mater. Chem. A, 2017, 5, 15669–15675 Search PubMed.
- H. B. Rong, M. Q. Xu, B. Y. Xie, W. Z. Huang, X. L. Liao, L. D. Xing and W. S. Li, J. Power Sources, 2015, 274, 1155–1161 CrossRef.
- Q. Y. Chen, C. Q. Du, D. Y. Qu, X. H. Zhang and Z. Y. Tang, RSC Adv., 2015, 5, 75248–75253 RSC.
- Y. X. Chen, P. L. Li, Y. J. Li, Q. Y. Su, L. L. Xue, Q. Han, G. L. Cao and J. G. Li, J. Mater. Sci., 2018, 53, 2115–2126 CrossRef.
- Y. Cho, M. H. Lee, H. Kim, K. Ku, G. Yoon, S. K. Jung, B. Lee, J. Kim and K. Kang, Mater. Res. Bull., 2017, 96, 524–532 CrossRef.
- R. R. Zhao, J. X. Liang, J. J. Huang, R. Y. Zeng, J. F. Zhang, H. Y. Chen and G. Shi, J. Alloys Compd., 2017, 724, 1109–1116 CrossRef.
- Y. K. Na, T. Yim, J. H. Song, J. S. Yu and Z. Lee, J. Power Sources, 2016, 307, 641–648 CrossRef.
- J. L. Fu, D. B. Mu, B. R. Wu, J. Y. Bi, X. J. Liu, Y. Y. Peng, Y. Q. Li and F. Wu, Electrochim. Acta, 2017, 246, 27–34 CrossRef.
- L. Wang, D. Mu, B. Wu, G. Yang, L. Gai, Q. Liu, Y. Fan, Y. Peng and F. Wu, Electrochim. Acta, 2016, 222, 806–813 CrossRef.
- M. H. Kim, H. S. Shin, D. Shin and Y. K. Sun, J. Power Sources, 2006, 159, 1328–1333 CrossRef.
- C. Hua, K. Du, C. Tan, Z. Peng, Y. Cao and G. Hu, J. Alloys Compd., 2014, 614, 264–270 CrossRef.
- X. Lu, X. Li, Z. Wang, H. Guo, G. Yan and X. Yin, Appl. Surf. Sci., 2014, 297, 182–187 CrossRef.
- B. Zhang, L. Li and J. Zheng, J. Alloys Compd., 2012, 520, 190–194 CrossRef.
- Y. Chen, Y. Zhang, F. Wang, Z. Wang and Q. Zhang, J. Alloys Compd., 2014, 611, 135–141 CrossRef.
- B. Zhang, P. Dong, H. Tong, Y. Yao, J. Zheng, W. Yu, J. Zhang and D. Chu, J. Alloys Compd., 2017, 706, 198–204 CrossRef.
- X. Li, H. Peng, M. S. Wang, X. Zhao, P. X. Huang, W. Yang, J. Xu, Z. Q. Wang, M. Z. Qu and Z. L. Yu, ChemElectroChem, 2016, 3, 130–137 CrossRef.
- X. Yan, L. Chen, S. A. Shah, J. Liang and Z. Liu, Electrochim. Acta, 2017, 249, 179–188 CrossRef.
- C. Zhang, J. Qi, H. Zhao, H. Hou, B. Deng, S. Tao, X. Su, Z. Wang, B. Qian and W. Chu, Mater. Lett., 2017, 201, 1–4 CrossRef.
- H. Konishi, M. Yoshikawa and T. Hirano, J. Power Sources, 2013, 244, 23–28 CrossRef.
- J. Zheng, W. H. Kan and A. Manthiram, ACS Appl. Mater. Interfaces, 2015, 7, 6926–6934 Search PubMed.
- X. Li, K. Zhang, M. Wang, Y. Liu, M. Qu, W. Zhao and J. Zheng, Sustainable Energy Fuels, 2018, 2, 413–421 Search PubMed.
- Z. Wang, Y. Sun, L. Chen and X. Huang, J. Electrochem. Soc., 2004, 151, A914–A921 CrossRef.
- Y. Li, X. Li, Z. Wang, H. Guo and J. Wang, J. Energy Chem., 2017, 27, 447–450 CrossRef.
- C. Liang, F. Kong, R. C. Longo, K. C. Santosh, J. S. Kim, S. H. Jeon, S. A. Choi and K. Cho, J. Phys. Chem. C, 2016, 120, 6383–6393 Search PubMed.
- H. J. Noh, S. Youn, S. Y. Chong and Y. K. Sun, J. Power Sources, 2013, 233, 121–130 CrossRef.
- Y. K. Sun, Z. Chen, H. J. Noh, D. J. Lee, H. G. Jung, Y. Ren, S. Wang, C. S. Yoon, S. T. Myung and K. Amine, Nat. Mater., 2012, 11, 942–947 CrossRef PubMed.
- W. Liu, P. Oh, X. Liu, M. J. Lee, W. Cho, S. Chae, Y. Kim and J. Cho, Angew. Chem., Int. Ed., 2015, 54, 4440–4457 CrossRef PubMed.
- P. He, H. Yu, D. Li and H. Zhou, J. Mater. Chem., 2012, 22, 3680–3695 RSC.
- S. W. Lee, H. Kim, M. S. Kim, H. C. Youn, K. Kang, B. W. Cho, K. C. Roh and K. B. Kim, J. Power Sources, 2016, 315, 261–268 CrossRef.
- Y. F. Xia, M. Nie, Z. B. Wang, F. D. Yu, Y. Zhang, L. L. Zheng, J. Wu and K. Ke, Ceram. Int., 2015, 41, 11815–11823 CrossRef.
- Y. Peng, Z. Wang, W. Peng, L. Li, W. Chen, H. Guo and X. Li, Powder Technol., 2011, 214, 279–282 CrossRef.
- F. Fu, G. L. Xu, Q. Wang, Y. P. Deng, X. Li, J. T. Li, L. Huang and S. G. Sun, J. Mater. Chem. A, 2013, 1, 3860–3864 Search PubMed.
- J. Yuan, J. Wen, J. Zhang, D. Chen and D. Zhang, Electrochim. Acta, 2017, 230, 116–122 CrossRef.
- M. Ghorbanzadeh, S. Farhadi, R. Riahifar and S. M. M. Hadavi, New J. Chem., 2018, 42, 3444–3451 RSC.
- J. Zhang, Z. Yang, R. Gao, L. Gu, Z. Hu and X. Liu, ACS Appl. Mater. Interfaces, 2017, 9, 29794–29803 Search PubMed.
- R. Zhao, J. Liang, J. Huang, R. Zeng, J. Zhang, H. Chen and G. Shi, J. Alloys Compd., 2017, 724, 1109–1116 CrossRef.
- S. U. Woo, C. S. Yoon, K. Amine, I. Belharouak and Y. K. Sun, J. Electrochem. Soc., 2007, 154, A1005–A1009 CrossRef.
- Y. Bi, W. Yang, R. Du, J. Zhou, M. Liu, Y. Liu and D. Wang, J. Power Sources, 2015, 283, 211–218 CrossRef.
- B. Li, M. Xu, T. Li, W. Li and S. Hu, Electrochem. Commun., 2012, 17, 92–95 CrossRef.
- J. Zheng, W. Shi, M. Gu, J. Xiao, P. Zuo, C. Wang and J. G. Zhang, J. Electrochem. Soc., 2013, 160, A2212–A2219 CrossRef.
- J. Zheng, P. Yan, W. H. Kan, C. Wang and A. Manthiram, J. Electrochem. Soc., 2016, 163, A584–A591 CrossRef.
- H. Gao, L. Jiao, W. Peng, G. Liu, J. Yang, Q. Zhao, Z. Qi, Y. Si, Y. Wang and H. Yuan, Electrochim. Acta, 2011, 56, 9961–9967 CrossRef.
- H. Liu, C. Li, Q. Cao, Y. P. Wu and R. Holze, J. Solid State Electrochem., 2008, 12, 1017–1020 CrossRef.
- X. Li, K. Zhang, D. Mitlin, Z. Yang, M. Wang, Y. Tang, F. Jiang, Y. Du and J. Zheng, Chem. Mater., 2018, 30, 2566–2573 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8se00192h |
| This journal is © The Royal Society of Chemistry 2018 |