Lactulose synergizes with CpG-ODN to modulate epithelial and immune cells cross talk
R.
Mukherjee
 *a,
M.
van de. Kaa†
b,
J.
Garssen
bc,
R. J.
Pieters
*a,
M.
van de. Kaa†
b,
J.
Garssen
bc,
R. J.
Pieters
 a,
A. D.
Kraneveld
bd and
L. E. M.
Willemsen
b
a,
A. D.
Kraneveld
bd and
L. E. M.
Willemsen
b
aDivision of Chemical Biology and Drug Discovery, Department of Pharmaceutical Sciences, Faculty of Science, Utrecht Institute of Pharmaceutical Sciences, Utrecht University, The Netherlands. E-mail: r.mukherjee@uu.nl; reshmi.mukherjee@gmail.com
bDivision of Pharmacology, Department of Pharmaceutical Sciences, Faculty of Science, Utrecht Institute of Pharmaceutical Sciences, Utrecht University, The Netherlands
cInstitute for Risk Assessment Sciences, Faculty of Veterinary Medicine, Utrecht University, The Netherlands
dNutricia Research, Utrecht, The Netherlands
First published on 4th January 2019
Abstract
Lactulose, a non-digestible oligosaccharide and functional food, promotes Bifidobacteria growth. Here we show that lactulose, beyond its prebiotic action, may have direct immunomodulatory effects as well. In synergy with CpG-ODN, a bacterial DNA mimetic, lactulose enhances basolateral concentrations of IFN-γ, IL-10, and galectin-9 in the co-culture model of epithelial and immune cells.
Lactulose, though not present in nature, occurs in heat-treated milk products. It was first produced in 1930 via catalyst-free Lobry de Bruyn-Alberda van Ekenstein (LA) rearrangement of lactose in a weak calcium hydroxide solution.1 Recently, various research groups have reported enzymatic syntheses of lactulose using the fungus Aspergillus oryzae, the archaea species Pyrococcus furiosus,2 and the bacterium Caldicellulosiruptor saccharolytisus.3
In contrast to lactose, humans cannot digest lactulose, mainly because human intestinal enzymes cannot cleave the β-(1,4)-glycosidic bond in lactulose. Most of the non-digested lactulose passes into the colon. The colonic bacteria, amongst others, Bifidobacteria, metabolize lactulose and produce short chain fatty acids (SCFAs).4 SCFAs are important for colon health and are proved to exert immunologic effects.5 These characteristics of lactulose have important biological and medical implications, thus creating many applications both in the food and pharmaceutical industries. Lactulose has been used medically since 1950.6 In the pharmaceutical industry, it is used for the treatment of various medical conditions, e.g. hyperammonemia/hepatic encephalopathy, constipation, small intestinal bacterial overgrowth, salmonella state carriers and for diagnostic applications.7 Lactulose is also used in veterinary medicine, mainly in hepatic encephalopathy and constipation.8 In the food industry, it is used as a prebiotic, a flavouring agent, and a sweetener.
Besides modifying the intestinal microbiota and promoting Bifidobacteria growth, lactulose also may show direct immunologic effects. Small amounts of lactulose are absorbed (0.25–2%), pass through the liver, and are available in the systemic circulation.6 These small amounts (∼30 μg mL−1) are sufficient for immunological reactions.6 When lactulose was administered intravenously, galactosamine-induced necrosis of hepatocytes and inflammatory reactions of liver tissue in rats were mostly prevented.9 In addition, the direct inhibitory effect of lactulose on endotoxin-induced tumor necrosis factor-α (TNFα) release by monocytes was observed as well.10
Other non-digestible oligosaccharides, e.g. a mixture of short chain galacto-oligosaccharides (scGOS) and long chain fructo-oligosaccharides (lcFOS), also show immunologic effects in the host. A diet containing scGOS/lcFOS (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and Bifidobacterium breve (containing immunomodulatory unmethylated CpG-DNA which is a Toll-like receptor (TLR)-9 ligand) suppresses allergic symptoms in mice and humans in association with induction of enhanced systemic galectin-9 levels.11 Galectin-9 is known to enhance regulatory T-cell function and to inhibit IgE-mediated mast cell degranulation.11,12 Furthermore, it was shown that galectin-9 enhances the secretion of regulatory-type IL-10 from T-cells and monocytes as well as Th1 polarization in an in vitro co-culture model of human intestinal epithelial cells (IECs) and peripheral blood mono-nuclear cells (PBMCs).13,14
1), and Bifidobacterium breve (containing immunomodulatory unmethylated CpG-DNA which is a Toll-like receptor (TLR)-9 ligand) suppresses allergic symptoms in mice and humans in association with induction of enhanced systemic galectin-9 levels.11 Galectin-9 is known to enhance regulatory T-cell function and to inhibit IgE-mediated mast cell degranulation.11,12 Furthermore, it was shown that galectin-9 enhances the secretion of regulatory-type IL-10 from T-cells and monocytes as well as Th1 polarization in an in vitro co-culture model of human intestinal epithelial cells (IECs) and peripheral blood mono-nuclear cells (PBMCs).13,14
The comparison of the chemical structures of scGOS, lcFOS, and lactulose, reveals that lactulose, a disaccharide, is a combination of the monosaccharides—galactose and fructose—that are present in both scGOS and lcFOS (Fig. 1).
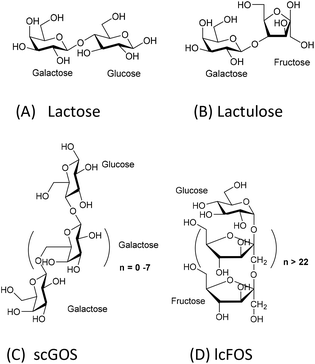 | ||
| Fig. 1 Chemical structures of prebiotics. A: lactose; B: lactulose; C: scGOS (only one representative structure is shown), scGOS is a mixture of many other structures; D: lcFOS. | ||
Therefore, we aimed to determine whether lactulose can promote some immunomodulatory effects like the scGOS/lcFOS mixture in the human co-culture model of IECs and PBMCs. The direct immunomodulatory effect of lactulose was compared to lactose upon exposure to IECs in the presence or absence of CpG-ODN, which is a mimetic of bacterial DNA as well as a TLR-9 ligand (agonist). The concentration of galectin-9, IFN-γ, and IL-10 were measured as a read-out. We chose lactose as a control since lactulose may be generated from lactose. Moreover, lactose is also a disaccharide, containing glucose and galactose. Hence, the degree of polymerization (thus roughly the molecular length and weight) was kept similar for the comparison of the observed effect.
Materials and methods
Lactulose was obtained from Fisher Scientific (99.3%) and lactose from Sigma, Zwijndrecht, the Nederlands. CD3 (clone CLB-T3/2), CD28 (clone CLB-CD28) antibodies as well as the buffy coats from healthy donors are purchased from Sanquin, Amsterdam, The Netherlands. CpG ODN M362 was purchased from Invitrogen San Diego USA.Transwell co-cultures
Human epithelial cells (HT-29 cell line), isolation of human PBMC and transwell cocultures were performed as previously described.10 HT-29 cells were grown till confluency on transwell insert filters (Corning, NY, USA). In the basolateral compartment 3 × 106 anti-CD3/CD28-activated PBMC were added below the IEC for 24 hours. IEC were exposed to medium, lactose (0.5% w/v) or lactulose (0.5% w/v) in absence or presence of the TLR9 ligand, CpG-ODN (5 μM). After 24 h the basolateral supernatant was collected and cytokines or galectin-9 were measured by means of ELISA.ELISA
The concentrations of IL10 (U-Cytech, Utrecht, The Netherlands) and IFNγ (Invitrogen, CA, USA) were measured according to the manufacturer's protocol. To assess the concentration of galectin-9, high-binding EIA/RIA 96-well plates (Costar; Corning Inc., Corning, N.Y., USA) were coated with 0.75 μg ml−1 primary antibodies (R&D Systems) and incubated overnight at 4 °C. 1% BSA in PBS was used to block the plates for 1 h. Samples or the human recombinant-galectin-9 (R&D systems) standard were then added for 2 h, washed and incubated with 0.75 μg ml−1 biotinylated secondary antibody (R&D Systems) in 1% BSA. Plates were washed and incubated with streptavidin-HRP (R&D Systems), washed and incubated with tetramethylbenzidine (Thermo Scientific, Rockford, Ill., USA). The reaction was stopped with 2 M H2SO4 and optical density was measured at 450 nm.11Statistics
Data were analysed using One-way ANOVA with Bonferroni post hoc test or for the ratios with Kruskall Wallis with Dunn's post hoc test in graph pad prism version 7.Results
Epithelial exposure to a combination of lactulose and CpG-ODN enhances IFN-γ and IL-10 production in the co-culture model
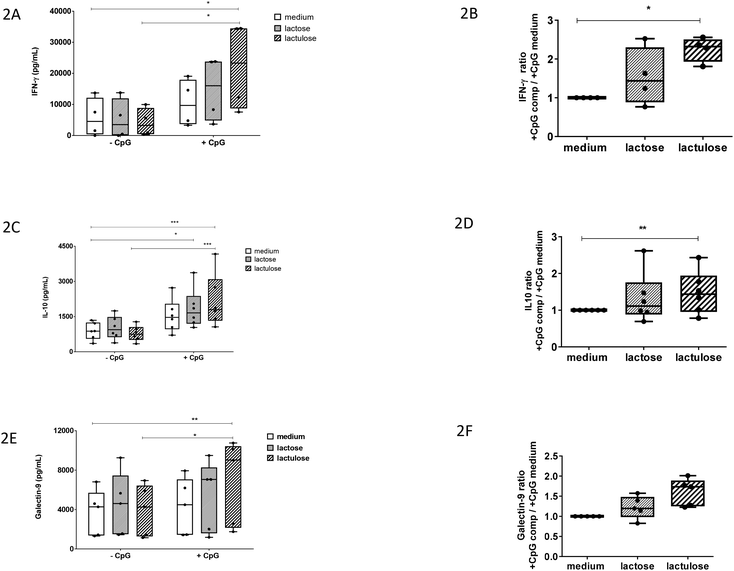
It was previously shown that apical scGOS/lcFOS potentiated the TLR9 ligand-induced IFN-γ secretion by the CD3/CD28-activated PBMCs via IECs dependent mechanisms.12 In our experiment with this co-culture model, apical exposure of IECs to lactulose or CpG-ODN alone did not enhance IFN-γ production by activated PBMCs. However, lactulose significantly enhanced the IFN-γ production by the PBMCs when added to the IECs simultaneously with CpG-ODN (Fig. 2A) indicating a synergistic effect between these components. Also, the effect of lactulose on the basolateral anti-inflammatory IL-10 production was determined in the IEC/PBMC co-culture model. Like the effect on IFN-γ, apical application of lactulose or CpG-ODN alone did not affect basolateral IL-10 production. By contrast, apical exposure of a combination of lactulose and CpG-ODN significantly enhanced the IL-10 concentration at the basolateral side (Fig. 2C). Apart from lactulose, it was observed that lactose was also capable to enhance the IL-10 production in the presence of CpG-ODN.In addition, we calculated the factor of increase in IFN-γ and IL-10 production induced by lactulose or lactose in the presence of CpG-ODN over the effect of CpG-ODN alone (Fig. 2B and D). These results confirm that apical IEC exposure to lactulose in the presence of CpG-ODN can potentiate the basolateral release of IFN-γ and IL-10 in the co-culture model.
Epithelial exposure to a combination of lactulose and CpG-ODN increases the galectin-9 concentration in the co-culture model
Previously, it was shown that IECs respond to the TLR-9 agonist CpG-ODN under inflammatory conditions by expressing and secreting galectin-9 in the co-culture model. It was also shown that this effect was further enhanced in the presence of scGOS/lcFOS.12 Therefore, we studied the effect of lactulose or lactose on the galectin-9 production in the co-culture model.Apical exposure of IECs to lactose, lactulose or CpG-ODN alone did not enhance the galectin-9 concentration (Fig. 2E). However, epithelial exposure to a combination of CpG-ODN and lactulose did enhance the basolateral galectin-9 concentration. On the contrary, the combination of lactose and CpG-ODN did not show the similar effect (Fig. 2E). The factor of increase in galectin-9 induced by combined exposure to lactulose and CpG-ODN over exposure to CpG-ODN alone showed a similar pattern compared to the ratio calculated for IFN-γ and IL-10 (Fig. 2F).
Discussion
The intestinal epithelium is the first line of defence against foreign/nonself-antigens. A single monolayer of IECs forms a tight barrier between the intestinal lumen and the lamina propria. It has been shown also that IECs take part actively in innate immune responses and shape adaptive immunity.15 IECs may be a potential target for intervention strategies to modulate immune responses in the gut.Beyond their function as a physical barrier, IECs can modulate innate and effector immune responses via cell–cell contact or soluble mediator release. IECs are known to express TLRs. TLRs recognize pathogens, e.g., fragments of bacteria. Human IECs were described to express TLRs at the surface at low levels during homeostasis and IECs are then unresponsive toward TLR ligands. However, during inflammation, TLR expression is increased allowing microbial components to modulate IEC function and mediator release.16–20
Dietary intervention using B. breve M-16 V and scGOS/lcFOS has been shown to partially prevent the development of allergic symptoms by increasing local intestinal and systemic galectin-9 levels.11 In mice as well as in a co-culture model of human IECs and activated PBMCs, IECs were identified as a source of galectin-9.11,12 In in vitro studies, using an IEC-PBMC co-culture model, it was demonstrated that genomic DNA from B. breve M-16V—a TLR-9 ligand, similar to synthetic agonist CpG-ODN—further enhances IFN-γ responses of activated PBMCs in an IEC-dependent manner.12 Furthermore, it was shown that galectin-9, a soluble mediator, released by IECs upon apical TLR9 ligand exposure, was essential for the IFN-γ and IL-10-polarized effector responses of the underlying PBMCs; this effect was further enhanced by scGOS/lcFOS. Interestingly, scGOS/lcFOS alone did not modulate the effector immune response in the IEC/PBMC co-culture model.
In the present study, we chose to evaluate the effects of lactulose in the co-culture model, which was previously used to identify the immunomodulatory effects of scGOS/lcFOS, because of its scientific merit. More recently, the effect of scGOS/lcFOS in inducing the production of galectin-9 by epithelial cells as well as enhancing in Th1 cells and regulatory IL-10 have been shown in a similar co-culture model using PBMCs from peanut allergy patients.21 Moreover, results derived from this co-culture model with IECs and PBMCs have been translated to a murine model of food allergy and infants affected with atopic dermatitis who were provided with a dietary intervention containing amongst others scGOS/lcFOS, which emphasized its validation.11,13
We show that lactulose in the presence of the TLR-9 agonist CpG-ODN, enhances basolateral galectin-9, IFN-γ, and regulatory IL-10 concentrations after 24 hours in the co-culture model. Lactulose on its own did not modulate these responses. This result suggests that lactulose has direct mucosal immunomodulatory effects like the scGOS/lcFOS mixture in combination with a TLR-9 ligand. Therefore, we hypothesize that, lactulose may have a potential to alleviate allergy development as well although further validation focussed on allergy management will be essential. We also showed that lactulose but not lactose synergizes with CpG-ODN to enhance the IFN-γ, IL-10, and galectin-9 concentrations in the co-culture model.
To understand the reason, we analyzed the chemical structural aspects of these oligosaccharides (Fig. 1). scGOS contains varying chain lengths of galactose with glucose at the reducing end. lcFOS contains various chain lengths of fructose with glucose at the reducing end. So, we hypothesized that the galactose and fructose moieties in the scGOS/lcFOS mixture might contribute at least in part to galectin-9 production and skewing of the immune response towards IL-10 and IFN-γ in the CpG-ODN exposed IEC/PBMC co-culture since both contain a glucose as a common reducing end. If that is true, a molecule containing fructose and galactose should show a comparable effect like the scGOS/lcFOS mixture for the specific immune effects as mentioned above. Indeed, lactulose, a non-digestible disaccharide composed of fructose and galactose within one molecule shows these similar immunomodulatory effects as was shown for scGOS/lcFOS in the human IEC/PBMC co-culture model regarding the specific immune effects measured.
The present study shows that lactose does not have similar immunomodulatory potential as lactulose, which may be attributed to its chemical structure. Lactose, a disaccharide, consists of glucose (at the reducing end) and galactose. The combination of these two monosaccharides may be not as effective in modulating the IEC response to CpG-ODN compared to the fructose and galactose combination in lactulose. Alternatively, lactose may be digested by epithelial enzymes, such as lactases, and therefore not functional. However, the IECs used in this study, HT29 cell line, do not express lactase.22 In addition, lactose is also a well-known galectin binder23 and in the used human co-culture model it may have interfered with the currently unknown mechanism that contributes to the increase in galectin-9 release by IECs or the mechanism by which galectin-9 exerts its immunomodulatory actions.
These findings may help to design potential drugs as well as food supplements to prevent or treat allergy and/or to alleviate allergic symptoms. Lactulose, being a disaccharide, opens opportunities for a small molecule carbohydrate-based drug design. Performing structure–activity relationships on lactulose would be relatively straightforward during the drug discovery process compared to longer oligosaccharides. Also, chemical modification on lactulose to improve its immunomodulatory potency would be relatively easier as well due to its smaller size. In the future, a lactulose-based drug may see its potential in the pharmaceutical industry.
Conclusions
Lactulose is present in heat-treated milk and is produced by non-catalytic rearrangement of lactose. Lactose and lactulose have different reducing ends—glucose and fructose, respectively. This study identified the difference in immunomodulatory potential between lactose and its derivative lactulose in a human co-culture model of IECs and activated PBMCs. Lactulose synergizes with CpG-ODN to enhance IFN-γ, IL-10 and galectin-9 concentrations in this co-culture model. These immunomodulatory effects were very similar as previously observed for some effects induced by a mixture of scGOS/lcFOS. On the contrary, lactose only enhanced IL-10 concentrations when combined with CpG-ODN. We think that the unique molecular structure of lactulose may contribute to its efficacy in enhancing the immune polarizing properties of CpG-ODN, which may link to its potential to reduce allergy risk. Lactulose may be further developed as an anti-allergy small molecule carbohydrate-based drug by coupling additional bioactive groups. Future studies in in vivo allergy models and clinical validation are essential to test lactulose's prophylactic and therapeutic potentials.Conflicts of interest
Authors do not have any conflict to declare. JG is partly employed at Nutricia Research, Utrecht, the Netherlands.Acknowledgements
This study is supported by the seed grant from the Utrecht Institute of Pharmaceutical Sciences (UIPS), Utrecht University, the Netherlands.Notes and references
- E. M. Montgomery and C. S. Hudson, J. Am. Chem. Soc., 1930, 52, 2101 CrossRef CAS.
- J. Mayer, J. Conrad, I. Klaiber, S. Lutz-Wahl, U. Beifuss and L. Fischer, J. Agric. Food Chem., 2004, 52, 6983 CrossRef CAS PubMed.
- E. Rentschler, K. Schuh, M. Krewinkel, C. Baur, W. Claaßen, S. Meyer, B. Kuschel, T. Stressler and L. Fischer, J. Dairy Sci., 2015, 98, 6767 CrossRef CAS PubMed.
- J. Ballongue, C. Schumann and P. Quignon, Scand. J. Gastroenterol., 1997, 32(sup 222), 41–44 CrossRef CAS PubMed.
- J. Tan, C. McKenzie, M. Potamitis, A. N. Thorburn, C. R. Mackay and L. Macia, Adv. Immunol., 2014, 121, 91–119 CAS.
- C. Schumann, Eur. J. Nutr., 2002, 41(Suppl 1), I/17 Search PubMed.
- A. Szilagyi, Handbook of prebiotics and probiotics ingredients, Health benefits and food applications, Tailor and Francis, 2010, p. 99 CrossRef CAS PubMed; P. S. Panesar and S. Kumari, Biotechnol. Adv., 2011, 29, 940 CrossRef CAS PubMed.
- G. Jankowski, J. Exot. Pet Med., 2009, 18, 156 CrossRef.
- H. Liehr and W. D. Heine, Hepatogastroenterology, 1981, 28, 296 CAS.
- J. W. Greve, D. J. Gouma, P. A. von Leeuwen and W. A. Buurman, Gut, 1990, 31, 198 CrossRef CAS.
- S. de Kivit, E. Saeland, A. D. Kraneveld, H. J. G. van de Kant, B. Schouten, B. C. A. M. van Esch, J. Knol, A. B. Sprikkelman, L. B. van der Aa, L. M. J. Knippels, J. Garssen, Y. van Kooyk and L. E. M. Willemsen, Allergy, 2012, 67, 343 CrossRef CAS PubMed.
- S. de Kivit, A. D. Kraneveld, L. M. J. Knippels, Y. van Kooyk, J. Garssen and L. E. M. Willemsen, J. Innate Immun., 2013, 5, 625 CrossRef CAS PubMed.
- S. de Kivit, A. I. Kostadinova, J. Kerperien, M. E. Morgan, V. A. Muruzabal, G. A. Hofman, L. M. J. Knippel, A. D. Kraneveld, J. Garssen and L. E. M. Willemsen, J. Leukocyte Biol., 2017, 102, 105 CrossRef CAS PubMed.
- S. de Kivit, A. I. Kostadinova, J. Kerperien, V. A. Muruzabal, M. E. Morgan, L. M. J. Knippels, A. D. Kraneveld, J. Garssen and L. E. M. Willemsen, J. Innate Immun., 2017, 9, 609 CrossRef CAS PubMed.
- I. D. Iliev, E. Mileti, G. Matteoli, M. Chieppa and M. Rescigno, Mucosal Immunol., 2009, 2, 340 CrossRef CAS PubMed.
- A. Uehara, Y. Fujimoto, K. Fukase and H. Takada, Mol. Immunol., 2007, 44, 3100 CrossRef CAS PubMed.
- J. B. Ewaschuk, J. L. Backer, T. A. Churchill, F. Obermeier, D. O. Krause and K. L. Madsen, Infect. Immun., 2007, 75, 2572 CrossRef CAS PubMed.
- G. Melmed, L. S. Thomas, N. Lee, S. Y. Tesfay, K. Lukasek, K. S. Michelsen, Y. Zhou, B. Hu, M. Arditi and M. T. Abreu, J. Immunol., 2003, 170, 1406 CrossRef CAS.
- J. C. Singh, S. M. Cruickshank, D. J. Newton, L. Wakenshaw, A. Graham, J. Lan, J. P. Lodge, P. J. Felsburg and S. R. Carding, Am. J. Physiol.: Gastrointest. Liver Physiol., 2005, 288, G514 CrossRef CAS PubMed.
- M. T. Abreu, P. Vora, E. Faure, L. S. Thomas, E. T. Arnold and M. Arditi, J. Immunol., 2001, 167, 1609 CrossRef CAS.
- S. M. Hayen, H. G. Otten, S. A. Overbeek, A. C. Knulst, J. Garssen and L. E. M. Willemsen, Front. Immunol., 2018, 9, 923 CrossRef PubMed.
- I. Chantret, A. Barbat, E. Dussaulx, M. G. Brattain and a. Zweibaum, Cancer Res., 1988, 48, 1936 CAS.
- M. Nagae, N. Nishi, T. Murata, T. Usui, T. Nakamura, S. Wakatsuki and R. Kato, J. Biol. Chem., 2006, 281, 35884 CrossRef CAS PubMed.
Footnote |
| † Present address: The division Internal Medicine and Dermatology, at the department Nephrology and Hypertension, University Medical Center Utrecht, The Netherlands. |
| This journal is © The Royal Society of Chemistry 2019 |