Alpha-lipoic acid and its protective role in fructose induced endocrine-metabolic disturbances
María Cecilia
Castro
,
Hernán Gonzalo
Villagarcía
,
María Laura
Massa
and
Flavio
Francini
 *
*
CENEXA (Centro de Endocrinología Experimental y Aplicada, UNLP-CONICET La Plata-FCM) (Centro asociado CICPBA), 1900 La Plata, Argentina. E-mail: f_francini@yahoo.com; Fax: +54 221 4222081
First published on 4th December 2018
Abstract
In recent decades a worldwide increase has been reported in the consumption of unhealthy high calorie diets associated with marked changes in meal nutrient composition, such as a higher intake of refined carbohydrates, which leads to the speculatation that changes in food habits have contributed to the current epidemic of obesity and type 2 diabetes. Among these refined carbohydrates, fructose has been deeply investigated and murine models of high fructose diet have emerged as useful tools to study dietary-induced insulin resistance, impaired glucose tolerance, dyslipidemia and alterations in glucose metabolism. Since oxidative stress has been demonstrated to play a key pathogenic role in the alterations described above, several lines of research have focused on the possible preventive effects of antioxidant/redox state regulation therapy, among which alpha-lipoic acid has been extensively investigated. The following references discussed support the fact that co-administration of alpha-lipoic acid normalized the changes generated by fructose rich diets, thereby making this compound a good therapeutic tool, also administered as a food supplement, to prevent endocrine–metabolic disturbances triggered by high fructose associated with obesity and type 2 diabetes at an early stage of development (prediabetes).
Introduction
Modern society is characterized by the consumption of unhealthy high calorie diets1–5 associated with marked changes in meal nutrient composition, such as a higher intake of refined carbohydrates, especially fructose.6–8 Several authors have suggested that this shift in meal composition has contributed to the current epidemic of obesity and type 2 diabetes (T2D).2,3,9,10In this sense, murine fructose-fed models have provided a useful tool to study dietary-induced insulin resistance, impaired glucose tolerance, dyslipidemia, alterations in glucose metabolism, and pathophysiological mechanisms associated with metabolic syndrome.11–18 At this point, however, it is important to keep in mind that fructose alone does not necessarily mirror what happens in the human food context, where fructose is normally incorporated as the disaccharide sucrose (fructose–glucose) or in high fructose corn syrups (HFCS) together with glucose. Nevertheless, there is evidence suggesting that the deleterious effects of sucrose and HFCS are basically mediated by fructose. In fact, besides studies in animals that have documented that fructose induces dyslipidemia and insulin resistance compared with glucose, Stanhope et al.19 have demonstrated in humans that consuming fructose-sweetened and non-glucose-sweetened beverages increases de novo lipogenesis, dyslipidemia and insulin resistance.
Several lines of evidence have highlighted the pathogenic role of glyco-oxidative stress in the development of the alterations listed above.20–23 As a consequence, antioxidant compounds and/or cellular redox status modulators could be useful tools to prevent/revert these pathological changes.
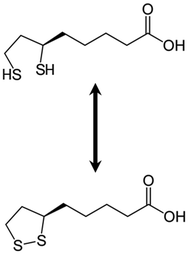
Alpha-lipoic acid (α-LA) (1,2-dithiolane-3-pentanoic acid) (Fig. 1), also known as thioctic acid, is a potent antioxidant and a natural cofactor of different mitochondrial enzymes such as pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase, involved in oxidative metabolism.24 α-LA scavenges reactive oxygen species (ROS), regenerates endogenous antioxidants such as glutathione, and vitamins E and C, chelates metals, and repairs oxidized proteins, contributing to establishing and maintaining the antioxidant defense network.24–26
α-LA is present in a wide variety of animals and vegetables, is absorbed from the diet, and once inside the cell, is converted to its reduced form: dihydrolipoic acid.27 Endogenously, it is synthesized from octanoic acid by the enzyme lipoic acid synthase (LASY), which is mainly present in the mitochondria.28
Due to its hydrophobic nature, α-LA can cross biologic membranes. Interestingly, in a model of Parkinson's disease, it was demonstrated that thioctic acid caused an increase in 14C-2DG incorporation into different areas of the brain, thus suggesting that the compound could enter the brain and modify neuronal activity.29
The aim of this review was to analyze the effects of α-LA administration on alterations induced by fructose rich diets – oxidative stress/redox state, dysmetabolism of lipid and carbohydrates, insulin resistance and inflammation – in different tissues and animal models.
Redox state
Fructose induced an increment in ROS production and a simultaneous reduction in antioxidant enzyme and non-enzymatic antioxidant availability, including molecules with potential to regulate cellular redox state.14,23In this regard, α-LA plays a key role in this protective network, not only by acting as an antioxidant but also for its capacity to increase GSH levels and provide thiol/redox regulation at several points.30 The redox environment is determined by the balance between the reducing capacity of the cell and oxidative processes, the equilibrium displaced to the latter condition in several pathological scenarios. α-LA acts as a modulator of cellular redox state, which may be the basis of a variety of protective effects that mechanistically account for cellular thiol/disulfide exchange reactions that in turn modulate the redox state and energy status at subcellular compartments. The redox balance can be estimated by quantification of thioredoxin, GSH/GSSG, and cysteine/cysteine redox control nodes.31 In this context, α-LA, due to its capacity to equilibrate between subcellular and extracellular compartments, can modulate these nodes and thus provide redox regulation of cellular proteins.30
In several animal models including treatment with a high fructose diet (HFD), a significant decrease in endogenous enzymatic and non-enzymatic antioxidants has been demonstrated, thus leading to the hypothesis that fructose is a pro-oxidant. However, the mechanistic basis of this capacity is still not well established.
In general, all these alterations induced by diet were reversed or prevented by administration of α-LA. Cummings et al.32 in a rat polygenic obese T2D model – UCD-T2DM rats treated with a fructose rich diet (20% of energy) – detected significantly lower glutathione (GSH) serum content that was restored by co-administration of α-LA (administered as a food supplement mixed with chow to provide a dose of 80 mg kg−1 day−1).
It was also found that male Wistar rats fed a HFD (60% for 20 days) evinced a decrease in non-enzymatic antioxidants (vitamin E, vitamin C and GSH) and a parallel increment in lipid hydroperoxides, conjugated dienes, and TBARS in the plasma, liver and kidney. These dysfunctions were ameliorated by α-LA treatment at two different doses (35 and 70 mg kg−1 day−1 i.p.).22,33 In the same model, increased lipid peroxidation of tail tendon in HFD rats was reversed by administration of α-LA.33
In accord with these results, our group described a decrease in GSH and a rise in protein carbonyl content in the liver of fructose treated male Wistar rats (10% fructose in drinking water for 21 days), normality restored by α-LA administration (35 mg kg−1 i.p. for the last 5 days of fructose treatment). α-LA also induced higher antioxidant capacity in α-LA treated rats as measured by the ABTS assay.34
As mentioned, GSH is a key metabolite in the maintenance of the cellular redox state, and higher GSH levels have a protective effect against oxidative damage to cell membranes. α-LA, in its dihydrolipoic acid form (DHLA), reduces extracellular cysteine to cysteine, thus favoring cellular uptake of the latter throughout ASC transporters, and as a consequence become available for GSH synthesis.35,36 More recently, a novel mechanism for explaining the beneficial effects of α-LA on cellular redox-state was demonstrated. The pathway involves Nf-e2 related factor (Nrf2), a transcription factor that regulates the expression of antioxidant and Phase II detoxification genes.37 Mechanistically, oxidation of sulfhydryles of Kelch-like actin-binding protein (Keap1), a protein that bridges Nrf2 to a ubiquitin ligase, disrupts this interaction and, once released from Keap1, Nrf2 translocates and accumulates into the nucleus, thus allowing the expression of stress response genes, including those associated with GSH synthesis and thiol redox control. Complementarily, α-LA induces Phase II detoxification enzymes in an Nrf2-dependent way via a new protein synthesis.38
In cardiac tissue, malondialdehyde content was increased significantly by a HFD (high fructose corn syrup with 30% fructose in drinking water) for 10 weeks, and was restored to control levels by α-LA administration (100 mg kg−1 day−1 orally for the last 6 weeks).39 This decrease in malondialdehyde level in plasma and tissues of α-LA treated animals is a consequence of its potent antioxidant capacity as a ROS quencher, avoiding free radical mediated lipid peroxidation.40,41
Thirunavukkarasu and Anuradha22 found that the activities of superoxide-dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione transferase (GST), and glutathione reductase (GR) in red blood cells, liver, and kidney of fructose fed rats were lower than those in control animals; and that treatment with α-LA induces a rise in these activities. Supporting their results, we found in HFD rats (10% fructose in drinking water for 21 days) a decrease in antioxidant enzyme protein content in the liver, which was restored by α-LA administration (35 mg kg−1 i.p. for the last 5 days).34 In cardiac tissue, a fructose rich diet (high fructose corn syrup with 30% fructose) in drinking water for 10 weeks induced a significant decrease in CAT activity, which was restored to control levels by α-LA administration (100 mg kg−1 day−1 orally during the last 6 weeks) in female Wistar rats.39 Cytosolic SOD is inactivated by glycation of specific lysine residues,42 and ROS themselves can reduce the activity of CAT and GPx.43 The α-LA quencher ROS capacity40,41 could preserve antioxidant enzymes and also maintained non-enzymatic antioxidant concentrations.16
At the pancreatic level, malondialdehyde content was increased and CAT activity was decreased in Wistar female rats fed a HFD (30% solution of HFCS, 24% fructose, and 28% dextrose) for 10 weeks. These changes were reverted by α-LA treatment during the last 4 weeks (100 mg kg−1, oral).44
Events associated with the respiratory chain in the mitochondria have been shown to be mainly responsible for the increased production and cell accumulation of ROS.45 Thus, an overload of nutrients such as that induced by the fructose rich diet could lead to ROS overproduction/accumulation and the consequent OS.14 The increased UCP2 protein levels measured in fructose fed rats could be part of a compensatory mechanism to reduce ROS production induced by the fructose overload.46,47 These changes in UCP2 expression were associated with PPARδ induction.46 After α-LA treatment and consequent normalized OS levels, UCP2 and PPARδ expressions returned to control values.
Another source of ROS is the NADPH oxidase complex. Our group studied the gene and protein expressions of some subunits of this complex and found an increment in gene expression of gp91phox and in gene and protein expressions of p22phox in the liver of fructose treated rats. These alterations were reversed by i.p. administration of 35 mg per kg body weight of α-LA.48 Since NADPH is a cofactor for recycling GSSG to GSH, the observed GSH reduction in fructose-fed animals could be associated in part with oxidation in NADPH due to the enhanced activity of NADPH oxidase. The capacity of α-LA to restore GSH content could lead to reduced expression of NADPH oxidase complex.
In conclusion, α-LA restores the antioxidant milieu by regenerating non-enzymatic antioxidant, preserving antioxidant enzymes, and even reducing ROS production in fructose fed rat models. On another front, the vicious circle that probably links mitochondrial OS to cytosolic OS via NADPH oxidase is also disrupted by α-LA administration.48
Lipid and carbohydrate metabolism
It has been recently demonstrated that the small intestine plays a central role in converting fructose to glucose and other circulating metabolites, thus protecting the liver from fructose exposure.49 However, fructose overload saturates intestinal clearance and as a consequence results in colonic microbiota and liver exposure to the sugar, and thereby toxicity on the latter.49 At the liver, fructose enters the cell via GLUT-2 and GLUT-8 transporters50 and is immediately phosphorylated to fructose-1-phosphate (F-1-P) by the enzyme fructokinase (FK). F-1-P can be metabolized into dihydroxiacetone phosphate and glyceraldehydes, and these trioses channeled into the triglyceride synthesis pathway, bypassing glycolysis rate-limiting steps (glucokinase – GK – and phospho-fructokinase-1 – PFK1). As a consequence, fructose provided carbons to lipogenic pathways in an unregulated way.51 Another mechanism to increase fatty acid synthesis is through fructose-1,6-bis-phosphate, which directly activates fatty acid synthase (FAS).52 When this flux is accompanied by a parallel glucose uptake, much of the triose pool converts into the backbone of triglycerides in the de novo lipogenesis.50In our model of a fructose (10%) rich diet, we described, in liver higher glycogen storage, triglyceride content, and an increment in GK, FK, glucose-6-phosphatase (G-6-Pase), and glucose-6-phosphate dehydrogenase (G-6-PDH) activities.13,14,48 α-LA administration (35 mg kg−1 i.p. for the last 5 days) restored values found in control animals.48
In cultured rat skeletal muscle cells and adipocytes, α-LA was reported to stimulate glucose uptake by redistributing the intracellular GLUT 1 and 4, an effect dependent on PI3K activity via the insulin-signaling pathway.53
The concentrations of cholesterol and free fatty acids in the plasma, liver, kidney, and skeletal muscle were also significantly increased in fructose-fed rats (60% in drinking water for 20 days) compared to normal rats. Animals simultaneously treated with fructose and α-LA (35 mg kg−1 day−1 i.p.) showed significant reductions in these altered lipid concentrations.54 In the same model, the authors found that the concentrations of VLDL-cholesterol and low-density lipoprotein LDL-cholesterol significantly increased whereas high-density lipoprotein-cholesterol was lowered in fructose-fed rats. Treatment with α-LA reversed cholesterol distribution in lipoprotein fractions. Similar results for total cholesterol, LDL-cholesterol and triglyceride were found by Abdelkarem et al.55
The activities of lipoprotein lipase (LPL) and lecithin cholesterol acyl transferase (LCAT) were lowered in the plasma and liver of fructose treated rats. LPL is an insulin-sensitive enzyme. Reduction in its activity in fructose-fed rats can be related to insulin resistance induced by fructose. These alterations were accompanied by an increment in lipogenic enzyme HMG-CoA reductase activity, and once again, α-LA treatment restored values found in control rats.22 Also, Huong and Ide56 showed that α-LA reduced serum insulin levels in a dose dependent manner (1, 2.5 and 5 g kg−1 for 21 days), a fact directly associated with a decrease in the mRNA levels and activity of lipogenic enzymes. Other authors demonstrated that insulin upregulates hepatic lipogenic enzymes through SREBP-1.57,58 Our group reported an increment in SREBP-1c, FAS and GPAT mRNA levels, reversed to control levels with α-LA administration in fructose induced insulin resistant rats.34 These changes were also accompanied by a reduction in PPARγ protein content in fructose fed animals, normalized after one week of α-LA treatment.34 Interestingly, it has been reported that α-LA may act as a PPARγ partial agonist59,60 and as a consequence the normalizing effects of α-LA on lipogenic genes could be at least partially mediated by PPARγ activation.60
In another study, fructose induced hepatic steatosis in male Sprague Dawley rats treated with 60% of calories from fructose for eight weeks, whereas administration of α-LA (60 mg kg−1 day−1 over the last 4 weeks) reversed this alteration.61
It is possible that α-LA lowers lipid content by reducing lipogenesis. Several authors also found that α-LA acts as a potent antilipemic agent reducing FFA, TG, and TC levels.54,62 α-LA was reported to reduce the mRNA levels and activity of enzymes involved in fatty acid synthesis and pyruvate kinase.56 In skeletal muscle, administration of α-LA significantly decreased lipid accumulation by activating AMPK, and is partially responsible for the improvement of insulin sensitivity.63 AMPK has an important role in regulating fatty acid and glucose metabolism. Phosphorylation of AMPK stimulates phosphorylation of acetyl-CoA carboxylase and reduces its activity, thereby reducing lipogenesis and increasing fatty acid oxidation through a decrease in hepatic concentration of malonyl-CoA-inhibitor of carnitine palmitoyl transferase I (CPT I).
It remains to be demonstrated whether α-LA's protective effects in fructose fed rats may be ascribed to the direct action of the compound on whole-body energy homeostasis by directly modulating AMPK or to indirect action through its effects as an antioxidant and a redox state modulator.
Insulin resistance
It has been clearly established that a fructose overload induces, in rats, insulin resistance, affecting several organs and tissues.12–14 This effect seems to be related to several unique metabolic and neuroendocrine features of sugar, such as those mentioned in the previous section, rather than to an unbalanced caloric intake.64 The hormone insulin has a major regulatory effect on free fatty acid (FFA) metabolism.65 Defects in the ability of insulin to promote lipid synthesis could lead to an increase in FFA levels, and in turn, high plasma FFA concentration impairs insulin action on glucose disposal via substrate competition in the glucose–FFA cycle.66 Conversely, diminished insulin-stimulated glucose disposal could lead to impaired FFA re-esterification and thereby to higher circulating FFA concentrations. These topics are reviewed in the lipid and carbohydrate metabolism section. Bieger et al.67 showed that an increase in blood triglyceride concentrations can decrease the number of insulin receptors, thereby reducing insulin sensitivity.Insulin resistance seems to be related to lipid dysmetabolism. As was discussed in the previous section, fructose stimulates hepatic de novo lipogenesis with a consequent enhanced triglyceride synthesis at the organ. This lipid accumulation leads to overproduction of VLDL particles enriched in apoC-III, a change that in turn is linked to enhanced small dense LDL levels. As a proof of concept, isocaloric restriction of dietary fructose in children with obesity and metabolic syndrome, improved glucose tolerance and enhanced insulin sensitivity unrelated to weight change.68 These changes were accompanied by an improvement in fasting serum lipids, including a reduction in circulating triglycerides64 as well as in apoC-III levels, and in a general improvement of the lipoprotein profile.69
Thirunavukkarasu et al.70 performed an oral glucose-tolerance test on male Wistar rats treated with a fructose rich diet (60% for 20 days) and α-LA (35 mg kg−1 day−1 i.p.). Fasting glucose level was higher in fructose-fed rats compared to control rats, and α-LA administration significantly restored these values to normal. Significant elevations in glucose level after oral glucose load were observed in fructose fed rats at all time points. All other experimental groups showed a response similar to that of control rats. Similar results were found by our group.34
To study glucose-stimulated insulin secretion in vivo, Tian et al.61 performed a hyperglycemic clamp in Sprague Dawley male rats fed a fructose rich diet (60% for 8 weeks) accompanied by low-dose lipopolysaccharide (LPS) infusion and α-LA (60 mg kg−1 day−1, orally) co-administration for the last 4 weeks. Fructose induces a reduction in glucose-stimulated insulin secretion which was not reversed by α-LA treatment. However, intraportal LPS infusion significantly impaired the glucose-stimulated insulin secretion, which was reversed in those with α-LA treatment.
Patel et al.71 treated male Wistar rats with 60% fructose in food, α-LA (1.6 g kg−1 food), and α-tocopherol or tocotrienol. In a prevention protocol, diets were administered for 16 weeks, whereas in a reversal protocol, antioxidants were administered only for the last 8 weeks. After an oral glucose load, rats treated with fructose presented higher plasma glucose concentrations compared to control rats, and the prevention group curve was similar to control animals. However, the reversal group showed increased glycaemia 120 minutes after glucose load (week 8) and normalization after antioxidant treatment (week 16).
The positive effect of α-LA on insulin-sensitization can be ascribed to an enhancement in tyrosine phosphorylation and activity of the insulin signaling pathway, i.e. insulin receptor (IR), insulin-receptor substrate 1 (IRS-1), phosphatidylinositol 3-kinase (PI3K), Akt1, and p38.72,73 α-LA also favors the autophosphorylation of the IR by a mechanism involving oxidation of cysteine residues in the alpha and beta subunits.74 α-LA also improves insulin sensitivity through inhibition of serine IRS-1 phosphorylation, thereby counteracting the negative effect of this late phosphorylation on IRS-1 protein.75
In this respect, we have shown that male Wistar rats treated with 10% fructose in drinking water for 21 days showed higher liver IR protein levels, but the tyrosine residue phosphorylation rate was lower. However, relative IRS-1 and IRS-2 (insulin receptor substrate-2) protein levels and the IRS-1 phosphorylation rate were lower in fructose treated rats. α-LA administration drove IR, pTyr-IR, IRS-1 and pTyr-IRS-1 protein levels to control values. These changes were not paralleled by IRS-1 and IRS-2 relative gene expression, thus suggesting a posttranscriptional regulation mechanism.15
Interestingly, in a recent publication, Maiztegui et al.76 have shown that rats receiving a high sucrose diet during three weeks evinced, together with hyperinsulinemia (and normoglycemia and thus insulin-resistance) and hyperleptinemia, a β-cell dysfunction characterized by an initial compensatory increase in glucose-induced insulin secretion accompanied by a decrease in insulin and leptin signaling pathway mediators.76 Development of these endocrine-metabolic abnormalities was prevented by co-administration of α-LA, thus showing that the mentioned mechanisms in the liver could be also operative in pancreatic islets, thus contributing to a more general insulin-sensitizing action.
Inflammatory process
Insulin resistance is associated with a state of chronic low-grade inflammation; adipocytes and immune cells secrete several mediators which have been identified to be involved in the development of insulin resistance.77 Removal of inflammatory mediators or pathway components protects against insulin resistance in obese mouse models.78,79 The most studied pro-inflammatory cytokines are Tumor Necrosis Factor α (TNFα), Interleukin-1 (IL-1) and 6 (IL-6) and among the adipocytokines, adiponectin and leptin.TNFα is an important inflammatory marker and is involved in deregulation of hepatic lipid metabolism and insulin signaling. Kanuri et al.,80 working with TNFα receptor 1 –/– (TNFR1) mice drinking 30% fructose for 8 weeks, demonstrated that TNFα plays a causal role in the onset of fructose-induced liver damage as well as insulin resistance in mice through signaling cascades downstream of this receptor. In the liver of TNFR1 –/– fructose drinking mice, content of triglyceride, FAS, SREBP-1 and PAI-1 mRNA levels, neutrophil infiltration, expression of ICAM-1, chemokine CCL2 and CCL19 mRNA expression, iNOS protein levels, and plasma ALT levels were reduced in comparison with wild-type control fed fructose mice. In accord with these results, exposure of cells to TNFα stimulated inhibitory phosphorylation of serine residues of IRS-1.81,82
We also demonstrated that fructose treated Wistar rats (10% in drinking water for 21 days) evinced significantly higher TNFα, IL1β, and PAI-1 gene expression compared to control animals, as well as protein levels of TNFα and COX2, thereby evincing an increased inflammatory state in these animals. α-LA administration (35 mg kg−1 i.p. for the last 5 days of treatment) to fructose fed rats fully prevented the abovementioned changes.15
Male rats fed a fructose rich diet (60% for 8 weeks) accompanied by a low-dose LPS infusion over the last 4 weeks evinced an inflammatory response (increment in TNFα and IL6 protein content) at the hepatic level. α-LA (60 mg kg−1 day−1) co-administration for the last 4 weeks suppressed these alterations.61 Under these conditions, monocyte liver infiltration was also reported and α-LA administration was effective in their reversion. In agreement with this, the authors detected higher CD-68 positive cells in the liver and pancreas of fructose-LPS treated animals, which was reversed with α-LA administration.
In cardiac tissue of fructose fed rats (30% F30-high fructose corn syrup with 30% fructose in drinking water for 10 weeks), we found higher mononuclear cell infiltration and TNFα and iNOS protein expression. Infiltration and elevation in protein levels were reduced to control levels by α-LA administration (100 mg kg−1 day−1 orally over the last 6 weeks). Using this model, the same results were found in aortic tissue.39
Abdelkarem et al.55 induced metabolic syndrome by feeding male Wistar rats 10% percent fructose (w/v) for 4 weeks. After four weeks of fructose feeding, rats received fructose and α-LA (200 mg kg−1 day−1 orally) for an additional four weeks. Fructose treatment induces a significant increment in serum leptin levels and a decrease in serum adiponectin. Administration of α-LA also attenuated serum adiponectin levels.
Taken together, the abovementioned results suggest that α-LA is able to modify and revert inflammatory events triggered by fructose.
Other alterations
Advanced glycation end products (AGE) are a consequence of a binding reaction between free amino groups in proteins and reducing sugars. Glycation could change the structure and function of proteins and is important for the etiology of secondary complications in diabetes.83,84 Several reports demonstrated the mechanisms through which glucose induces AGE formation. However, the role of fructose-mediated AGE generation via Maillard reaction is still controversial.50 Fructose is several times more reactive than glucose in Maillard reaction product formation; however, traditional methods for identifying glucose glycation are not able to detect these products of fructose-mediated adducts.85 As a consequence, the potential role of fructose glycation in the pathogenesis of diabetes and its complications are underscored.50 In addition, as mentioned elsewhere in this review, fructose metabolism generates trioses in an unregulated way, and this flux leads to an overproduction of methylglioxal, another key source of oxidative stress, which also damages proteins by glycation.50Collagen is a slow turnover rate protein containing several basic amino acids with free amino groups, and is a strong candidate for extensive glycation.86 Male Wistar rats treated with fructose (60%) for 45 days present higher collagen content and higher glycation, AGE linked fluorescence, aldehyde content, peroxidation, and shrinkage temperature in skin collagen and in tail tendon collagen. Administration of α-LA (35 mg kg−1 day−1) to these rats restored normal control levels.33,87 These positive effects could be due to the antioxidant capacity of α-LA and/or the reduction in glycemia and improvement of glucose metabolism.
More recently, in an in vitro study, Ghelani et al.88 demonstrated that co-incubation of myoglobin, fructose and α-LA (1, 2 and 4 mM) inhibited the formation of AGEs during a 30 day study period. The authors showed a protective effect of α-LA against myoglobin oxidative damage, evinced by decreased protein carbonyl content and increased protein thiols.88
Alterations in renal function also play an important role in the development of hypertension in fructose-fed rats. Saygin et al.39 studied biomarkers of cardiac tissue damage such as creatinine kinase MB (CKMB), lactate dehydrogenase (LDH), and uric acid in female Wistar rats treated with a fructose (30% F30-high fructose corn syrup with 30% fructose in drinking water for 10 weeks). The sugar induced an increment in these markers, whereas administration of α-LA (100 mg kg−1 day−1 for the last 6 weeks) lowered these parameters to control levels. Thirunavukkarasu et al.70 found that creatinine and urea clearance rates were lower in fructose treated rats, whereas α-LA enhances these rates.
In the abovementioned protocol applied by Patel et al.,71 blood pressure was higher in rats treated with fructose, an effect prevented/reversed by α-LA administration.
Interestingly, in the male UCD-T2DM rat model, Cummings et al.32 showed that chronic consumption of a high fructose diet (20% of energy intake) accelerated diabetes onset, developing fasting hyperglycemia 2.6 months earlier than control animals. Interestingly, α-LA supplementation (80 mg kg−1 day−1) delayed T2D onset.
Finally, Wistar female rats fed a HFD (30% solution of HFCS; 24% fructose, 28% dextrose) for 10 weeks evinced a decrease in insulin and glucagon secreting cell scores, whereas α-LA (100 mg kg−1, orally) improved these pathologic conditions.44
Conclusions
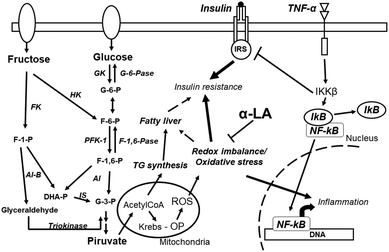
The increase in refined carbohydrate intake is considered a plausible cause of the current epidemic of diabetes and obesity. Several studies have clearly shown the detrimental effect of fructose on endocrine–metabolic processes, OS and an imbalance in cellular redox state being pathological players in these effects. Most of the animal models assayed evinced a rapid effect of HFD on OS and consequent IR and dyslipidemia while maintaining, normoglycemia, thus establishing a prediabetic scenario. A word of caution is needed here: even when it was demonstrated that consuming fructose-sweetened and not glucose-sweetened beverages induces endocrine-metabolic disturbances,19 fructose is rarely incorporated alone into human food and normally it is incorporated as sucrose or in HFCS (fructose plus glucose). In this context, extrapolation of the results obtained in animal models to humans must be done with caution.The above discussed references support the fact that α-LA co-administration normalized changes generated by fructose rich diets, thus diminishing OS burden and modifying redox state that in turn reverts/prevents all metabolic, endocrine, and inflammatory events associated with the sugar (Fig. 2). It is possible to hypothesize that high fructose administration triggers two initial processes: an imbalance in the redox state (for instance a marked reduction in GSH) associated with oxidative stress, together with inflammation, that in turn triggers impairment of insulin sensitivity. This pathological triad establishes an active vicious circle that self-sustains the development of wider fructose induced dysfunction. This circle can be disrupted by α-LA administration; as a consequence α-LA may be considered a potential therapeutic agent for the treatment of these pathologies at early stages of its development (prediabetes). Although several previous studies utilized α-LA i.p., other reports show that oral administration as a food supplement is also possible. However, a second word of caution is needed here: a systematic review and meta-analysis of α-LA in the treatment of patients with diabetic peripheral neuropathy showed that the dose employed was 600 mg day−1, much lower than the doses described in the current review for rodents. As a consequence the results should be taken with caution when a correlation with human therapy is proposed.89
It has been clearly proved that gut commensal bacteria, as well as several metabolites derived from them, play a key role in maintaining gastrointestinal homeostasis by the modulation of inflammation, insulin resistance, and intestinal permeability.90,91 On the other hand, gut microbiota is highly influenced by the diet92 and in fact, it has been demonstrated that long-term intake of fructose affects gut permeability leading to bacterial endotoxins’ translocation into blood circulation with biological consequences on the development of obesity, T2D and liver disease.90 Since nutritional supplementation with probiotics may restore gut commensal bacteria and thus achieve a new healthy balance, to evaluate a possible modulatory effect of α-LA supplementation on gut microbiota could help to better understand some of the beneficial effects recorded for this compound.
Interestingly, GK, a key enzyme in glucose metabolism and considered a glucose sensor in tissues where it is located (particularly in the liver and endocrine pancreas), has shown changes in its activities under OS conditions.13 Since these changes were reversed by α-LA treatment,14 it is possible to conclude that this enzyme could also have a role of a redox state sensor. Complementarily, it would be also interesting to evaluate whether the abovementioned effects were associated with changes in the redox-state of the cell or they were the product of a direct effect of α-LA.
In brief, we believe that the current review is relevant for the Food & Function audience since the manuscript provides a comprehensive view of the deleterious effects of a nowadays common dietary compound, the sugar fructose, and the preventive molecular and physiological effects of α-LA on the endocrine-metabolic disturbances triggered by the sugar. Since diabetes and obesity are becoming epidemic, and since enhanced fructose intake as a food sweetener has been postulated as a possible causal agent of these pathologies, the review provides a basement for physicists, biochemists, nutritionists and food scientists on the problem of health at an early stage of its development (prediabetes).
Conflicts of interest
All authors declare that no competing financial interests exist.Acknowledgements
This study was partially supported by an unrestricted grant from the CONICET to MLM (PIP 0371) and FF (PIP 0181). The authors are grateful to Susan Rogers for English editing.References
- J. F. Guthrie and J. F. Morton, Food sources of added sweeteners in the diets of Americans, J. Am. Diet. Assoc., 2000, 100(1), 43–51 CrossRef CAS PubMed.
- G. A. Bray and B. M. Popkin, Calorie-sweetened beverages and fructose: what have we learned 10 years later, Pediatr. Obes., 2013, 8(4), 242–248 CrossRef CAS PubMed.
- G. A. Bray and B. M. Popkin, Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes?: health be damned! Pour on the sugar, Diabetes Care, 2014, 37(4), 950–956 CrossRef CAS PubMed.
- S. W. Ng, M. M. Slining and B. M. Popkin, Use of caloric and noncaloric sweeteners in US consumer packaged foods, 2005–2009, J. Acad. Nutr. Diet., 2012, 112(11), 1828–1834 CrossRef PubMed.
- E. S. Ford and W. H. Dietz, Trends in energy intake among adults in the United States: findings from NHANES, Am. J. Clin. Nutr., 2013, 97(4), 848–853 CrossRef CAS PubMed.
- J. Putnam and J. Allshouse, Trends in U.S. Per Capita Consumption of Dairy Products, 1909 to 2001, Amber Waves, 2013, 42, 12–13 Search PubMed.
- M. Moss, Salt Sugar Fat: How the Food Giants Hooked Us, Random House, New York City, 2013 Search PubMed.
- Economic Research Service, Food Availability (Per Capita) Data System, Department of Agriculture, Washington, DC, United States, 2013 Search PubMed.
- L. S. Gross, L. Li, E. S. Ford and S. Liu, Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment, Am. J. Clin. Nutr., 2004, 79(5), 774–779 CrossRef CAS PubMed.
- V. S. Malik, B. M. Popkin, G. A. Bray, J. P. Després, W. C. Willett and F. B. Hu, Sugar sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis, Diabetes Care, 2010, 33(11), 2477–2483 CrossRef PubMed.
- C. V. Anuradha and S. D. Balakrishnan, Taurine attenuates hypertension and improves insulin sensitivity in fructose- fed rat, an animal model of insulin resistance, Can. J. Physiol. Pharmacol., 1999, 77(10), 749–754 CrossRef CAS PubMed.
- A. Alzamendi, A. Giovambattista, A. Raschia, V. Madrid, R. C. Gaillard, O. Rebolledo, J. J. Gagliardino and S. Spinedi, Fructose-rich diet-induced abdominal adipose tissue endocrine dysfunction in normal male rats, Endocrine, 2009, 35(2), 227–232 CrossRef CAS PubMed.
- F. Francini, M. C. Castro, J. J. Gagliardino and M. L. Massa, Regulation of liver glucokinase activity in rats with fructose-induced insulin resistance and impaired glucose and lipid metabolism, Can. J. Physiol. Pharmacol., 2009, 87(9), 702–710 CrossRef CAS PubMed.
- F. Francini, M. C. Castro, G. Schinella, M. E. Garcia, B. Maiztegui, M. A. Raschia, J. J. Gagliardino and M. L. Massa, Changes induced by a fructose-rich diet on hepatic metabolism and the antioxidant system, Life Sci., 2010, 86(25–26), 965–971 CrossRef CAS PubMed.
- M. C. Castro, M. L. Massa, L. González Arbeláez, G. Schinella, J. J. Gagliardino and F. Francini, Fructose-induced inflammation, insulin resistance and oxidative stress: A liver pathological triad effectively disrupted by lipoic acid, Life Sci., 2015, 137, 1–6 CrossRef CAS PubMed.
- V. Thirunavukkarasu, A. T. Nandhini and C. V. Anuradha, Lipoic acid restores antioxidant system in tissues of hyperinsulinaemic rats, Indian J. Med. Res., 2003, 118, 134–140 CAS.
- P. J. Havel, Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism, Nutr. Rev., 2005, 63(5), 133–157 CrossRef PubMed.
- K. L. Stanhope and P. J. Havel, Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup, Am. J. Clin. Nutr., 2008, 88(6), 1733S–1737S CrossRef CAS PubMed.
- K. L. Stanhope, J. M. Schwarz, N. L. Keim, S. C. Griffen, A. A. Bremer, J. L. Graham, B. Hatcher, C. L. Cox, A. Dyachenko, W. Zhang, J. P. McGahan, A. Seibert, R. M. Krauss, S. Chiu, E. J. Schaefer, M. Ai, S. Otokozawa, K. Nakajima, T. Nakano, C. Beysen, M. K. Hellerstein, L. Berglund and P. J. Havel, Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans, J. Clin. Invest., 2009, 119(5), 1322–1334 CrossRef CAS PubMed.
- J. Busserolles, W. Zimowska, E. Rock, Y. Rayssiguier and A. Mazur, Rats fed a high sucrose diet have altered heart antioxidant enzyme activity and gene expression, Life Sci., 2002, 71(11), 1303–1312 CrossRef CAS PubMed.
- A. Girard, S. Madani, F. Boukortt, M. Cherkaoui-Maiki, J. Belleville and J. Prost, Fructose enriched diet modifies antioxidant status and lipid metabolism in spontaneously hypertensive rats, Nutrition, 2006, 22(7–8), 758–766 CrossRef CAS PubMed.
- V. Thirunavukkarasu and C. V. Anuradha, Influence of α-lipoic acid on lipid peroxidation and antioxidant defense system in blood of insulin-resistant rats, Diabetes, Obes. Metab., 2004, 6(3), 200–207 CrossRef CAS PubMed.
- M. C. Castro, F. Francini, G. Schinella, C. I. Caldiz, M. G. Zubiría, J. J. Gagliardino and M. L. Massa, Apocynin administration prevents the changes induced by a fructose-rich diet on rat liver metabolism and the antioxidant system, Clin. Sci., 2012, 123(12), 681–692 CrossRef CAS PubMed.
- D. Tibullo, G. Li Volti, C. Giallongo, S. Grasso, D. Tomassoni, C. D. Anfuso, G. Lupo, F. Amenta, R. Avola and V. Bramanti, Biochemical and clinical relevance of alpha lipoic acid: antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential, Inflammation Res., 2017, 66(11), 947–959 CrossRef CAS PubMed.
- L. Packer, E. H. Witt and H. J. Tritschler, Alpha-Lipoic acid as a biological antioxidant, Free Radicals Biol. Med., 1995, 19(2), 227–250 CrossRef CAS PubMed.
- D. Han, G. Handelman, L. Marcocci, C. K. Senk, S. Roy, H. Kobuchi, H. J. Tritschler, L. Flohé and L. Packer, Lipoic acid increases de novo synthesis of cellular glutathione by improving cysteine utilization, Biofactors, 1997, 6(3), 321–338 CrossRef CAS PubMed.
- U. Cakatay, A. Telci, S. Salman, L. Satman and A. Sivas, Oxidative protein damage in type I diabetic patients with and without complications, Endocr. Res., 2000, 26(3), 365–379 CrossRef CAS PubMed.
- T. Morikawa, R. Yasuno and H. Wada, Do mammalian cells synthesize lipoic acid? Identification of a mouse cDNA encoding a lipoic acid synthase located in mitochondria, FEBS, 2001, 498(1), 16–21 CrossRef CAS PubMed.
- T. A. Seaton, P. Jenner and C. D. Marsden, The isomers of thioctic acid alter 14C-deoxyglucose incorporation in rat basal ganglia, Biochem. Pharmacol., 1996, 51(7), 983–986 CrossRef CAS PubMed.
- L. Packer, E. H. Witt and H. J. Tritschler, Alpha-Lipoic acid as a biological antioxidant, Free Radicals Biol. Med., 1995, 19(2), 227–250 CrossRef CAS PubMed.
- L. Packer and E. Cadenas, Lipoic acid: energy metabolism and redox regulation of transcription and cell signaling, J. Clin. Biochem. Nutr., 2011, 48(1), 26–32 CrossRef CAS PubMed.
- B. P. Cummings, K. L. Stanhope, J. L. Graham, J. L. Evans, D. G. Baskin, S. C. Griffen and P. J. Havel, Dietary fructose accelerates the development of diabetes in UCD-T2DM rats: amelioration by the antioxidant, α lipoic acid, Am. J. Physiol.: Regul., Integr. Comp. Physiol., 2010, 298(5), R1343–R1350 CrossRef CAS PubMed.
- V. Thirunavukkarasu, A. T. Nandhini and C. V. Anuradha, Lipoic acid prevents collagen abnormalities in tail tendon of high-fructose-fed rats, Diabetes, Obes. Metab., 2005, 7(3), 294–297 CrossRef CAS PubMed.
- M. C. Castro, M. L. Massa, G. Schinella, J. J. Gagliardino and F. Francini, Lipoic acid prevents liver metabolic changes induced by administration of a fructose-rich diet, Biochim. Biophys. Acta, 2013, 1830(1), 2226–2232 CrossRef CAS PubMed.
- D. Han, G. Handelman, L. Marcocci, C. K. Sen, S. Roy, H. Kobuchi, H. J. Tritschler, L. Flohé and L. Packer, Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization, Biofactors, 1997, 6(3), 321–338 CrossRef CAS.
- D. Han, H. J. Tritschler and L. Packer, Alpha-lipoic acid increases intracellular glutathione in a human T-lymphocyte Jurkat cell line, Biochem. Biophys. Res. Commun., 1995, 207(1), 258–264 CrossRef CAS PubMed.
- J. M. Lee and J. A. Johnson, An important role of Nrf2-ARE pathway in the cellular defense mechanism, J. Biochem. Mol. Biol., 2004, 37(2), 139–143 CAS.
- K. P. Shay, A. J. Michels, W. Li, A. N. T. Kong and T. M. Hagen, Cap-independent Nrf2 translation is part of a lipoic acid-stimulated detoxification stress response, Biochim. Biophys. Acta, 2012, 1823(6), 1102–1109 CrossRef CAS PubMed.
- M. Saygin, H. Asci, F. N. Cankara, D. Bayram, S. Yesilot, I. A. Candan and H. H. Alp, The impact of high fructose on cardiovascular system: Role of α-lipoic acid, Hum. Exp. Toxicol., 2016, 35(2), 194–204 CrossRef CAS.
- G. R. Haenen, N. P. Vermeulen, H. Timmerman and A. Bast, Effect of thiols on lipid peroxidation in liver microsomes, Chem.-Biol. Interact., 1989, 71(2–3), 201–212 CrossRef CAS PubMed.
- R. Sumathi, S. Jayanthi, V. Kalpanadevi and P. Varalakshmi, Effect of DL alpha-lipoic acid on tissue lipid peroxidation and antioxidant systems in normal and glycollate treated rats, Pharmacol. Res., 1993, 27(4), 309–318 CrossRef CAS PubMed.
- A. Oda, C. Bannai, T. Yamoka, T. Kotari, T. Matsushima and K. Yamashita, Inactivation of Cu, Zn-superoxide dismutase by in vitro glycosylation in erithrocytes of diabetic patients, Horm. Metab. Res., 1994, 26(1), 1–4 CrossRef CAS PubMed.
- K. Datta, S. Sinha and P. Chattopadhyay, Reactive oxygen species in health and disease, Natl. Med. J., 2000, 13(6), 304–310 CAS.
- S. Topsakal, O. Ozmen, F. N. Cankara, S. Yesilot, D. Bayram, N. Genç Özdamar and S. Kayan, Alpha lipoic acid attenuates high-fructose-induced pancreatic toxicity, Pancreatology, 2016, 16(3), 347–352 CrossRef CAS PubMed.
- M. Brownlee, The pathobiology of diabetic complications: a unifying mechanism, Diabetes, 2005, 54(6), 1615–1625 CrossRef CAS PubMed.
- M. C. Castro, M. L. Massa, H. Del Zotto, J. J. Gagliardino and F. Francini, Rat liver uncoupling protein 2: changes induced by a fructose-rich diet, Life Sci., 2011, 89(17–18), 609–614 CrossRef CAS PubMed.
- J. W. Joseph, V. Koshkin and C. Y. Zhang, et al., Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet, Diabetes, 2002, 51(11), 3211–3219 CrossRef CAS PubMed.
- M. C. Castro, F. Francini, J. J. Gagliardino and M. L. Massa, Lipoic acid prevents fructose-induced changes in liver carbohydrate metabolism: role of oxidative stress, Biochim. Biophys. Acta, 2014, 1840(3), 1145–1151 CrossRef CAS PubMed.
- C. Jang, S. Hui, W. Lu, A. J. Cowan, R. J. Morscher, G. Lee, W. Liu, G. J. Tesz, M. J. Bimbaum and J. D. Rabinowitz, The small intestine converts dietary fructose into glucose and organic acids, Cell Metab., 2018, 27(2), 351–361 CrossRef CAS PubMed.
- A. Gugliucci, Formation of fructose-mediated advanced glycation end products and their roles in metabolic and inflammatory diseases, Adv. Nutr., 2017, 8(1), 54–62 CrossRef CAS PubMed.
- P. A. Mayes, Intermediary metabolism of fructose, Am. J. Clin. Nutr., 1993, 58(5 Suppl), 754S–765S CrossRef CAS PubMed.
- D. Huang, T. Dhawan, S. Young, W. H. Yong, L. G. Boros and A. P. Heaney, Fructose impairs glucose-induced hepatic triglyceride synthesis, Lipids Health Dis., 2011, 10, 20, DOI:10.1186/1476-511X-10-20.
- D. E. Estrada, H. S. Ewart, T. Tsakiridus, A. Volchuk, T. Ramlal, H. Tritschler and A. Klip, Stimulation of glucose uptake by the natural coenzyme alpha-lipoic acid/thioctic acid: participation of elements of the insulin signaling pathway, Diabetes, 1996, 45(12), 1798–1804 CrossRef CAS.
- V. Thirunavukkarasu, A. T. Nandhini and C. V. Anuradha, Effect of alpha lipoic acid on lipid profile in rats fed a high-fructose diet, Exp. Diabesity Res., 2004, 5(3), 195–200 CrossRef CAS PubMed.
- H. M. Abdelkarem, L. H. Fadda and A. A. G. Hassan, Potential intervention of α- Lipoic acid and carnitine on insulin sensitivity and anti- inflammatory cytokines levels in fructose-fed rats, a model of metabolic syndrome, J. Diet. Suppl., 2016, 14, 54–64 CrossRef PubMed.
- D. T. Huong and T. Ide, Dietary lipoic acid-dependent changes in the activity and mRNA levels of hepatic lipogenic enzymes in rats, Br. J. Nutr., 2008, 100(1), 79–87 CrossRef CAS PubMed.
- J. D. Horton, J. L. Goldstein and M. S. Brown, SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver, J. Clin. Invest., 2002, 109(9), 1125–1131 CrossRef CAS PubMed.
- B. Desvergne, L. Michalik and W. Wahli, Transcriptional regulation of metabolism, Physiol. Rev., 2006, 86(2), 465–514 CrossRef CAS PubMed.
- J. P. Berger, A. E. Petro, K. L. Macnaul, L. J. Kelly, B. B. Zhang, K. Richards, A. Elbrecht, B. A. Johnson, G. Zhou, T. W. Doebber, C. Biswas, M. Parikh, N. Sharma, M. R. Tanen, G. M. Thompson, J. Ventre, A. D. Adams, R. Mosley, R. S. Surwit and D. E. Moller, Distinct properties and advantages of a novel peroxisome proliferator-activated protein γ selective modulator, Mol. Endocrinol., 2013, 17(4), 662–676 CrossRef PubMed.
- H. A. Pershadsingh, Alpha lipoc acid: physiologic mechanisms and indications for the treatment of metabolic syndrome, Expert Opin. Invest. Drugs, 2007, 16(3), 291–302 CrossRef CAS PubMed.
- Y. F. Tian, C. T. He, Y. T. Chen and P. S. Hsieh, Lipoic acid suppresses portal endotoxemia-induced steatohepatitis and pancreatic inflammation in rats, World J. Gastroenterol., 2013, 19(18), 2761–2771 CrossRef CAS PubMed.
- H. Ansar, Z. Mazloom, F. Kazemi and N. Hejazi, Effect of alpha-lipoic acid on blood glucose, insulin resistance, and glutathione peroxidase of type 2 diabetic patients, Saudi Med. J., 2011, 32(6), 584–588 Search PubMed.
- W. J. Lee, K. H. Song, E. H. Koh, J. C. Won, H. S. Kim, H. S. Park, M. S. Kim, S. W. Kim, K. U. Lee and J. Y. Park, Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle, Biochem. Biophys. Res. Commun., 2005, 332(3), 885–891 CrossRef CAS PubMed.
- R. H. Lustig, K. Mulligan, S. M. Noworolski, V. W. Tai, M. J. Wen, A. Erkin-Cakmak, A. Gugliucci and J. M. Schwarz, Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome, Obesity, 2016, 24(2), 453–460 CrossRef CAS.
- V. Tokarz, P. E. MacDonald and A. Klip, The cell biology of systemic insulin function, J. Cell Biol., 2018, 217(7), 2273–2289 CrossRef CAS PubMed.
- A. W. Thorburn, L. H. Storlien, A. B. Jenkins, S. Khouri and E. W. Kraegen, Fructose induced in vitro insulin resistance and elevated plasma triglyceride levels in rats, Am. J. Clin. Nutr., 1989, 49(6), 1155–1163 CrossRef CAS PubMed.
- W. P. Bieger, G. Michel, D. Barwich, K. Biehl and A. Wirth, Diminished insulin receptors on monocytes and erythrocytes in hypertriglyceridemia, Metabolism, 1984, 33(11), 982–987 CrossRef CAS PubMed.
- J. M. Schwarz, S. M. Noworolski, A. Erkin-Cakmak, N. J. Korn, M. J. Wen, V. W. Tai, G. M. Jones, S. P. Palii, M. Velasco-Alin, K. Pan, B. W. Patterson, A. Gugliucci, R. H. Lustig and K. Mulligan, Effects of Dietary Fructose Restriction on Liver Fat, De Novo Lipogenesis, and Insulin Kinetics in Children With Obesity, Gastroenterology, 2017, 153(3), 743–752 CrossRef CAS PubMed.
- A. Gugliucci, R. H. Lustig, R. Caccavello, A. Erkin-Cakmak, S. M. Noworolski, V. W. Tai, M. J. Wen, K. Mulligan and J. M. Schwarz, Short-term isocaloric fructose restriction lowers apoC-III levels and yields less atherogenic lipoprotein profiles in children with obesity and metabolic syndrome, Atherosclerosis, 2016, 253, 171–177 CrossRef CAS PubMed.
- V. Thirunavukkarasu, A. T. Nandhini and C. V. Anuradha, Lipoic acid attenuates hypertension and improves insulin sensitivity, kallikrein activity and nitrite levels in high fructose-fed rats, J. Comp. Physiol., B, 2004, 174(8), 587–592 CrossRef CAS PubMed.
- J. Patel, N. A. Matnor, A. Iyer and L. Brown, A regenerative antioxidant protocol of vitamin E and α-lipoic acid ameliorates cardiovascular and metabolic changes in fructose-fed rats, J. Evidence-Based Complementary Altern. Med., 2011, 120801, DOI:10.1155/2011/120801.
- D. Konrad, R. Somwar, G. Sweeney, K. Yaworsky, M. Hayashi, T. Ramlal and A. Klip, The antihyperglycemic drug alpha-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: potential role of p38 mitogen-activated protein kinase in GLUT4 activation, Diabetes, 2001, 50(6), 1464–1471 CrossRef CAS.
- K. Yaworsky, R. Somwar, T. Ramlal, H. J. Tritschler and A. Klip, Engagement of the insulin-sensitive pathway in the stimulation of glucose transport by alpha lipoic acid in 3T3-L1 adipocytes, Diabetologia, 2000, 43(3), 294–303 CrossRef CAS PubMed.
- H. Moini, O. Tirosh, Y. C. Park, K. J. Cho and L. Packer, R-alpha-lipoic acid action on cell redox status, the insulin receptor, and glucose uptake in 3T3-L1 adipocytes, Arch. Biochem. Biophys., 2002, 397(2), 384–391 CrossRef CAS.
- A. A. Gupte, G. L. Bomhoff, J. K. Morris, B. K. Gorres and P. C. Geiger, Lipoic acid increases heat shock protein expression and inhibits stress kinase activation to improve insulin signaling in skeletal muscle from high-fat-fed rats, J. Appl. Physiol., 2009, 106(4), 1425–1434 CrossRef CAS PubMed.
- B. Maiztegui, C. L. Román, J. J. Gagliardino and L. E. Flores, Impaired endocrine-metabolic homeostasis: underlying mechanism of its induction by unbalanced diet, Clin. Sci., 2018, 132(8), 869–881 CrossRef CAS PubMed.
- H. Tilg and A. R. Moschen, Inflammatory mechanisms in the regulation of insulin resistance, Mol. Med., 2008, 14(3–4), 222–231 CAS.
- G. S. Hotamisligil, N. S. Shargill and B. M. Spiegelman, Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance, Science, 1993, 259(5091), 87–91 CrossRef CAS PubMed.
- J. Hirosumi, G. Tuncman, L. Chang, C. Z. Görgün, K. T. Uysal, K. Maeda, M. Karin and G. S. Hotamisligil, A central role for JNK in obesity and insulin resistance, Nature, 2002, 420(6931), 333–336 CrossRef CAS PubMed.
- G. Kanuri, A. Spruss, S. Wagnerberger, S. C. Bischoff and I. Bergheim, Role of tumor necrosis factor α (TNFα) in the onset of fructose-induced nonalcoholic fatty liver disease in mice, J. Nutr. Biochem., 2011, 22(6), 527–534 CrossRef CAS PubMed.
- V. Aguirre, T. Uchida, L. Yenush, R. Davis and M. F. White, The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307), J. Biol. Chem., 2000, 275(12), 9047–9054 CrossRef CAS PubMed.
- G. S. Hotamisligil, P. Peraldi, A. Budavari, R. Ellis, M. F. White and B. M. Spiegelman, IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance, Science, 1996, 271(5249), 665–668 CrossRef CAS PubMed.
- H. Vlassara, R. Bucala and L. Striker, Pathogenic effects of advanced glycosylation: biochemical, biologic and clinical implications for diabetes and ageing, Lab. Invest., 1994, 70(2), 138–151 CAS.
- J. Uribarri, M. D. del Castillo, M. P. de la Maza, R. Filip, A. Gugliucci, C. Luevano-Contreras, M. H. Macías-Cervantes, D. H. Markowicz Bastos, A. Medrano, T. Menini, M. Portero-Otin, A. Rojas, G. R. Sampaio, K. Wrobel, K. Wrobel and M. E. Garay-Sevilla, Dietary advanced glycation end products and their role in health and disease, Adv. Nutr., 2015, 6(4), 461–473 CrossRef PubMed.
- N. Ahmed and A. J. Furth, Failure of common glycation assays to detect glycation by fructose, Clin. Chem., 1992, 38(7), 1301–1303 CAS.
- K. M. Reiser, Non-enzymatic glycation of collagen in ageing and diabetes, Proc. Soc. Exp. Biol. Med., 1998, 218(1), 23–37 CrossRef CAS PubMed.
- V. Thirunavukkarasu, A. T. Nandhini and C. V. Anuradha, Fructose diet-induced skin collagen abnormalities are prevented by lipoic acid, Exp. Diabesity Res., 2004, 5(4), 237–244 CrossRef CAS PubMed.
- H. Ghelani, V. Razmovski-Naumovski, R. R. Pragada and S. Nammi, (R)-α-Lipoic acid inhibits fructose-induced myoglobin fructation and the formation of advanced glycation end products (AGEs) in vitro, BMC Complementary Altern. Med., 2018, 18(1), 13, DOI:10.1186/s12906-017-2076-6.
- T. Han, J. Bai, W. Liu and Y. Hu, A systematic review and meta-analysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy, Eur. J. Endocrinol., 2012, 167(4), 465–471 CAS.
- J. Lambertz, S. Weiskirchen, S. Landert and R. Weiskirchen, Fructose: a dietary sugar in crosstalk with microbiota contributing to the development and progression of non-alcoholic liver disease, Front. Immunol., 2017, 8, 1159, DOI:10.3389/fimmu.2017.01159.
- J. Chen, Y. Li, Y. Tian, C. Huang, D. Li, Q. Zhong and X. Ma, Interaction between microbes and host intestinal health: modulation by dietary nutrients and gut-brain-endocrine-immune axis, Curr. Protein Pept. Sci., 2015, 16(7), 592–603 CrossRef CAS PubMed.
- P. Fan, P. Song, L. Li, C. Huang, J. Chen, W. Yang, S. Qiao, G. Wu, G. Zhang and X. Ma, Roles of biogenic amines in intestinal signaling, Curr. Protein Pept. Sci., 2017, 18(6), 532–540, DOI:10.2174/1389203717666160627073048.
| This journal is © The Royal Society of Chemistry 2019 |