Amphibious superamphiphilic fabrics with self-healing underwater superoleophilicity†
Sida
Fu‡
a,
Hua
Zhou‡
a,
Hongxia
Wang
 *a,
Haitao
Niu
a,
Weidong
Yang
b,
Hao
Shao
a and
Tong
Lin
*a,
Haitao
Niu
a,
Weidong
Yang
b,
Hao
Shao
a and
Tong
Lin
 *a
*a
aInstitute for Frontier Materials, Deakin University, Geelong, VIC 3216, Australia. E-mail: hong.wang@deakin.edu.au; tong.lin@deakin.edu.au
bFuture Manufacturing Flagship, CSIRO, Clayton South, VIC 3169, Australia
First published on 27th September 2018
Abstract
Most of the superamphiphilic surfaces reported so far show an oleophobic feature in underwater environments because once they are wetted with water their wettability is governed by the water layer adsorbed on the surface. In contrast, underwater oleophilic surfaces often show superhydrophobicity–oleophilicity in a dry state in air. A challenge remains in developing a superamphiphilic surface that shows both superhydrophilicity and underwater superoleophilicity (i.e. underwater superamphiphilicity). Herein, we demonstrate a novel strategy to prepare a surface that simultaneously possesses superamphiphilicity in air and underwater environments (also referred to as “amphibious superamphiphilicity” in this paper). A single-step wet-chemical coating method was employed to apply a crosslinkable polymer material, which consists of hydrophilic and oleophilic functional groups, onto a fabric substrate. The fabric after coating treatment exhibited amphibious superamphiphilicity with a contact angle of 0° for water and oils. In the dry state, it took less than 1 second for water and oil fluids of surface tension in the range of 18.4–50.8 mN m−1 to spread completely on the surface. In water, although the fabric was quickly wetted, it still allowed oils to spread completely into the wetted fabric matrix in less than 1 minute. More interestingly, the underwater superoleophilicity is self-healable against chemical damage. We further showed that such amphibious superamphiphilicity has great potential for recovery of oil from water. No matter whether the fabric was in a dry or pre-wetted state, it showed a similar oil absorption capability. The high resilience against moisture environments made oil recovery very stable. In addition, the coating is durable enough against various types of harsh damage. Such unusual superamphiphilicity may offer novel properties and applications in diverse fields.
Conceptual insightsIn this study, a novel strategy to endow fabrics with amphibious superamphiphilicity (i.e. superamphiphilicity simultaneously in air and underwater environments) has been demonstrated. The fabrics have a contact angle of 0° for water and oils. In the dry state, it took less than 1 second for water and oil fluids to spread completely on the surface. In water, the fabric was quickly wetted but still allowed oils to spread completely into the matrix in less than 1 minute. Such amphibious superamphiphilicity is significantly different to the previously reported superamphiphilic surfaces in that the previous superamphiphilicity just works either in the dry state in air or in underwater environments. The in-air only superamphiphilicity, however, shows an underwater oleophobic feature because once the surface is wetted with water its wettability is governed by the surface water layer, whereas underwater superoleophilicity often shows superhydrophobicity–oleophilicity in air. Our amphibious superamphiphilicity offers great potential for applications in oil recovery from water. Its amphibiousness considerably enhances the resilience of the recovery materials against moisture environments, ensuring stable oil recovery performance. In addition, the amphibious superamphiphilicity shows a self-healing underwater superoleophilicity against chemical damage. |
Superamphiphilicity possesses both superhydrophilicity and superoleophilicity with a contact angle close to 0° for water and oil fluids, and water and oil fluids can spread on the surface in a very short time.1,2 Superhydrophilic/superoleophilic surfaces have received tremendous interest in both academic and industrial areas owing to their novel properties and potential applications for self-cleaning,3 antifogging,4 liquid separation,5–7 liquid transport,8,9 gas bubble manipulation,10 and fabrication of polymer films.11 Superamphiphilic surfaces have been prepared by various methods such as electrospinning,12 coating with nanoparticle-containing polymer followed by plasma etching,13 etching and oxidation of solid surfaces11 and dip-coating.14
The above superamphiphilicity works under the condition that the surface is dry and exposed to air (also referred to “in air superamphiphobicity”). However, when the surfaces are in underwater environments, they must show a diffident wettability. Taking cotton as an example, cotton fabrics have a superamphiphilic surface. In underwater environments, however, they show a superoleophobic feature. When cotton is pre-wetted with oil, it shows a hydrophobic property. A similar underwater superoleophobic feature was also reported on other superamphiphilic surfaces.15–18 In contrast, most of the underwater superoleophilic surfaces reported so far show in air superhydrophobicity,19–21 and the underwater superoleophilicity was realized chiefly through a plastron layer formed in water, which allows oil drops to break air bubbles easily and wick on surfaces. Recently, Zhang et al.22 reported an organic gel which showed super-spreading capability for oil in underwater environments when it was pre-swollen with oil. The special wettability originated from a combination of hydraulic pressure and liquid-like properties. Gao et al.23 fabricated an organohydrogel that showed underwater superoleophilic properties, though the in-air wettability was not reported. Despite these reports, it still remains a challenge to develop a surface that simultaneously possesses superamphiphilicity in air and underwater environments (also referred to as “amphibious superamphiphilicity” in this paper).
To achieve amphibious superamphiphilicity, oil fluid needs to overcome the interfacial adhesion between water and the solid surface. Because no air bubbles are entrapped on the surface, the interfacial adhesion between water and the hydrophilic surface is large. The oil should have stronger interfacial adhesion to the surface than water. A novel surface design at a molecular level would facilitate this. Amphibious superamphiphilicity is expected to enhance the resilience of superamphiphilic surfaces against aqueous environments. It will be particularly useful for applications in areas where the oleophilicity is frequently interfered by water.
Herein, we report a novel strategy to prepare a superamphiphilic fabric that works simultaneously in air and underwater environments. A polyester fabric was used as a substrate, and a specially designed polymer, which is crosslinkable and consists of both hydrophilic and oleophilic functional groups, was used as a coating material. A one-step wet-chemical coating method was employed to apply the coating materials onto a fabric substrate. The fabric after treatment showed a contact angle (CA) of 0° for water. Water spread completely into the fabric within 1 second. Sixteen types of commonly used oil fluids (all water-immiscible with surface tension of 18.4–50.8 mN m−1) were chosen for a superoleophilic test. No matter whether the surface was dry (in air), pre-wetted with water, or immersed in water, it showed a CA of 0° for the oils. In water, the oil fluids can spread on the fully water wetted fabric within 1 minute. Different from the previously reported underwater superoleophilic surfaces, our treated fabric achieves underwater oil spreading through a competitive repulsion mechanism to repel water off the surface. More interestingly, the coating showed a self-healing ability to restore the underwater superamphiphilicity after being damaged by a chemical method (e.g. oxygen plasma treatment or UV irradiation). As a demonstration, we further showed that the amphibious superamphiphilic fabric had a high resilience against moisture environments when used for recovery of oil from water. No matter whether the fabric was in a dry or pre-wetted state, it showed a similar oil absorption capability.
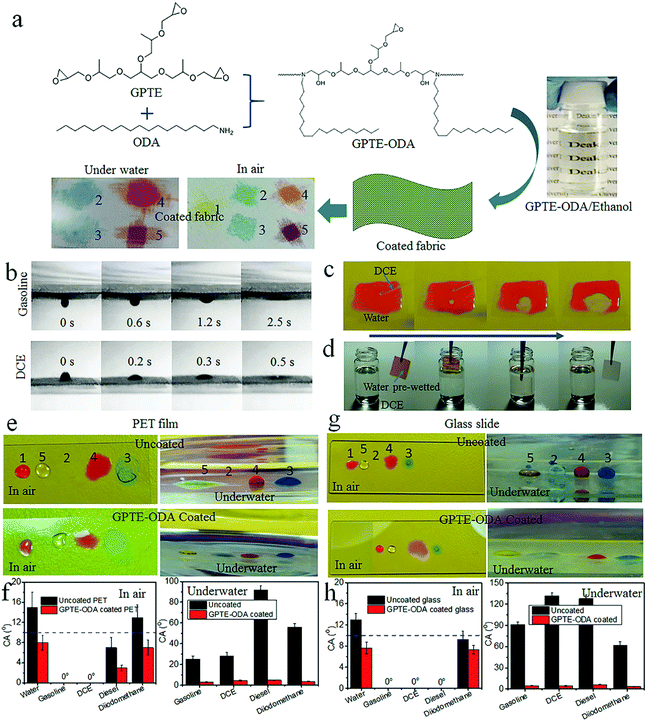
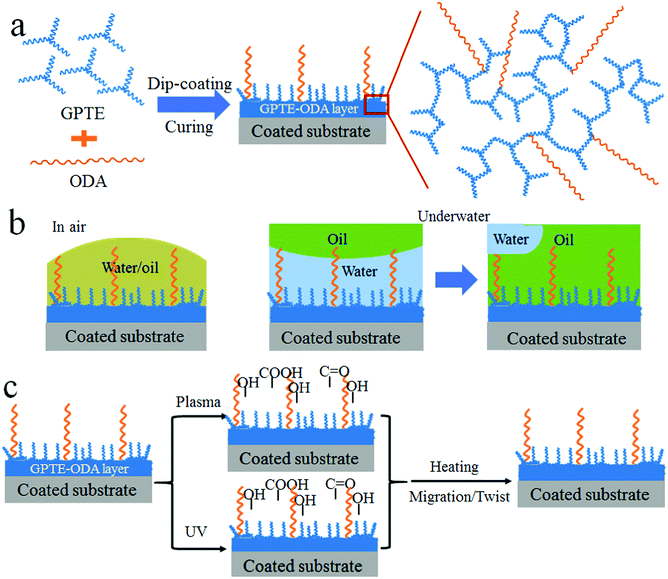
To prepare the amphibious superamphiphilic fabric, a woven polyester fabric was used as a substrate, and a dip coating technique was used for coating treatment. The coating solution was prepared by mixing glycerol propoxylate triglycidyl ether (GPTE) with octadecylamine (ODA) in ethanol. Fig. 1a shows the chemical structures of GPTE and ODA and the possible reaction product (GPTE–ODA). GPTE can react with ODA via an epoxide-amino coupling reaction. After completing the reaction, the solution looks transparent. It was very stable under ambient conditions. After storage at room temperature for one month, the solution was still very transparent without precipitation (Fig. S1, ESI†).
The GPTE–ODA epoxide-amino coupling product was verified by Fourier transform infrared (FTIR) spectroscopy (Fig. S2, ESI†) and nuclear magnetic resonance (Fig. S3, ESI†). The gel permeation chromatography (GPC) test indicated that the reaction product had a weight-average molecular weight (Mw) of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 653 g mol−1 when GPTE and ODA in the reaction solution was at the ratio of 5
653 g mol−1 when GPTE and ODA in the reaction solution was at the ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (wt/wt) (Fig. S4, ESI†).
1 (wt/wt) (Fig. S4, ESI†).
Fig. 1a also shows the coating procedure for fabric treatment. A dip-coating method was employed to apply the coating solution onto polyester fabric. After drying and curing, a conformal coating layer was formed on fiber, and a crosslinking reaction took place between epoxide and secondary alcohol in the GPTE–ODA. The reaction of epoxide with secondary alcohol was well reported in the field of epoxy resin.24–26 Such an etherification reaction took place during the curing process. The reaction mechanism is illustrated in Fig. S5 (ESI†). After curing, the C–O vibration band was slightly strengthened as shown in Fig. S6 (ESI†).
The surface morphology of the polyester fabric before and after coating treatment was observed using a scanning electron microscope (SEM). For the uncoated fabric, the fibers had a smooth surface, whereas the fiber after coating treatment became rougher (Fig. S7, ESI†). Atomic force microscope (AFM) imaging indicated that the fiber after coating treatment increased the root-mean-square (RMS) roughness from 7.9 nm to 22.4 nm (Fig. S8, ESI†).
The surface chemistry of the polyester fabric before and after coating treatment was examined by FTIR and X-ray photoelectron spectroscopy (XPS) (see the spectra in Fig. S9 and S10, ESI†). After coating treatment, new peaks at 2927 cm−1 and 2863 cm−1 occurred in the FTIR spectra, which were assigned to the C–H stretching vibrations of methylene in an alkyl group. The new peak at 1371 cm−1 was assigned to the CH3 symmetric deformation. The peaks at 1096 cm−1 strengthened, corresponding to the stretching vibration of C–O–C. The new peaks at 931 cm−1 corresponded to the epoxy group asymmetric vibration. These results indicate that ODA and GPTE exist on the fiber surface. The XPS survey spectrum of the coated fabric indicated the presence of elements N, confirming that ODA exists on the fiber surface (see Fig. S10a and Table S1, ESI†). The high-resolution XPS C1s spectra showed the peaks at 289.1, 287.7, 286.5, 285.7, and 285.0 eV (Fig. S10b, ESI†), corresponding to the C–N, epoxy, C–O, C–OH, and C–C/C–H groups, respectively.
The photos in Fig. 1a show the wettability of the polyester fabric before and after coating treatment (see more results in Fig. S11, ESI†). In a dry state in air, the control polyester fabric was hydrophobic but oleophilic, with a contact angle (CA) of about 130° (Fig. S12, ESI†) for water and 0° for oil fluids (e.g. gasoline, dichloromethane (DCE), diesel, and diiodomethane). After coating treatment (GPTE and ODA, weight ratio 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the fabric became both hydrophilic and oleophilic with a CA of 0° for water and the above mentioned oil fluids. A water droplet (∼5 μL) can spread into the fabric within 1 s (Fig. S13 and Video S1, ESI†).
1), the fabric became both hydrophilic and oleophilic with a CA of 0° for water and the above mentioned oil fluids. A water droplet (∼5 μL) can spread into the fabric within 1 s (Fig. S13 and Video S1, ESI†).
When the uncoated polyester fabric was immersed in water, some air bubbles were trapped in the fibrous matrix, due to the hydrophobic feature. When oil is dropped onto the fabric in water, oil fluids easily spread into the fabric. Upon removing the air bubbles forcefully, e.g. through long time vacuuming, oil fluids were repelled strongly by the wetted fabric (Fig. S14, ESI†).
For the coated polyester fabric, once immersed in water it became fully wetted without a plastron layer at the liquid–solid interface. When dropping gasoline, DCE, diesel, and diiodomethane onto the fabric, however, all the oil droplets can spread into the fabric matrix easily in a short time. Fig. 1b shows still frames taken from two videos recording gasoline and DCE on the coated fabric in water (see Video S2, ESI†). Both oil drops spread into the wetted fabric in a short time (<3 second). Fig. S15 (ESI†) shows diesel and diiodomethane spreading in the coated fabric in water. The times for gasoline, DCE, diesel, and diiodomethane (10 μL each) to spread completely into the coated fabric were 2.5 s, 0.5 s, 6.5 s, and 10.5 s, respectively.
To exclude the effect of fabric structure on wetting performance, we tested the wettability of a GPTE–ODA-coated dense PET film and glass slide (the coating method is similar to that of the polyester fabric). Without coating treatment, both the PET film and glass slide show in-air amphiphilicity. As seen in Fig. 1e and g, the uncoated substrates in water show an oleophobic surface. For uncoated PET film, the CAunderwater for diesel was 92°. The uncoated glass slide showed a similar feature with a diesel CAunderwater of 91.5° (Fig. 1f and h). After GPTE–ODA coating treatment, however, both the PET film and glass slide showed superamphiphilicity in air and underwater states. They showed CAs less than 10° for all the liquids studied, no matter whether in the normal state or underwater environments. These results clearly indicate that our superamphiphilic coating is general, and suitable for not only fabrics but also dense solid substrates.
Fig. 1c shows dropping DCE onto the coated PET film which was pre-wetted with water. As expected, DCE quickly spread on the surface; meanwhile water was repelled away (also see Video S3, ESI†), suggesting stronger affinity of the surface to DCE than water.
To verify the stronger affinity of the coated fabric to oil fluids than water, we dropped water onto the coated fabric which was fully immersed in oil. As expected, water droplets cannot spread into the oil-wetted fabric (Fig. S16, ESI†). In the oil environment, the fabric had large water CA (168° in gasoline, 166° in diesel, and 165° in soybean oil). In another experiment, water pre-wetted fabric (GPTE–ODA coated) was immersed into DCE. In this case, the water was substituted with oil rapidly (Fig. 1d, also see Video S4, ESI†). Similar results were also observed when a water pre-wetted fabric (GPTE–ODA coated) was immersed into gasoline and diesel (see Fig. S17 and Video S5, ESI†).
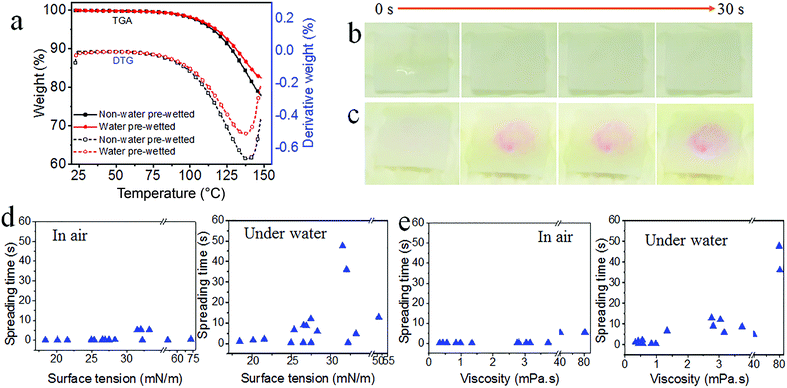
To probe the extent of water being substituted by oil, we conducted thermogravimetric analysis (TGA) on the GPTE–ODA fabric. To do this, the fabric was pre-wetted with water and then immersed in hexadecane. After taking from hexadecane, the samples were subjected to a TG scan. For comparison, the fabric just immersed in hexadecane without pre-wetting with water was also tested. As shown in Fig. 2a, both the water pre-wetted and the non-pre-wetted samples show almost identical TG features. No weight loss associated water was observed in the curves.
To further prove the replacement of water with oil, the GPTE–ODA coated fabric was firstly wetted with an aqueous NaOH solution (pH = 9), and the pre-wetted fabric was then immersed in diesel. After gently shaking with hand for 30 s, the fabric was taken out, phenolphthalein-ethanol solution was then dropped onto the fabric. No color change was observed in the fabric (Fig. 2b), suggesting the neutral surface. In contrast, when the uncoated fabric was subjected to the same test, a pink color was developed just in a few seconds (Fig. 2c). These results indicate that the majority of water is removed from the pre-wetted fabric after immersing into the oil fluid.
It is known that cotton fabric has a superamphiphilic surface in air, but is oleophobic in underwater environments. When cotton fabric was pre-wetted with water and then immersed in oils, water was still kept within the cotton fabric (Fig. S18, ESI†). This wetting behavior is different from our GPTE–ODA treated polyester fabric because the GPTE–ODA treated polyester fabric showed amphibious superamphiphilicity. To our knowledge, such amphibious superamphiphilicity has not been reported in the literature. Our GPTE–ODA treated polyester fabric should be the first report of its type.
A series of liquids having different surface tension values in the range of 18.4–72.8 mN m−1 were used to probe the wettability of the coated fabric both in air and underwater states (Table S2, ESI†). All the oils selected were immiscible to water. These oil fluids showed different spreading time, suggesting the effect of fluid properties on spreading behavior. Fig. 2d and e show the effect of liquid surface tension and viscosity on the spreading time. In both air and underwater states, the spreading time increased generally with increasing viscosity, while the surface tension was not linearly related to spreading time. The in air spreading time was shorter than that in an underwater state. This is probably because oils in water move more slowly due to the larger fluid resistance.
The role of GPTE and ODA in the coating was examined by varying their weight ratio in the coating solution (Table S3, ESI†). When the polyester fabric was treated by GPTE only, the fabric became superhydrophilic and underwater superoleophilic for cyclohexane, DCE, chloroform, 1,2-dichloroethane, and terpineol. At the GPTE/ODA weight ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the underwater superoleophilicity extended to three other oils, gasoline, diesel, and diiodomethane. When the weight ratio changed to 5
1, the underwater superoleophilicity extended to three other oils, gasoline, diesel, and diiodomethane. When the weight ratio changed to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the fabric showed superoleophilicity both in air and underwater for all the oils. At the weight ratio of 1
1, the fabric showed superoleophilicity both in air and underwater for all the oils. At the weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, however, the fabric becomes hydrophobic in air with water CA about 140°. The fabric treated by the weight ratio of 5
1, however, the fabric becomes hydrophobic in air with water CA about 140°. The fabric treated by the weight ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 GPTE/ODA showed the best results.
1 GPTE/ODA showed the best results.
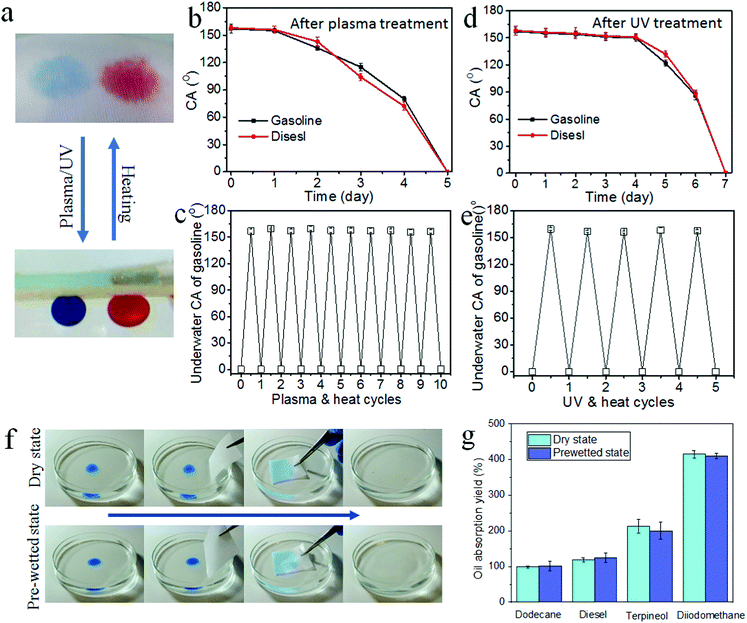
More interestingly, the coated fabric showed a self-healing underwater superoleophilicity (Fig. 3a). To prove it, we deliberately damaged the coated fabric by vacuum plasma treatment using an air source or UV irradiation. After 5 min plasma treatment, the fabric turned underwater superoleophobic with CAunderwater over 150° for gasoline and diesel. Such an underwater superoleophobicity can be maintained for at least 1 day. After 3 days, the fabric still showed oleophobicity, and the CAunderwater was 115° and 104° for gasoline and diesel, respectively (Fig. 3b). The self-healing test was conducted just after the plasma treatment of UV irradiation. In this case, the fabric had stable superoleophobicity. When the plasma damaged fabrics were heated at 150 °C for 30 min, underwater superoleophilicity was restored. This self-healing property was repeatable and worked for many cycles. Fig. 3c shows the change of underwater gasoline CA with plasma-and-heat cycles. After 10 cycles, the fabric still showed amphibious superamphiphilicity (also see a similar result on diesel in Fig. S19a, ESI†).
Similarly, the self-healing property also worked after damage by UV light. When the coated fabric was irradiated under UV for 10 h, the fabric turned underwater superoleophobic as well, with CAunderwater greater than 150° for gasoline and diesel. This underwater superoleophobicity was stable for at least 4 days (Fig. 3d). However, when the just UV irradiated fabric was subjected to a heating treatment at 150 °C for 30 minutes, it restored the underwater superoleophilicity (Fig. 3e and Fig. S19b, ESI†). This self-healing property was repeatable and worked for many cycles as well.
The amphibious superamphiphilic fabric is expected to have advanced applications in oil absorption and recovery. As a demonstration, we showed the oil absorption ability of the coated polyester fabric at both dry and underwater states. No matter whether the fabric was dry or pre-wetted with water, it showed almost identical oil absorption capability (Fig. 3f and g). Such a stable oil absorption ability is superior to the normal superoleophilic materials, which lose the oil absorption ability once the surface is completely wetted with water. Because of the strong resilience against water and moisture, our amphibious superamphiphilicity will offer great applications for oil absorption/recovery.
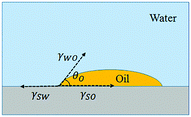
In underwater environments, when an oil droplet stays stably on a solid surface, a thermodynamic equilibrium is reached among the oil phase, solid phase and water phase (Fig. 4). According to Young's equation,
γSW = γSO + γWO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θO θO |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θO − 1)/cos
θO − 1)/cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θO.
θO.
For an underwater oleophilic surface, θO ≤ 90, thus cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θO − 1 ≤ 0. To allow automatic replacement of water with oil, ΔG < 0, thus γSW − γSO > 0. γSW > γSO implies that oil spreading on the solid surface will require less energy than water. The solid surface should have stronger affinity to oil than water.
θO − 1 ≤ 0. To allow automatic replacement of water with oil, ΔG < 0, thus γSW − γSO > 0. γSW > γSO implies that oil spreading on the solid surface will require less energy than water. The solid surface should have stronger affinity to oil than water.
A possible mechanism was proposed to explain this amphibious superamphiphilicity, as illustrated in Fig. 5. ODA has a hydrophobic/oleophilic feature while GPTE is amphiphilic. ODA can graft onto GPTE via the epoxide-amino reaction. After curing, a crosslinking network is formed to immobilize the functional groups in the coating layer (Fig. 5a). In air, although hydrophobic/oleophilic ODA segments are on the surface, the dominant component on the fiber surface is GPTE. As a result, water/oil can spread on the surface rapidly (Fig. 5b). When the fabric is immersed into water, the fiber surface was trapped by water. Upon oil molecules contacting with the surface, they were captured firstly by ODA segments, facilitating oil to spread on the fiber surface. Meanwhile, water was substituted with oil rapidly (Fig. 5b).
The self-healing property was explained by the change of surface energy. When the coated fabric was subjected to plasma treatment, high polarity groups such as hydroxyl, carbonyl and carboxyl groups were introduced onto the surface. For the UV irradiation, a part of the crosslinked coating was decomposed as well as the introduced hydrophilic groups by oxidation in air. After heating, the low surface moieties in the coating layer migrated to the surface to cover the polarity groups. In this way, the underwater superoleophilicity is restored (Fig. 5c).
In addition, the coated polyester fabric was durable enough against harsh damages such as repeated washing and abrasion, as well as acid/base etching (Fig. S20 and Table S4, ESI†). Since dip coating has been widely used in industry, the method shows potential for making amphibiously superamphiphilic fabrics on a large scale.
Conclusions
We have demonstrated a novel method to make fabric have a superamphiphilic surface working simultaneously in air and underwater by using a novel polymer that is crosslinkable and consists of both hydrophilic and oleophilic functional groups. The co-existence of hydrophilic and oleophilic functionality plays a key role in achieving the amphibious superamphiphilicity. Such amphibious superamphiphilicity is self-healable against self-healing properties. The resilience to moisture environments offers the amphibious superamphiphilicity great potential for oil recovery and other innovative applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Funding support from Australian Research Council through a Discovery Project (DP150100406), Alfred Deakin Postdoctoral Fellowship (to H. Z.), and Institute for Frontier Materials (IFM) Research Excellence Grants scheme 2018 is acknowledged.Notes and references
- Z. Zhu, S. Zheng, S. Peng, Y. Zhao and Y. Tian, Adv. Mater., 2017, 29, 1703120 CrossRef PubMed.
- M. Liu, S. Wang and L. Jiang, Nat. Rev. Mater., 2017, 2, 17036 CrossRef CAS.
- X. Du, S. You, X. Wang, Q. Wang and J. Lu, Chem. Eng. J., 2017, 313, 398 CrossRef CAS.
- Y. Chang, Y. Li, J. Wang and C.-W. Wang, Mater. Lett., 2017, 187, 162 CrossRef CAS.
- G. Cao, W. Zhang, Z. Jia, F. Liu, H. Yang, Q. Yu, Y. Wang, X. Di, C. Wang and S.-H. Ho, ACS Appl. Mater. Interfaces, 2017, 9, 36368 CrossRef CAS PubMed.
- M. Tao, L. Xue, F. Liu and L. Jiang, Adv. Mater., 2014, 26, 2943 CrossRef CAS PubMed.
- P. Zhu, T. Kong, Y. Tian, X. Tang, X. Tian and L. Wang, Mater. Horiz., 2018 10.1039/c8mh00964c.
- H. Zhou, H. Wang, H. Niu and T. Lin, Sci. Rep., 2013, 3, 2964 CrossRef PubMed.
- Z. Wang, X. Yang, Z. Cheng, Y. Liu, L. Shao and L. Jiang, Mater. Horiz., 2017, 4, 701 RSC.
- P. Zhang, J. Zhang, Z. Xue, J. Wang and L. Jiang, Mater. Horiz., 2017, 4, 665 RSC.
- Z. Zhu, Y. Tian, Y. Chen, Z. Gu, S. Wang and L. Jiang, Angew. Chem., 2017, 129, 5814 CrossRef.
- H. S. Lim, S. H. Park, S. H. Koo, Y.-J. Kwark, E. L. Thomas, Y. Jeong and J. H. Cho, Langmuir, 2010, 26, 19159 CrossRef CAS PubMed.
- V.-T. Bui, X. Liu, S. H. Ko and H.-S. Choi, J. Colloid Interface Sci., 2015, 453, 209 CrossRef CAS PubMed.
- X. Zhang, D. Liu and G. Sui, Adv. Mater. Interfaces, 2018, 5, 1701094 CrossRef.
- Z. Xue, S. Wang, L. Lin, L. Chen, M. Liu, L. Feng and L. Jiang, Adv. Mater., 2011, 23, 4270 CrossRef CAS PubMed.
- W. Zhang, Y. Zhu, X. Liu, D. Wang, J. Li, L. Jiang and J. Jin, Angew. Chem., Int. Ed., 2014, 53, 856 CrossRef CAS PubMed.
- J. Li, D. Li, Y. Yang, J. Li, F. Zha and Z. Lei, Green Chem., 2016, 18, 541 RSC.
- X. Yang, Y. He, G. Zeng, X. Chen, H. Shi, D. Qing, F. Li and Q. Chen, Chem. Eng. J., 2017, 321, 245 CrossRef CAS.
- M. Jin, J. Wang, X. Yao, M. Liao, Y. Zhao and L. Jiang, Adv. Mater., 2011, 23, 2861 CrossRef CAS PubMed.
- J. Yong, F. Chen, M. Li, Q. Yang, Y. Fang, J. Huo and X. Hou, J. Mater. Chem. A, 2017, 5, 25249 RSC.
- S. Huang, J. Song, Y. Lu, F. Chen, H. Zheng, X. Yang, X. Liu, J. Sun, C. J. Carmalt and I. P. Parkin, ACS Appl. Mater. Interfaces, 2016, 8, 2942 CrossRef CAS PubMed.
- P. Zhang, F. Zhang, C. Zhao, S. Wang, M. Liu and L. Jiang, Angew. Chem., Int. Ed., 2016, 55, 3615 CrossRef CAS PubMed.
- H. Gao, Z. Zhao, Y. Cai, J. Zhou, W. Hua, L. Chen, L. Wang, J. Zhang, D. Han and M. Liu, Nat. Commun., 2017, 8, 15911 CrossRef CAS PubMed.
- K. Fryauf, V. Strehmel and M. Fedtke, Polymer, 1993, 34, 323 CrossRef CAS.
- L. Xu and J. R. Schlup, J. Appl. Polym. Sci., 1998, 67, 895 CrossRef CAS.
- H. Yamasaki and S. Morita, Appl. Spectrosc., 2012, 66, 926 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8mh00898a |
| ‡ Authors who equally contributed to the paper. |
| This journal is © The Royal Society of Chemistry 2019 |