White-emitting organometallo-silica nanoparticles for sun-like light-emitting diodes†
Cintia
Ezquerro‡
a,
Elisa
Fresta‡
b,
Elena
Serrano
 c,
Elena
Lalinde
c,
Elena
Lalinde
 a,
Javier
García-Martínez
a,
Javier
García-Martínez
 *c,
Jesús R.
Berenguer
*c,
Jesús R.
Berenguer
 *a and
Rubén D.
Costa
*a and
Rubén D.
Costa
 *b
*b
aDepartamento de Química-Centro de Investigación en Síntesis Química (CISQ), Universidad de La Rioja, Madre de Dios, 53, E-26006, Logroño, La Rioja, Spain. E-mail: jesus.berenguer@unirioja.es
bIMDEA Materials Institute, Calle Eric Kandel 2, E-28906 Getafe, Madrid, Spain. E-mail: ruben.costa@imdea.org
cLaboratorio de Nanotecnología Molecular, Departamento de Química Inorgánica, Universidad de Alicante, Ctra. Alicante-S. Vicente s/n, E-03080 Alicante, Spain. E-mail: j.garcia@ua.es
First published on 10th September 2018
Abstract
This work discloses a radically new way to prepare white-emitting hybrid nanoparticles, whose implementation in lighting devices provides encouraging proof-of-concept performances towards alternative sunlight sources. In detail, the new synthetic approach is based on the kinetic control of the formation of organometallic dots, built via the condensation of three emitting iridium(III) complexes, which are subsequently transformed into mesoporous silica nanoparticles. Our novel hybrid systems, which are exceptionally stable under harsh irradiation and thermal stress environments, show a bright white emission with a record photoluminescence quantum yield. Their remarkable performance prompted us to implement them into single-component hybrid light-emitting diodes (HLEDs), achieving a high-quality sunlight source that is stable for >2000 hours with linearly extrapolated stabilities of >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h. This represents one of the most stable HLEDs reported so far, while the versatility of our synthesis approach with respect to the type of emitters opens new opportunities for the design and fabrication of white-emitting color down-converters for HLEDs in the future.
000 h. This represents one of the most stable HLEDs reported so far, while the versatility of our synthesis approach with respect to the type of emitters opens new opportunities for the design and fabrication of white-emitting color down-converters for HLEDs in the future.
Conceptual insightsWhite hybrid light-emitting diodes (WHLEDs) combine a high-energy emitting LED with low-energy emitting organic color down-converting packings, which are easy-to-prepare and eco-friendly. Here, one of the most outstanding down-converting materials is luminescent organometallo-silica nanoparticles. To date, the synthesis methodology has been limited by the incorporation of a single complex, leading to a moderate color stability in WHLEDs due to different aging rates. Herein, we disclose a new synthesis protocol based on the kinetic control of the formation of organometallic dots with three emitting iridium(III) complexes, which are subsequently transformed into mesoporous silica nanoparticles. The white organometallo-silica nanoparticles show record photoluminescence quantum yields stable under severe scenarios. Noteworthily, WHLEDs prepared by using this novel down-converting material provide encouraging proof-of-concept performances including a stable (>2000 h) emission with a sun-like spectrum. |
Introduction
To design the next generation of artificial illumination, two main routes have been developed. In a first approach, white organic light-emitting diodes (WOLEDs) have raised great expectations in both industrial and scientific communities.1 However, they require a multilayered structure based on several organic semiconductors, which are needed for charge injection, charge transport/blocking, and light emission.2 Although there are large-area devices featuring luminous efficiencies of 50–60 lm W−1 and stabilities of thousands of hours,2a this is not sufficient for their large scale application as it is still necessary to develop (i) simple architectures to enhance charge transport and recombination, as well as light out-coupling, (ii) stable blue emitters, and (iii) easy and low-cost fabrication processes.2a,b In a second approach, all-inorganic white light-emitting diodes (IWLEDs) are dominating the lighting market. IWLEDs are highly efficient UV- or blue-emitting inorganic chips covered by color down-converting coatings that partially transform the high-energy chip emission into visible light.1,3 The most widely used color down-converters are based on yellow-emitting rare-earth doped crystals, such as cerium-doped yttrium aluminium garnet (Y3Al5O12:Ce3+; YAG:Ce).4 They feature excellent photo- and thermal-stabilities, high photon flux saturations and photoluminescence quantum yields (ϕ) > 70%. However, reliance on rare-earth materials implies high production costs, as well as uncertain availability. In addition, and despite many efforts, the possibility to use chemical routes to tune the color emission of these compounds is still a challenge, which greatly limits the LED color quality of these systems.1,3,4 Certainly, this is a major concern, because a high blue-light component is hazardous for the human retina of both children and elderly, as well as the circadian cycle and the hormonal system of adults.5 Hence, much effort is put into enhancing the white light quality of LEDs by better mimicking natural sunlight.5The current limitations in the development of high quality lighting systems have, therefore, encouraged further efforts to produce hybrid inorganic/organic WLED (HWLED) architectures.6 Here, organic or inorganic color down-converters, such as polymers, coordination complexes, carbon nanodots or fluorescent proteins, have been tested in new packaging matrices like polymers, cellulose, metal organic frameworks (MOFs), etc.7 State-of-the-art HWLEDs involve luminous efficiencies up to 120 lm W−1 and high color quality with x/y CIE color coordinates of 0.30–3/0.30–3, color rendering index (CRI) above 80–90, and correlated color temperatures (CCT) ranging from 2700 K to 6500 K. Their major drawback is the device stability, which is typically limited to a maximum of a few hundred hours.6,7 Among the color down-converters mentioned above, organometallic complexes stand out.7i,k,m,8
In light of the aforementioned discussion, there are two major challenges for the preparation of high quality and stable HWLEDs. On the one hand, it is highly desirable to develop new color down-converting coatings with enhanced photostabilities and thermal management, which will allow for high irradiation intensities under ambient conditions. On the other hand, there is a strong need for white-emitting single-component down-converting coatings. The use of mixtures of emitters leads to a loss of performance stemming from (i) reabsorption, (ii) phase separation over time, and (iii) poor color stability due to differences in both thermal quenching and aging rates of the emitters.
Tackling both challenges simultaneously constitutes the major thrust of the work at hand. Our approach builds on the synthetic strategies developed to modify silica nanoparticles via encapsulation or grafting of a myriad of chromophores, which have produced highly promising results. These studies include perovskite nanocrystals, quantum dots, organic dyes or carbon nanodots.9 In an effort to better integrate the active moiety in the silica matrix, we10 and others7m,11 have developed the so-called Sol–Gel Coordination Chemistry, which is based on the co-condensation of an emissive organometallic complex bearing alkoxysilane terminal groups with a silica source – i.e. tetraethyl orthosilicate (TEOS). This approach allows for preparing hybrid materials with the chromophore homogeneously incorporated into the silica framework. Recently, this strategy has also been used to obtain luminescent organometallo-silica materials featuring enhanced stability and photophysical properties compared to bare complexes.10 However, to date, this methodology has only been focused on the incorporation of a single organometallic complex.7m,11,12
Herein, we report a novel strategy for the synthesis of the first white-emitting organometallo-silica nanoparticles, in which blue-, green-, and red-emitting organometallic Ir(III) complexes are simultaneously encapsulated (three-in-one) via a sol–gel process inside silica nanoparticles. A key aspect of our synthetic approach is the kinetic control of the formation of the white-emitting organometallic dots (ODs), built prior to the growth of the silica nanoparticles (NPs). But the most remarkable contribution of our method is that the combination of the emission characteristics of the three complexes leads to an exceptionally high quality white emission that is stable under harsh environments. As such, the white-emitting nanoparticles have been used as color down-converters to develop single-component HWLEDs, featuring excellent color quality (sun-like light) that are stable over thousands of hours (>2000 h continuous and >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h using a linear extrapolation up to 50% brightness) measured in air. While their performance stands out among the state-of-the-art HWLEDs,6,7 this works presents an original and versatile synthesis approach to design highly emissive white emitters, which paves the road for future breakthroughs in the HWLED field.
000 h using a linear extrapolation up to 50% brightness) measured in air. While their performance stands out among the state-of-the-art HWLEDs,6,7 this works presents an original and versatile synthesis approach to design highly emissive white emitters, which paves the road for future breakthroughs in the HWLED field.
Results
Synthesis and photophysical properties of the organometallic precursors
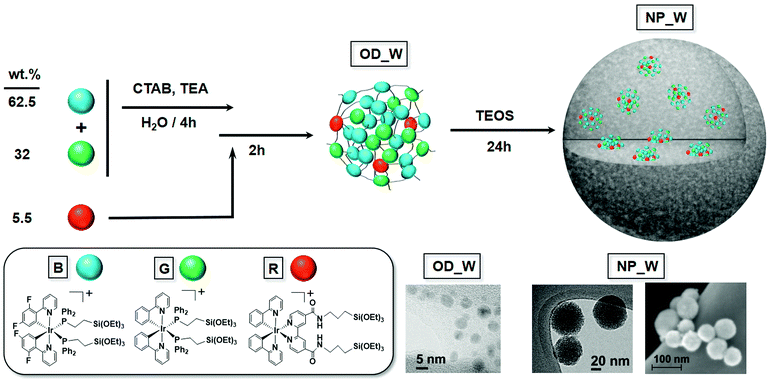
To prepare the white-emitting organometallo-silica nanoparticles, we focused on cyclometalated Ir(III) complexes, which are currently among the most efficient, stable, and emission tunable color phosphorescent emitters.13 In detail, we have recently reported the bis-phosphine complex [Ir(ppy)2(PPETS)2]OTf (G) [ppy = 2-phenylpyridinyl, PPETS = PPh2(CH2)2Si(OEt)3]10a – Scheme 1 – which features a green unstructured emission band in the solid-state (298 K) – Fig. 1; maximum emission wavelength or λem = 512 nm, ϕ = 31.2%, excited-state lifetime or τ = 0.97 μs – associated to a triplet excited state with a mixed 3ILCT(ppy)/3MLCT(Ir → ppy) nature – i.e., ILCT refers to intra-ligand charge transfer and MLCT refers to metal-to-ligand charge transfer. Blue emission was attained using the dfppy ligand to obtain the complex [Ir(dfppy)2(PPETS)2]PF6 (B) [dfppy = 2-(2,4-difluorophenyl)pyridinyl] – Scheme 1. The latter exhibits a blue-shifted emission in the solid-state (298 K) – Fig. 1; λem = 480 nm, ϕ = 23.5%, τ = 1.55 μs – also ascribed to a triplet excited state with a mixed 3ILCT(dfppy)/3MLCT(Ir → dfppy) nature. Finally, the red-emitting chromophore was obtained by replacing the PPETS ligands of the G derivative by a bipyridine ligand bearing two alkoxysilane groups – i.e., dasipy = 4,4′-[CONH(CH2)3Si(OEt)3]-bipyridine. As expected, the resulting complex [Ir(ppy)2(dasipy)]OTf (R; Scheme 1) shows a red emission in the solid-state (298 K) – Fig. 1; λem = 620 nm, ϕ = 16.5%, τ = 0.13 μs – attributed to a triplet excited state with a mixed 3ML′CT(Ir → dasipy)/3LL′CT(ppy → dasipy) having a remarkable ML′CT character – i.e., LL′CT refers to ligand-to-ligand charge transfer. The three alkoxysilane derivatives have been obtained from their respective solvato precursors – i.e., [Ir(ppy)2(MeCN)2]+ and [Ir(dfppy)2(MeCN)2]+ – and fully characterized by the usual analytical and spectroscopic means, as well as theoretical calculations – see Experimental procedures, Tables S1–S4 and Fig. S1–S6 in the ESI† for further details.Preparation and characterization of the organometallo-silica nanoparticles
The synthetic procedure to prepare the hybrid white-emitting organometallo-silica nanoparticles (NP_W) is based on a two-step sol–gel process performed at room temperature. The white emission was achieved using an optimized content of the three Ir(III) complexes, namely 0.125 wt% B, 0.064 wt% G, and 0.011 wt% R, which yields a mass ratio of 62.5 B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 G
32 G![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 R. It should be noted that a low content of the red-emitting complex in the mixture was required, as the increase in the concentration of R always gives rise to emissions with a majority contribution of the red component. This fact, along with the good overlap between the low energy absorption feature of R and the emissions of complexes B and G in the solid state (450–550 nm, see Fig. S7, ESI†), points to the occurrence of a certain degree of energy transfer from the blue and green chromophores to the red one.
5.5 R. It should be noted that a low content of the red-emitting complex in the mixture was required, as the increase in the concentration of R always gives rise to emissions with a majority contribution of the red component. This fact, along with the good overlap between the low energy absorption feature of R and the emissions of complexes B and G in the solid state (450–550 nm, see Fig. S7, ESI†), points to the occurrence of a certain degree of energy transfer from the blue and green chromophores to the red one.
In detail, the three alkoxysilane derivatives B, R, and G were first prehydrolyzed in an ethanolic–water mixture under kinetic control, in the presence of hexadecyltrimethylammonium bromide (CTAB) to moderate the growth of the organometallic dots formed (OD_W) – Scheme 1. Taking into account the different rates of hydrolysis of PPETS complexes when compared to the dasipy derivative, complexes B and G were firstly hydrolyzed for 4 h followed by the addition of R. The mixture was stirred for 2 additional hours – see the ESI† for further details. In this way, the alkoxysilane groups of the complexes condensate under mild conditions forming small organometallic dots (OD_W) of about 5 nm in diameter as estimated by Transmission Electron Microscopy (TEM) – Scheme 1 and Fig. S8 (ESI†). According to their volume in relation with the average volume of the molecules of the organometallic complexes, the OD_W organometallic dots must be formed by less than 50 molecules that are covalently bonded together through Si–O bonds. The OD_W organometallic dots were then subjected to further sol–gel condensation with TEOS added to the reaction media and then reacted for 24 hours at room temperature, yielding the final hybrid white-emitting organometallo-silica nanoparticles NP_W – Scheme 1 and Fig. S9 (ESI†). Similarly, monochromatic emitting blue (NP_B), green (NP_G), and red (NP_R) hybrid silica nanoparticles, with a nominal iridium content of 0.2 wt%, were also obtained from their respective B, G, and R complexes, through the previous formation of the corresponding OD_B,G,R organometallic dots – Scheme S1 and Fig. S9 (ESI†). Finally, Field Emission Scanning Electron Microscopy (FESEM) analysis of both the monochromatic NP_B,G,R and the white-emitting NP_W also corroborated nearly monodispersed spherical shaped particles with sizes in the 50–70 nm range – Fig. S10 (ESI†) for NP_W. ICP analyses of these solids gave incorporation yields between 80–90% – Table S5 (ESI†).
Physisorption experiments were carried out to study the porosity of the organometallo-silica nanoparticles. For comparative purposes, the isotherm and pore size distribution of related organometallo-free silica nanoparticles (Control NP; see ESI†) are also included in Fig. S11 (ESI†). All samples yielded similar type IV isotherms with Brunauer–Emmett–Teller (BET) surface areas of ca. 1000 m2 g−1 and mesopore volumes in the 0.5–0.7 cm3 g−1 range – Fig. S11 and Table S5 (ESI†).14 An additional adsorption process takes place at P/P0 > 0.8, which is a characteristic of interparticle porosity. Thanks to the use of CTAB as a surfactant, the mesopores are quite homogeneous in size, as evidenced by their narrow pore size distribution with an average pore size in the 2.1–2.4 nm range – Fig. S11 (ESI†). This feature was also observed via TEM. Both the monochromatic NP_B,G,R and the white emitting NP_W nanoparticles showed the typical morphology of mesoporous discrete silica nanoparticles prepared in the presence of CTAB – Fig. S9 (ESI†). Moreover, both the mesopore volume and the average mesopore diameter remained almost the same after incorporating the organometallic complexes into the silica NPs. This fact is consistent with the integration of the initially formed emissive ODs through the silica matrix,10 and with the photophysical properties of the hybrid silica as described below.
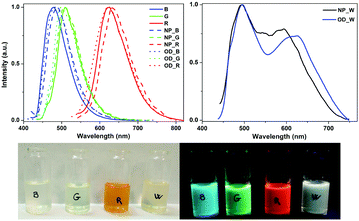
The organometallo-silica nanoparticles NP_B,G,R and their corresponding dots OD_B,G,R display similar emission properties to those observed for the corresponding B, G, and R complexes in the solid-state – Fig. 1, Fig. S12–S19, and Tables S6, S7 (ESI†). This indicates that the ODs are generated without any significant structural change of their constituent complexes. Notably, although smaller than those found for the pure complexes, the NP_B,G,R nanoparticles show ϕ ranging from 10 to 20%.
In contrast, NP_W nanoparticles, which are formed from a mixture of the B, G, and R complexes in the appropriate mass content – vide supra, exhibit a white emission associated to a broad (full width at half maximum of ca. 6250 cm−1) band with maxima at 490 and 595 nm along with a tail extending up to 750 nm – Fig. 1. Not unexpectedly, the photoluminescence response is excitation wavelength (λexc) dependent. As shown in Fig. S18 (right) (ESI†), the lowest energy emission feature intensifies upon exciting at lower energies. The excitation spectrum (Fig. S18, left, ESI†) monitored at the red low-energy emission peak (580 nm) shows a peak at 500 nm, which overlaps with the emission for both the blue and green emitters. This fact also suggests the occurrence of an energy transfer process from the high-energy emitting complexes to the red-emitting one inside the NP_W, as noted before on the basis of absorption and emission spectra (see Fig. S7, ESI†). Further confirmation was obtained upon comparing the τ values of NP_W monitored at 480 nm (0.57 μs), 510 nm (0.64 μs), and 595 nm (0.63 μs) with those of NP_B (0.99 μs), NP_G (0.89 μs), and NP_R (0.12 μs) at their respective emission maxima – Table S7 (ESI†). Here, the energy transfer process is pinpointed by the slight τ decrease of the blue and green emitters and a clear τ increase of the red one.15 The variation in the excited state lifetimes suggests a certain degree of Förster resonance energy transfer. However, in multimetallic systems incorporating phosphorescent emitters (Ru(II), Os(II), and Ir(III)), a significant triplet–triplet Dexter contribution to the energy transfer rate has been reported, although chromophores are not able to get a good conjugation in the ground state.15b
In order to investigate the importance of having the three complexes in close proximity, we mixed the appropriate amounts of NP_B, NP_G, and NP_R nanoparticles, obtaining a homogeneous mixture with the same weight ratio of chromophores as in NP_W. As shown in Fig. S19 (ESI†), the mixture shows a weaker broad emission band, likely due to some degree of reabsorption, in which the low energy maximum (ca. 600 nm) is missing. Hence, the energy transfer is only feasible in a short distance range process – i.e., the OD_W size of ca. 5 nm formed with the three complexes in the NP_W sample,15a,16 and it cannot happen in the homogeneous mixture of NP_B,G,R, where each single emitter is localized inside the silica nanoparticles, which are 50–70 nm in diameter. Indeed, similar emission properties were noted for the OD_W dots compared to that of the NP_W dots – Table S7 and Fig. S18 and S19 (ESI†). All-in-all, the occurrence of a certain degree of energy transfer in the in situOD_W and NP_W from the blue and green chromophores to the red one explains the high contribution of the low energy emission, despite the small relative amount of R in NP_W (5.5 wt%). Noteworthily, the white emission is associated to a ϕ value of 20.5% (λexc = 390 nm), which represents one of the highest values reported for white emitting silica nanoparticles.9a–e,g,i
Design and study of HWLEDs
These findings encouraged us to shed light on the prospect of the novel white-emitting hybrid silica nanoparticles as color down-converting coatings and/or packagings for HWLEDs. Following our previous reports,6a,c,7g–i both pure organometallic complexes (B, G, R) and monochromatic emitting nanoparticles NP_B,G,R were implemented into a rubber-like coating – i.e., hereafter OC- or NP-rubbers – based on a mixture of branched and linear poly(ethylene oxide) polymers – see Fig. 2 and the ESI† for more details.The photoluminescence features of these OC- and NP-rubbers (5 mm thick) were firstly investigated. Similar emission spectra to those observed for both the B, G or R complexes and NP_B,G,R nanoparticles were noted – Fig. S20 and S21 (ESI†), indicating that the emitter–matrix interaction is not very strong. Moreover, the rubbers show excellent photo- and thermal-stabilities under both ambient conditions and harsh environments, such as long-term storage, UV irradiation (310 nm, 8 W), and thermal treatment up to 70 °C – Fig. S20 and S21 (ESI†). While all of them show excellent stabilities over months upon storage, the rubbers with the emitting nanoparticles show superior photo- and thermal-stabilities – i.e., no changes were observed under extreme conditions (UV irradiation at 70 °C) compared to rubbers with only coordination complexes, which quickly degrade after a few hours – Fig. S20 and S21 (ESI†).
Following these assays, we decided to fabricate a series of HLEDs using a commercial UV-LED with a 400 nm emitting chip – i.e., WINGER® WEPUV3-S2 UV Power LED Star (Blacklight) 1.2 W – covered by thick (1 mm) color down-converting coatings based on rubbers with the organometallic complexes (OC-HLEDs) and the monochromatic NP_B, NP_G, and NP_R hybrid nanoparticles (NP-HLEDs). These devices were driven (i) at different applied currents, to evaluate their color conversion properties, and (ii) at constant current, to determine the device stability. In both experiments, the changes in the overall spectrum, the luminous efficiency, and the temperature of the HLEDs were monitored – see the ESI† for more details. Upon applying different currents ranging from 20 to 200 mA, the superior performance of NP-HLEDs was attested by the color down-conversion efficiency (ηcon), which is defined as the ratio between the maxima of the down-converting coatings and the LED emission bands – Fig. S22 (ESI†), while both OC- and NP-HLEDs showed a linear increase of the ηconversus applied current, indicating that there is no saturation, bleaching, and/or non-linear related quenching effects.
Next, the beneficial effect of incorporating the complexes into the silica nanoparticles during their synthesis was clearly revealed by the stability tests, which were performed at a constant applied current of 150 mA. As reference devices, blue and green OC-HLEDs showed luminous efficiencies of 0.27 and 1.65 lm W−1, respectively. They show a quick degradation (blue OC-HLED) or an exponential decrease (green OC-HLED) in luminous efficiency within the first hour – Fig. S23 and S24 (ESI†). In addition, the emission maxima of the color down-conversion band for blue OC-HLEDs significantly changed from 495 to 520 nm, along with the x/y CIE color coordinates from 0.18/0.18 to 0.31/0.44 after 4 hours of operation. In contrast, red-emitting OC-HLEDs showed a stable emission spectrum during several hours – i.e., a maximum emission centered at 630 nm and x/y CIE color coordinates of 0.61/0.29 – Fig. S25 (ESI†). This was associated with a low luminous efficiency of 0.03 lm W−1. In stark contrast, the NP-HLEDs showed much higher and stable luminous efficiencies, reaching values of 1.24, 1.30, and 1.50 lm W−1 for blue-, green-, and red-emitting NP-HLEDs, respectively – Fig. S23–S25 (ESI†). Importantly, the temperature of the down-converting coatings reached 30–32 °C for both OC- and NP-HLEDs. This fact strongly suggests that the main impact of the silica encapsulation is the isolation of the emitters from both the ambient oxygen and the residual solvent present in the matrix, along with the geometry constraint of the organometallic molecules which limits non-radiative vibrational relaxation motion upon continuous excitation. Hence, the use of the hybrid NPs (NP_B,G,R) leads to HLEDs with enhanced luminous efficiency and stability features.
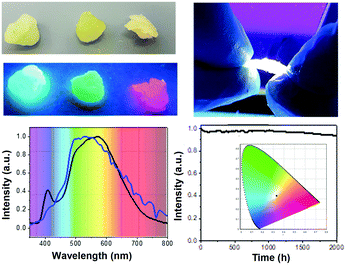
Similar to the NP-HLEDs, we fabricated single-component NP-HWLEDs using the NP_W nanoparticles. Remarkably, these devices showed a broad emission spectrum with a maximum at 590 nm closely flanked by shoulders at ca. 500 and 620 nm, which perfectly matches with that of the natural sunlight measured in Madrid on December; 2017 – Fig. 2. Moreover, NP-HWLEDs showed minimal changes with respect to the emission spectrum at different measuring angles – Fig. S26 (ESI†), suggesting a uniform spherical light distribution. Finally, these devices show a remarkable stability over 2000 h under continuous operation conditions, as shown in – Fig. 2. This is confirmed by the neglectable changes in luminous efficiency of 2.5 lm W−1 (<5% decrease) and constant x/y CIE color coordinates of 0.34/0.33, CRI of 85, and CCT of 5143 K, representing one of the most stable HWLEDs reported so far.7d–f,b,i,17 What is more, these devices can reach values of ca. 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h of stability using a linear extrapolation up to 50% of the maximum luminance – Fig. S27 (ESI†). To highlight the importance of using a single-component device, we assembled related multi-component devices using a mixture of NP_B, NP_G, and NP_R with the same weight ratio to that of the organometallic complexes in NP_W. As expected, the lack of an efficient energy transfer between monochromatic NPs – vide supra, leads to devices failing to provide white light due to the lack of emission in the low-energy region – i.e., λ > 600 nm, Fig. S28 (ESI†).
000 h of stability using a linear extrapolation up to 50% of the maximum luminance – Fig. S27 (ESI†). To highlight the importance of using a single-component device, we assembled related multi-component devices using a mixture of NP_B, NP_G, and NP_R with the same weight ratio to that of the organometallic complexes in NP_W. As expected, the lack of an efficient energy transfer between monochromatic NPs – vide supra, leads to devices failing to provide white light due to the lack of emission in the low-energy region – i.e., λ > 600 nm, Fig. S28 (ESI†).
Conclusions
In conclusion, we have disclosed an innovative and efficient way of realizing the first white-emitting organometallo-silica nanoparticles. They have been obtained by kinetically controlling the covalent bonding at the molecular level of three different organometallic chromophores prior to the formation of the silica matrix around them. These white-emitting nanoparticles show a high photoluminescence quantum yield value (20%) that is stable over months under harsh environments. In addition, these white hybrid silica nanoparticles have been implemented into a rubber-like down-converting coating, achieving one of the most stable single-component HWLEDs to date. Moreover, the excellent light distribution and color quality of the new HWLEDs were demonstrated by comparing them with the sunlight spectrum. As such, this work provides a ground-breaking contribution towards eye-friendly, exceptionally efficient, and highly stable HWLEDs. These unique features, which are usually mutually exclusive, have been achieved, thanks to the use of a new three-in-one approach that makes it possible to overcome quenching, poor color stability, and phase separation issues, which are common drawbacks of organic color down-converting coatings for HWLEDs. Our strategy has successfully overcome these limitations by using white-emitting silica nanoparticles (NP_W), which combine a better preservation of the integrity of the complexes, thanks to the geometry constraint produced by the silica matrix, and the proximity of the chromophores within the white-emitting organometallic dots (OD_W), which are incorporated within the protective silica shell.Although the device stability values represent a breakthrough, the maximum efficiency is far from the state-of-the-art. As such, future work will be directed towards obtaining organometallic complexes with higher photoluminescence quantum yields and closer surface nanoparticles to enhance the environmental protection and to avoid undesired light reflections. This can be controlled by reducing the time exposition of the surfactant, as well as using other surfactant derivatives. In addition, a more comprehensive photophysical study is required to fully understand the energy transfer mechanism within the Ir(III) complexes in the core of the hybrid silica nanoparticles. Finally, we strongly believe that our approach can easily be extrapolated to other emitters. Here, excellent candidates are thermally stable perylene diimide derivatives that provide WHLEDs with stabilities over 500 h measured in air and on chip as recently reported by Song and Qu groups.18
Overall, the versatility and simplicity of our new synthesis approach, as well as the excellent optical properties and stability of our hybrid nanoparticles and devices, are likely to open new and exciting opportunities for the design and fabrication of new white emitters and HWLEDs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. E., E. L. and J. R. B. acknowledge Spanish MINECO and AEI/FEDER (ref. CTQ2016-78463-P). C. E. also thanks Universidad de La Rioja for a grant. E. F. and R. D. C. acknowledge the program “Ayudas para la atracción de talento investigador – Modalidad 1 of the Consejería de Educación, Juventud y Deporte – Comunidad de Madrid with the reference number 2016-T1/IND-1463”. R. D. C. acknowledges Spanish MINECO for the Ramón y Cajal program (RYC-2016-20891). J. G. M. acknowledges Spanish MINECO and AEI/FEDER (ref. CTQ2014-60017-R). E. S. thanks Spanish MINECO and AEI/FEDER (ref. CTQ2015-74494-JIN) and the University of Alicante (“Ayudas para la captación de talento” program with the reference number UATALENTO16-03).Notes and references
- J. Kido, K. Hongawa, K. Okuyama and K. Nagai, Appl. Phys. Lett., 1994, 64, 815–817 CrossRef CAS.
- (a) D. Zhao, Z. Qin, J. Huang and J. Yu, Org. Electron., 2017, 51, 220–242 CrossRef CAS; (b) J. Chen, F. Zhao and D. Ma, Mater. Today, 2014, 17, 175–183 CrossRef CAS; (c) LG OLED (http://www.lgoledlight.com/); (d) OLEDWorks (https://www.oledworks.com/); (e) Konoca Minolta Pioneer (https://www.kompo.jp/en/).
- J. Cho, J. H. Park, J. K. Kim and E. F. Schubert, Laser Photonics Rev., 2017, 11, 1600147 CrossRef.
- (a) T. M. Tolhurst, S. Schmiechen, P. Pust, P. J. Schmidt, W. Schnick and A. Moewes, Adv. Opt. Mater., 2016, 4, 584–591 CrossRef CAS; (b) P.-P. Dai, C. Li, X.-T. Zhang, J. Xu, X. Chen, X.-L. Wang, Y. Jia, X. Wang and Y.-C. Liu, Light: Sci. Appl., 2016, 5, e16024 CrossRef CAS PubMed; (c) W. Cheng, F. Rechberger and M. Niederberger, ACS Nano, 2016, 10, 2467–2475 CrossRef CAS PubMed.
- (a) U.S. Department of Energy (https://energy.gov/sites/prod/files/2016/06/f32/ssl_rd-plan_%20jun2016_0.pdf); (b) J. Denneman, Voice of the Lighting Industry in Europe Opens Exciting Opportunities for the European Lighting Industry, 2016.
- (a) P. Schlotter, R. Schmidt and J. Schneider, Appl. Phys. A: Mater. Sci. Process., 1997, 64, 417–418 CrossRef CAS; (b) V. Fernández-Luna, P. Coto and R. D. Costa, Angew. Chem., Int. Ed., 2018, 57, 8826–8836 CrossRef PubMed; (c) E. Fresta, V. F. Luna, P. B. Coto and R. D. Costa, Adv. Funct. Mater., 2018, 28, 1707011 CrossRef.
- (a) I. O. Huyal, U. Koldemir, T. Ozel, H. V. Demir and D. Tuncel, J. Mater. Chem., 2008, 18, 3568–3574 RSC; (b) N. J. Findlay, J. Bruckbauer, A. R. Inigo, B. Breig, S. Arumugam, D. J. Wallis, R. W. Martin and P. J. Skabara, Adv. Mater., 2014, 26, 7290–7294 CrossRef CAS PubMed; (c) H. Yoo, H. S. Jang, K. Lee and K. Woo, Nanoscale, 2015, 7, 12860–12867 RSC; (d) C.-Y. Sun, X.-L. Wang, X. Zhang, C. Qin, P. Li, Z.-M. Su, D.-X. Zhu, G.-G. Shan, K.-Z. Shao, H. Wu and J. Li, Nat. Commun., 2013, 4, 2717 CrossRef PubMed; (e) Y. Cui, T. Song, J. Yu, Y. Yang, Z. Wang and G. Qian, Adv. Funct. Mater., 2015, 25, 4796–4802 CrossRef CAS; (f) D. Zhou, H. Zou, M. Liu, K. Zhang, Y. Sheng, J. Cui, H. Zhang and B. Yang, ACS Appl. Mater. Interfaces, 2015, 7, 15830–15839 CrossRef CAS PubMed; (g) L. Niklaus, S. Tansaz, H. Dakhil, K. T. Weber, M. Pröschel, M. Lang, M. Kostrzewa, P. B. Coto, R. Detsch, U. Sonnewald, A. Wierschem, A. R. Boccaccini and R. D. Costa, Adv. Funct. Mater., 2017, 27, 1601792 CrossRef; (h) M. D. Weber, L. Niklaus, M. Pröschel, P. B. Coto, U. Sonnewald and R. D. Costa, Adv. Mater., 2015, 27, 5493–5498 CrossRef CAS PubMed; (i) L. Niklaus, H. Dakhil, M. Kostrzewa, P. B. Coto, U. Sonnewald, A. Wierschem and R. D. Costa, Mater. Horiz., 2016, 3, 340–347 RSC; (j) W. Liu, Y. Fang, G. Z. Wei, S. J. Teat, K. Xiong, Z. Hu, W. P. Lustig and J. Li, J. Am. Chem. Soc., 2015, 137, 9400–9408 CrossRef CAS PubMed; (k) G. Meng, Z. Chen, H. Tang, Y. Liu, L. Wei and Z. Wang, New J. Chem., 2015, 39, 9535–9542 RSC; (l) E.-P. Yao, Z. Yang, L. Meng, P. Sun, S. Dong, Y. Yang and Y. Yang, Adv. Mater., 2017, 29, 1606859 CrossRef PubMed; (m) O.-H. Kim, S.-W. Ha, J. I. Kim and J.-K. Lee, ACS Nano, 2010, 4, 3397–3405 CrossRef CAS PubMed.
- (a) R. D. Costa, E. Ortí, H. J. Bolink, F. Monti, G. Accorsi and N. Armaroli, Angew. Chem., Int. Ed., 2012, 51, 8178–8211 CrossRef CAS PubMed; (b) V. K. Praveen, C. Ranjith and N. Armaroli, Angew. Chem., Int. Ed., 2014, 53, 365–368 CrossRef CAS PubMed; (c) E. Fred Schubert, J. Cho and J. Kyu Kim, Reference Module in Materials Science and Materials Engineering, Elsevier, 2016 Search PubMed.
- (a) X. Di, L. Shen, J. Jiang, M. He, Y. Cheng, L. Zhou, X. Liang and W. Xiang, J. Alloys Compd., 2017, 729, 526–532 CrossRef CAS; (b) Y. Qu, L. Feng, B. Liu, C. Tong and C. Lü, Colloids Surf., A, 2014, 441, 565–571 CrossRef CAS; (c) F. Gai, T. Zhou, L. Zhang, X. Li, W. Hou, X. Yang, Y. Li, X. Zhao, D. Xu, Y. Liu and Q. Huo, Nanoscale, 2012, 4, 6041–6049 RSC; (d) M. N. Luwang, R. S. Ningthoujam, S. K. Srivastava and R. K. Vatsa, J. Mater. Chem., 2011, 21, 5326–5337 RSC; (e) J. Malinge, C. Allain, A. Brosseau and P. Audebert, Angew. Chem., Int. Ed., 2012, 51, 8534–8537 CrossRef CAS PubMed; (f) D. Chen, G. Fang and X. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 40477–40487 CrossRef CAS PubMed; (g) C. Liu, Z. Zhao, R. Zhang, L. Yang, Z. Wang, J. Yang, H. Jiang, M.-Y. Han, B. Liu and Z. Zhang, J. Phys. Chem. C, 2015, 119, 8266–8272 CrossRef CAS; (h) H.-S. Jung, Y.-J. Kim, S.-W. Ha and J.-K. Lee, J. Mater. Chem. C, 2013, 1, 5879–5884 RSC; (i) M. Melucci, M. Zambianchi, G. Barbarella, I. Manet, M. Montalti, S. Bonacchi, E. Rampazzo, D. C. Rambaldi, A. Zattoni and P. Reschiglian, J. Mater. Chem., 2010, 20, 9903–9909 RSC; (j) W. Nianfang, K. Sungjun, J. Byeong Guk, L. Dongkyu, K. Whi Dong, P. Kyoungwon, N. Min Ki, L. Kangha, K. Yewon, L. Baek-Hee, L. Kangtaek, B. Wan Ki and C. L. Doh, Nanotechnology, 2017, 28, 185603 CrossRef PubMed.
- (a) C. Ezquerro, A. E. Sepulveda, A. Grau-Atienza, E. Serrano, E. Lalinde, J. R. Berenguer and J. Garcia-Martinez, J. Mater. Chem. C, 2017, 5, 9721–9732 RSC; (b) M. Rico, A. E. Sepulveda, S. Ruiz, E. Serrano, J. R. Berenguer, E. Lalinde and J. Garcia-Martinez, Chem. Commun., 2012, 48, 8883–8885 RSC.
- (a) D. J. Lewis, V. Dore, N. J. Rogers, T. K. Mole, G. B. Nash, P. Angeli and Z. Pikramenou, Langmuir, 2013, 29, 14701–14708 CrossRef CAS PubMed; (b) S. Liu, J. Zhang, D. Shen, H. Liang, X. Liu, Q. Zhao and W. Huang, Chem. Commun., 2015, 51, 12839–12842 RSC; (c) S. Zanarini, E. Rampazzo, L. Ciana, M. Marcaccio, E. Marzocchi, M. Montalti, F. Paolucci and L. Prodi, J. Am. Chem. Soc., 2009, 131, 2260–2267 CrossRef CAS PubMed; (d) S. Bonacchi, D. Genovese, R. Juris, M. Montalti, L. Prodi, E. Rampazzo and N. Zaccheroni, Angew. Chem., Int. Ed., 2011, 50, 4056–4066 CrossRef CAS PubMed.
- (a) Q. Zhao, Y. Liu, Y. Cao, W. Lv, Q. Yu, S. Liu, X. Liu, M. Shi and W. Huang, Adv. Opt. Mater., 2015, 3, 233–240 CrossRef CAS; (b) G. Valenti, E. Rampazzo, S. Bonacchi, L. Petrizza, M. Marcaccio, M. Montalti, L. Prodi and F. Paolucci, J. Am. Chem. Soc., 2016, 138, 15935–15942 CrossRef CAS PubMed; (c) H. Sun, J. Zhang, K. Y. Zhang, S. Liu, H. Liang, W. Lv, W. Qiao, X. Liu, T. Yang, Q. Zhao and W. Huang, Part. Part. Syst. Charact., 2015, 32, 48–53 CrossRef CAS; (d) L. Donato, Y. Atoini, E. A. Prasetyanto, P. Chen, C. Rosticher, C. Bizzarri, K. Rissanen and L. De Cola, Helv. Chim. Acta, 2018, 101, e1700273 CrossRef.
- (a) A. F. Henwood and E. Zysman-Colman, Chem. Commun., 2017, 53, 807–826 RSC; (b) D. Ma, T. Tsuboi, Y. Qiu and L. Duan, Adv. Mater., 2017, 29, 1603253 CrossRef PubMed; (c) F. Lafolet, S. Welter, Z. Popovic and L. D. Cola, J. Mater. Chem., 2005, 15, 2820–2828 RSC.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CAS.
- (a) V. E. Pritchard, D. Rota Martir, E. Zysman-Colman and M. J. Hardie, Chem. – Eur. J., 2017, 23, 8839–8849 CrossRef CAS PubMed; (b) R. Muñoz-Rodríguez, E. Buñuel, N. Fuentes, J. A. G. Williams and D. J. Cárdenas, Dalton Trans., 2015, 44, 8394–8405 RSC.
- (a) L. Grösch, Y. Lee, F. Hoffmann and M. Fröba, Chem. – Eur. J., 2015, 21, 331–346 CrossRef PubMed; (b) F. de Trindade, E. Triboni, B. Castanheira and S. Brochsztain, J. Phys. Chem. C, 2015, 119, 26989–26998 CrossRef; (c) G. Schwartz, S. Reineke, T. C. Rosenow, K. Walzer and K. Leo, Adv. Funct. Mater., 2009, 19, 1319–1333 CrossRef CAS.
- (a) Q. Gong, Z. Hu, B. J. Deibert, T. J. Emge, S. J. Teat, D. Banerjee, B. Mussman, N. D. Rudd and J. Li, J. Am. Chem. Soc., 2014, 136, 16724–16727 CrossRef CAS PubMed; (b) H. Tetsuka, A. Nagoya and R. Asahi, J. Mater. Chem. C, 2015, 3, 3536–3541 RSC.
- J. He, S. Yang, K. Zheng, Y. Zhang, J. Song and J. Qu, Green Chem., 2018, 20, 3557–3565 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental procedures and theoretical calculations of complexes B and R. Textural and morphological characterization of the nanoparticles. Photophysical properties of the complexes, nanoparticles and rubber-like materials. Fabrication, characterization and photostability measurements of WHLEDs. See DOI: 10.1039/c8mh00578h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |