Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bio-inspired temporal regulation of ion-transport in nanochannels†
K. P.
Sonu
 a,
Sushmitha
Vinikumar
a,
Sushmitha
Vinikumar
 a,
Shikha
Dhiman
a,
Shikha
Dhiman
 b,
Subi J.
George
b,
Subi J.
George
 *b and
Muthusamy
Eswaramoorthy
*b and
Muthusamy
Eswaramoorthy
 *a
*a
aNanomaterials and Catalysis Laboratory, Chemistry and Physics of Materials Unit, School of Advanced Materials (SAMat), Jawaharlal Nehru Centre for Advanced Scientific Research, Jakkur P.O., Bangalore 560064, India. E-mail: eswar@jncasr.ac.in
bSupramolecular Chemistry Laboratory, New Chemistry Unit, School of Advanced Materials (SAMat), Jawaharlal Nehru Centre for Advanced Scientific Research, Jakkur P.O., Bangalore 560064, India. E-mail: george@jncasr.ac.in; subijg@gmail.com
First published on 12th March 2019
Abstract
Temporal regulation of mass transport across the membrane is a vital feature of biological systems. Such regulatory mechanisms rely on complex biochemical reaction networks, often operating far from equilibrium. Herein, we demonstrate biochemical reaction mediated temporal regulation of mass transport in nanochannels of mesoporous silica sphere. The rationally designed nanochannels with pH responsive electrostatic gating are fabricated through a hetero-functionalization approach utilizing propylamine and carboxylic acid moieties. At basic pH, cationic small molecules can diffuse into the nanochannels which release back to the solution at acidic pH. The transient ion transport is temporally controlled using a base as fuel along with esterase enzyme as the mediator. The slow enzymatic hydrolysis of a dormant deactivator (ethyl acetate) determines the lifetime of transient encapsulated state, which can be programmed easily by modulating the enzymatic activity of esterase. This system represents a unique approach to create autonomous artificial cellular models.
Introduction
Biological systems have been a long standing inspiration for temporal control over self-assembled nanostructures and functions. From precise control over circadian rhythms to translocation of molecules across membranes, natural systems achieve the same by complex loops of chemical reaction networks facilitated by the cascade of enzymes resulting in structural changes that execute corresponding functions of the cellular motifs.1,2 Inspired by this, a recent move towards complex adaptive materials with temporal control over structure and function has begun. Many active self-assembled materials have been synthetically designed recently via fuel-driven, non-equilibrium structural/conformational changes.3–6 However, autonomous, temporal control on biomimetic functions still remain a challenge and needs to be addressed to design life-like materials.Recent attempts to achieve temporal regulation are, in general, concerned with transient assembly or conformational changes of supramolecular polymers and of colloidal systems.7–18 Most of these systems are examples of fundamental design strategies to build a transient change in self-assembled structures. However, temporally programmed functions similar to natural systems are very few.19–22 Prins and co-workers designed systems showing transient signalling and transient catalysis.23–25 Using a transient pH switch, Walther and co-workers have exemplified the applications in out-of-equilibrium photonics.26 Very recently Ulijn and co-workers elegantly designed enzyme responsive chromophores for transient electronics.27
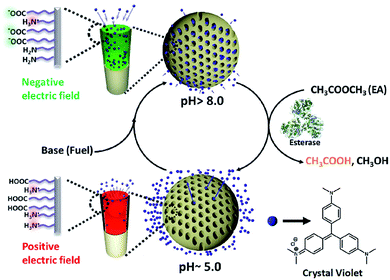
Amongst a plethora of autonomous functions, selective transport of molecules in and out of the cellular compartments is a vital feature of biological systems. In order to achieve this selective transport, cell employs various sophisticated membrane channel proteins.28 There have been significant efforts to mimic such selective transport of molecules across the cell membrane in artificial nanochannels.29–33 However, all such artificial systems that demonstrated selective transport of molecules often operate in a passive manner and lack autonomous behaviour, unlike their natural counterparts.34–45 To alleviate this scenario, herein we report a unique temporal regulation of ion transport in synthetic nanochannels using a bio-inspired, chemical fuel-driven strategy. In order to show this concept of ‘transient ion transport’, we have used pH responsive, heterogeneously functionalized nanochannels of mesoporous silica particles as a model system (Scheme 1). Moreover, we obtain a control over the slow release of encapsulated cationic molecule which can be used for controlled payload/drug release.
Results and discussion
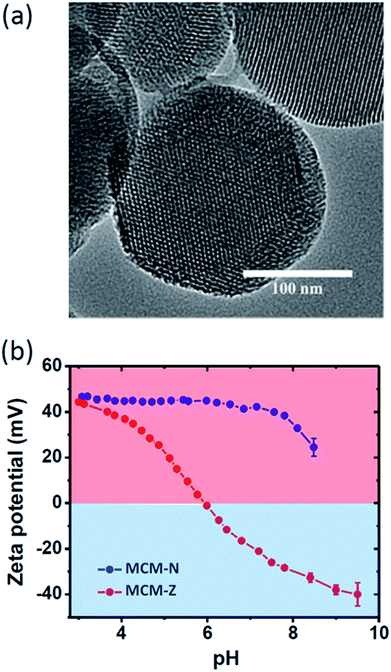
For a transient ion transport, the first condition is to synthesize charge-switchable nanochannels that can undergo charge reversal on the addition of an external chemical fuel (activation). The second requirement is to have a chemical reagent that can revert the changes in a temporal manner (deactivation). Importantly, the steps should work in tandem, with the rate of activation higher than the rate of deactivation. As the chemical fuel consumed, rate of deactivation becomes higher and the system comes to equilibrium. For this, we have heterogeneously functionalized nanochannels of mesoporous silica particles with pH responsive amine and carboxylic acid functional groups (Scheme 1). Mesoporous silica spheres (MCM-41, hereafter referred as MCM) (Fig. S1 and S2†) were synthesized following a well-known sol–gel procedure and the surface was covalently modified with (3-aminopropyl)triethoxysilane to obtain MCM-N with amine functionalized surface.46,47 A portion of amine groups was then reacted with succinic anhydride to form MCM-Z with zwitterionic nature having carboxyl and amine groups on the nanochannel surface (Fig. S1†). The XRD patterns of MCM, MCM-N and MCM-Z exhibited a small angle peak (at around 2θ = 2.5 degree) suggesting that the hexagonal mesostructure was retained during functionalization (Fig. S3†). Retention of mesoporosity of nanochannels was further confirmed via transmission electron microscopy (Fig. 1a). Thermogravimetric analysis (TGA) suggested propylamine functionalization in MCM-N was around ca. 2.0 mmol g−1 (Fig. S4†). The carboxylic acid groups (–COOH) in MCM-Z was determined to be around ca. 1.1 mmol g−1 using TGA and ninhydrin test (Fig. S5†), which suggest that approximately 56% of amine groups were covalently connected (via amide linkage) to carboxylic acid groups in MCM-Z.Nitrogen adsorption–desorption analyses were carried out at 77 K to evaluate the progress of functionalization inside the nanochannels (Fig. S6a†). The Barrett–Joyner–Halenda (BJH) pore size distributions showed a gradual reduction in average pore size from ca. 2.9 nm for MCM to 2.5 nm for MCM-N which further decreased to 2.2 nm for MCM-Z suggesting progress of functionalization inside the nanochannels (Fig. S6b†). Further, the number of propylamine groups on MCM-N was calculated to be 1.25 molecules per nm2 area from N2 sorption studies and TGA (see ESI† text for details). Similarly, in the case of MCM-Z, the number of carboxylic acid groups present was around 0.71 molecule per nm2.
To monitor the surface charge of these biomimetic nanochannels, we employed zeta potential measurements. The variation of zeta potential with pH for both MCM-N and MCM-Z were shown in Fig. 1b. As expected, propylamine functionalized MCM (MCM-N) showed a zeta potential of ca. +46 ± 0.5 mV at pH 3 that reduced to +24 ± 2 mV at pH 8.5. In the case of MCM-N, no charge reversal occurred on going from low pH to high pH (Fig. 1b, S7†). On the other hand MCM-Z (that have both amine groups and carboxylic acid groups on the surface) showed a clear charge reversal with respect to pH. At pH 3, MCM-Z was positively charged with a zeta potential of ca. +44 ± 0.5 mV associated with the protonation of free amines in MCM-Z. The surface charge became zero at around pH 6 (isoelectric point) and a further increase in pH caused charge reversal from positive to negative with a zeta potential of ca. −32 ± 1 mV at around pH 8.5. This can be attributed to the generation of carboxylate groups along with the neutralization of amines. As a result, the functionalized nanochannels become anionic above the isoelectric point of pH 6.0 and cationic below pH 6.0.
The pH dependent charge switchable property of nanochannels was explored to encapsulate and release of cationic dye molecule. For this purpose, a cationic dye – crystal violet (CV+) was chosen which maintain the cationic nature in the pH range of 3 to 8.5.48,49 The dye uptake and release were quantitatively probed via UV-Vis spectroscopy (Scheme 1, Fig. S8†). To investigate the amount of CV+ encapsulation, 10 mg MCM-Z was soaked in a 3.34 μM aqueous solution of CV+ dye at pH 8 for 10 minutes. The absorption spectra of CV+ stock solution and supernatant solution after soaking with MCM-Z was compared (Fig. 2a). The disappearance of CV+ absorption band for supernatant solution suggested an efficient encapsulation of cationic dye in negatively charged nanochannels at pH 8 via electrostatic attraction. The encapsulation is driven by electrostatic attraction between the CV+ and silica surface. There are no physically entrapped dye molecules inside the pore as it is evident from ‘zero’ leak release of CV+ from silica pores at pH > 8.0 (Fig. 2c). The uptake of CV+ calculated from the UV-vis spectra was found to be ca. 1 μmol g−1 (Fig. 2b). Similar encapsulation experiment was done at pH 3 and only a negligible uptake was observed, confirming the strong electrostatic gating of MCM-Z nanochannels at pH 3 towards the cationic dye (Fig. 2b).
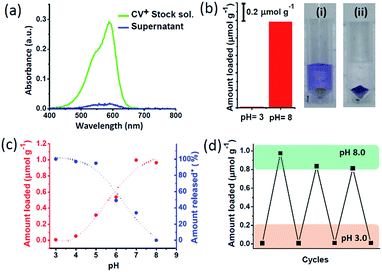 | ||
| Fig. 2 Transportation of CV+ in MCM-Z nanochannels: (a) absorption spectra of CV+ stock solution and the supernatant after incubating with MCM-Z at pH 8. (b) Amount of encapsulation of CV+ into MCM-Z at pH 3 and pH 8 (CV+ stock solution concentration was ca. 3.34 μM and MCM-Z was ca. 10 mg). Photographs showing the supernatant solution after soaking CV+ with MCM-Z for 10 min in (i) pH 3 and (ii) pH 8. (c) Encapsulation and release of CV+ in MCM-Z at different pH. *For release studies, CV+ was first encapsulated in to MCM-Z at pH 8 and the released amount at different pH was normalized w.r.t. loading at pH 8. (d) Cycling of CV+ encapsulation into MCM-Z at pH 3 and 8 (see ESI† for the details). | ||
Next we studied the CV+ dye uptake in MCM-Z and its release as a function of pH (Fig. 2c). The MCM-Z (10 mg) was incubated in a 3.34 μM CV+ solution at different pHs (in the range of 3–8) for 10 minutes and the encapsulation was probed with absorption spectra. A gradual increase in the uptake of CV+ was observed with increasing pH and a maximum uptake was seen at pH 8 (ca. 1 μmol g−1) which can be explained based on the nature of surface charge developed at different pH as shown in Fig. 1b. Release profile of CV+ encapsulated in MCM-Z at pH 8.0 (high uptake due to electrostatic interactions) showed maximum release at pH 3.0 due to the charge repulsion between the positively charged nanochannels and the cationic dye (Fig. 2c). We further investigated the recyclability of the charge reversal and dye encapsulation by sequentially changing the pH of the MCM-Z dispersion containing 1 μmol g−1 CV+ between 3 and 8. The encapsulation and release of CV+ in and out of MCM-Z was studied and a significant amount of dye uptake was observed up to three cycles (>80%) (Fig. 2d). Thus, we obtained an efficient recycling, validating the adaptability and reversibility of these nanochannels to solution pH variations.
Further, we investigated the structural stability of MCM-Z in basic aqueous solution (pH > 8.0) to evaluate the robustness of the nanochannels. The MCM-Z was dispersed in Tris buffer of pH 9.0 (10 mM) and the zeta potential was measured at regular time intervals up to 3 hours, which showed no change even after 2.5 hours (Fig. S9a†). The XRD pattern (Fig. S9b†) and N2 sorption analysis (Fig. S9c†) also confirmed the structural stability of MCM-Z nanochannels in basic conditions.
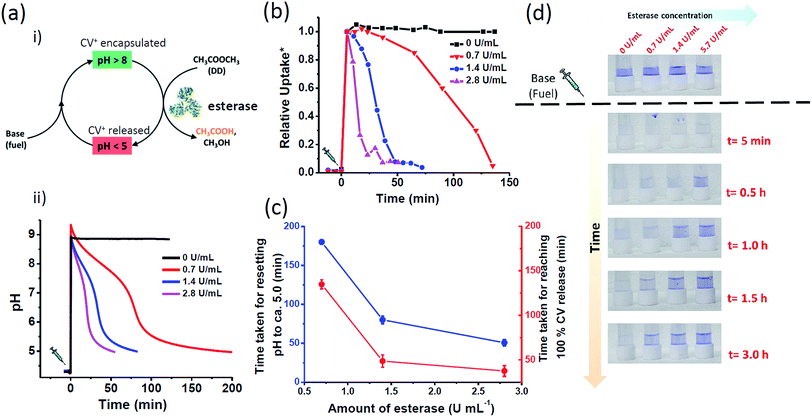
With the understanding of switchable charged nanochannels of MCM-Z at varying pH between 3 to 8 and corresponding cationic dye encapsulation and release, we next envisioned to couple this to a temporal change in pH to realize “transient ion transport”. For this, we have exploited a feedback-controlled chemical processes which temporally modulates the pH of the surrounding solution.50 When we first inject a base to the acidic dispersion of MCM-Z, the increase in pH (∼9.0) deprotonates the carboxylic acid functional groups (and ammonium cations) resulting in the formation of anionic nanochannels (activation). Furthermore, the high pH initiates hydrolysis of ethyl acetate (by esterase enzyme) that gradually decreases the pH (due to the formation of acetic acid). As a result amine and carboxylate functional groups get protonated and nanochannels become cationic (deactivation). Since ethyl acetate decreases the pH of the system only via its kinetically controlled hydrolysis (by esterase enzyme) it is referred as dormant deactivator. The instantaneous activation (formation of anionic nanochannels) by the addition of base and the slow deactivation (leading to cationic nanochannels) due to ethyl acetate hydrolysis allow the nanochannels to exist in transient anionic state (Fig. 3a(i)).
We first investigated the efficiency to obtain temporal control on pH by varying the amount of esterase. The chemical fuel (tris(hydroxymethyl)aminomethane (base)) and ethyl acetate (DD) in appropriate concentrations were mixed and injected to a dispersion of MCM-Z containing ∼1.0 μmol g−1 CV+ and esterase enzyme (0–3 U mL−1) and a time dependent pH variation was monitored (Fig. 3a(ii)). An initial increase in pH to ∼9 was observed that gradually decayed to equilibrium state acidic pH ∼5.0 due to the formation of acetic acid by the hydrolysis of ethyl acetate (DD). The kinetics of ethyl acetate hydrolysis is exclusively proportional to the concentration of esterase. The increase in concentration of enzyme (at constant concentration of ethyl acetate) resulted in higher rate of ethyl acetate hydrolysis and a corresponding decrease in lifetime of transient alkaline pH state. For ca. 0.7 U mL−1 of esterase enzyme, it took more than 180 ± 3 min to return the pH back to ca. 5; however, it took only 50 ± 4 min when the enzyme amount was increased to ca. 2.8 U mL−1 (Fig. 3c). On the other hand, the pH remained around 9.0 when no esterase was used attributing the importance of esterase enzyme to build transient profile. Interestingly, the temporal variation of solution pH showed three distinct slopes which were in agreement with the previously reported activity profile of esterase.50
The temporal control achieved on pH was next coupled to CV+ dye encapsulation in MCM-Z. Due to the above described in situ modulation of pH, the permeation of ions in and out of the pH adaptive MCM-Z nanochannels was expected to adjust autonomously (Fig. 2b, d). To achieve a temporal control on ion transport, MCM-Z, CV+ and esterase enzyme at pH 4.2 were taken. The system stays at equilibrium and insignificant encapsulation of CV+ by MCM-Z was observed even after 10 min of soaking (Fig. 3b). However, on addition of chemical fuel base (Tris) and ethyl acetate, resulted in an instantaneous increase in pH that facilitated a spontaneous CV+ encapsulation in MCM-Z nanochannels. Subsequently, hydrolysis of ethyl acetate (DD) by esterase occurs that gradually decreased the pH and a slow CV+ release back into the solution is observed. Thus, a transient ion transport or transient molecule encapsulation is attained.
Further, the lifetime of the transient ‘CV+ encapsulated state’ was programmed by modulating deactivation rate via enzyme concentration. In the case of 2.8 U mL−1 esterase concentration, within 38 ± 6 min complete release was seen; on the other hand, when the esterase concentration was reduced to 0.7 U mL−1, it took longer time to reach complete release (>135 ± 5 min) (Fig. 3b–d). This clearly demonstrates the temporal programmability of CV+ encapsulation and modular transient ion transport. Interestingly, we noted that for any esterase concentration, time taken for resetting pH back to the equilibrium pH (i.e. 5.0) (tlead) was higher than the time required for the complete release (tresponse) (Fig. 3c).
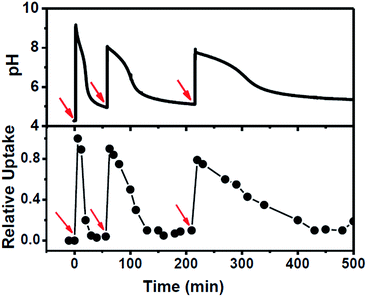
The system was further scrutinized to test its ability to undergo multiple cycles on refuelling by chemical fuel (Tris) (Fig. 4). For this purpose, a dispersion containing MCM-Z, CV+ (∼1.0 μmol g−1) and 2.8 U mL−1 esterase was taken at a pH around 4.2 and the pH and CV+ uptake was monitored (Fig. 4). As expected no uptake was observed at this equilibrium state. However, an instantaneous encapsulation of CV+ was observed up on the addition of chemical fuel (Tris) (same amount used in the previous experiments). The CV+ uptake was followed by gradual release as ethyl acetate (DD) hydrolysis proceeds (ethyl acetate was added along with chemical fuel). Once the system reached back to the equilibrium state, it was refuelled by adding same amount of chemical fuel (Tris) and ethyl acetate (DD) and a similar temporal profile was observed. Third refuelling also resulted in a similar trend. Thus system could be refuelled multiple times by subsequent addition of fuel, however, there was a dampening observed in the time required for complete release of CV+ as the cycles proceeded. This can be attributed to the accumulation of waste products in the closed system. Nevertheless, we could obtain a transient ion transport and successfully modulate its temporal profile and refuel it multiple times.
Conclusions
In summary, we demonstrated the construction of mesoporous silica based biomimetic nanochannels having enzyme mediated autonomous (temporal) regulation over ion transport. The fuel driven encapsulation of ions into the nanochannels and temporal control of release have been shown for the first time in such nanochannels. A modular lifetime of transient ion transport was shown along with the refuelability of the system. The nanochannels were also found to be very robust in terms of structural stability in basic medium. These temporally switchable and adaptive nanochannels shall pave the way for future on demand devices or delivery vehicles.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Prof. C. N. R. Rao, F. R. S. for his support and encouragement. S. D. and S. J. G. thank Nanomission, DST (SR/NM/TP-25/2016), JNCASR and Sheikh Saqr Laboratory (SSL) for financial support. S. J. G. gratefully acknowledges Swarna Jayanti Fellowship Award (DST/SJF/CSA-01/2016–2017). K. P. S. thanks B. V. V. S. Pavan Kumar for helpful discussions. M. E. thanks Sheikh Saqr Career Award Fellowship.Notes and references
- T. Mitchison and M. Kirschner, Nature, 1984, 312, 237 CrossRef CAS PubMed.
- E. D. Korn, Physiol. Rev., 1982, 62, 672–737 CrossRef CAS PubMed.
- R. Merindol and A. Walther, Chem. Soc. Rev., 2017, 46, 5588–5619 RSC.
- S. A. P. van Rossum, M. Tena-Solsona, J. H. van Esch, R. Eelkema and J. Boekhoven, Chem. Soc. Rev., 2017, 46, 5519–5535 RSC.
- L. Heinen and A. Walther, Soft Matter, 2015, 11, 7857–7866 RSC.
- G. Ashkenasy, T. M. Hermans, S. Otto and A. F. Taylor, Chem. Soc. Rev., 2017, 46, 2543–2554 RSC.
- J. Boekhoven, A. M. Brizard, K. N. K. Kowlgi, G. J. M. Koper, R. Eelkema and J. H. van Esch, Angew. Chem., Int. Ed., 2010, 49, 4825–4828 CrossRef CAS PubMed.
- J. Boekhoven, W. E. Hendriksen, G. J. M. Koper, R. Eelkema and J. H. van Esch, Science, 2015, 349, 1075–1079 CrossRef CAS PubMed.
- B. G. P. van Ravensteijn, W. E. Hendriksen, R. Eelkema, J. H. van Esch and W. K. Kegel, J. Am. Chem. Soc., 2017, 139, 9763–9766 CrossRef CAS PubMed.
- S. Dhiman, A. Jain, M. Kumar and S. J. George, J. Am. Chem. Soc., 2017, 139, 16568–16575 CrossRef CAS PubMed.
- S. Dhiman, A. Jain and S. J. George, Angew. Chem., Int. Ed., 2017, 56, 1329–1333 CrossRef CAS PubMed.
- K. Jalani, S. Dhiman, A. Jain and S. J. George, Chem. Sci., 2017, 8, 6030–6036 RSC.
- J. P. Wojciechowski, A. D. Martin and P. Thordarson, J. Am. Chem. Soc., 2018, 140, 2869–2874 CrossRef CAS PubMed.
- B. Kemper, L. Zengerling, D. Spitzer, R. Otter, T. Bauer and P. Besenius, J. Am. Chem. Soc., 2018, 140, 534–537 CrossRef CAS PubMed.
- B. Dong, L. Liu and C. Hu, Biomacromolecules, 2018, 19, 3659–3668 CrossRef CAS PubMed.
- L. S. Kariyawasam and C. S. Hartley, J. Am. Chem. Soc., 2017, 139, 11949–11955 CrossRef CAS PubMed.
- C. G. Pappas, I. R. Sasselli and R. V. Ulijn, Angew. Chem., Int. Ed., 2015, 54, 8119–8123 CrossRef CAS PubMed.
- J. K. Sahoo, C. G. Pappas, I. R. Sasselli, Y. M. Abul-Haija and R. V. Ulijn, Angew. Chem., Int. Ed., 2017, 56, 6828–6832 CrossRef CAS PubMed.
- H. Che, S. Cao and J. C. M. van Hest, J. Am. Chem. Soc., 2018, 140, 5356–5359 CrossRef CAS PubMed.
- P. Rangadurai, M. R. Molla, P. Prasad, M. Caissy and S. Thayumanavan, J. Am. Chem. Soc., 2016, 138, 7508–7511 CrossRef CAS PubMed.
- E. Del Grosso, A. Amodio, G. Ragazzon, L. J. Prins and F. Ricci, Angew. Chem., Int. Ed., 2018, 57, 10489–10493 CrossRef CAS PubMed.
- H. Che, B. C. Buddingh' and J. C. M. van Hest, Angew. Chem., Int. Ed., 2017, 56, 12581–12585 CrossRef CAS PubMed.
- C. Pezzato and L. J. Prins, Nat. Commun., 2015, 6, 7790 CrossRef CAS PubMed.
- S. Maiti, I. Fortunati, C. Ferrante, P. Scrimin and L. J. Prins, Nat. Chem., 2016, 8, 725–731 CrossRef CAS PubMed.
- F. della Sala, S. Maiti, A. Bonanni, P. Scrimin and L. J. Prins, Angew. Chem., Int. Ed., 2018, 57, 1611–1615 CrossRef CAS PubMed.
- T. Heuser, R. Merindol, S. Loescher, A. Klaus and A. Walther, Adv. Mater., 2017, 29, 1606842 CrossRef PubMed.
- M. Kumar, N. L. Ing, V. Narang, N. K. Wijerathne, A. I. Hochbaum and R. V. Ulijn, Nat. Chem., 2018, 10, 696–703 CrossRef CAS PubMed.
- B. Hille, Ion Channels of Excitable Membranes, Sinauer Associates, Inc., U.S.A., 2001 Search PubMed.
- W. Guo, Y. Tian and L. Jiang, Acc. Chem. Res., 2013, 46, 2834–2846 CrossRef CAS PubMed.
- B. Yameen, M. Ali, R. Neumann, W. Ensinger, W. Knoll and O. Azzaroni, J. Am. Chem. Soc., 2009, 131, 2070–2071 CrossRef CAS PubMed.
- X. Hou, F. Yang, L. Li, Y. Song, L. Jiang and D. Zhu, J. Am. Chem. Soc., 2010, 132, 11736–11742 CrossRef CAS PubMed.
- A. Brunsen, C. Díaz, L. I. Pietrasanta, B. Yameen, M. Ceolín, G. J. A. A. Soler-Illia and O. Azzaroni, Langmuir, 2012, 28, 3583–3592 CrossRef CAS PubMed.
- Q. Liu, K. Xiao, L. Wen, Y. Dong, G. Xie, Z. Zhang, Z. Bo and L. Jiang, ACS Nano, 2014, 8, 12292–12299 CrossRef CAS PubMed.
- A. D. Dinsmore, M. F. Hsu, M. G. Nikolaides, M. Marquez, A. R. Bausch and D. A. Weitz, Science, 2002, 298, 1006–1009 CrossRef CAS PubMed.
- A. J. Dzieciol and S. Mann, Chem. Soc. Rev., 2012, 41, 79–85 RSC.
- J. Gao, B. Zhao, M. Wang, M. A. C. Serrano, J. Zhuang, M. Ray, V. M. Rotello, R. W. Vachet and S. Thayumanavan, J. Am. Chem. Soc., 2018, 140, 2421–2425 CrossRef CAS PubMed.
- I. R. Fernando, D. P. Ferris, M. Frasconi, D. Malin, E. Strekalova, M. D. Yilmaz, M. W. Ambrogio, M. M. Algaradah, M. P. Hong, X. Chen, M. S. Nassar, Y. Y. Botros, V. L. Cryns and J. F. Stoddart, Nanoscale, 2015, 7, 7178–7183 RSC.
- R. Liu, Y. Zhang, X. Zhao, A. Agarwal, L. J. Mueller and P. Feng, J. Am. Chem. Soc., 2010, 132, 1500–1501 CrossRef CAS PubMed.
- S. Wu, X. Huang and X. Du, Angew. Chem., Int. Ed., 2013, 52, 5580–5584 CrossRef CAS PubMed.
- X. Yao, X. Chen, C. He, L. Chen and X. Chen, J. Mater. Chem. B, 2015, 3, 4707–4714 RSC.
- A. Popat, J. Liu, G. Q. Lu and S. Z. Qiao, J. Mater. Chem., 2012, 22, 11173–11178 RSC.
- L. Yuan, Q. Tang, D. Yang, J. Z. Zhang, F. Zhang and J. Hu, J. Phys. Chem. C, 2011, 115, 9926–9932 CrossRef CAS.
- C.-H. Lee, S.-H. Cheng, I.-P. Huang, J. S. Souris, C.-S. Yang, C.-Y. Mou and L.-W. Lo, Angew. Chem., Int. Ed., 2010, 49, 8214–8219 CrossRef CAS PubMed.
- L. Xing, H. Zheng, Y. Cao and S. Che, Adv. Mater., 2012, 24, 6433–6437 CrossRef CAS PubMed.
- J.-T. Sun, C.-Y. Hong and C.-Y. Pan, J. Phys. Chem. C, 2010, 114, 12481–12486 CrossRef CAS.
- S. Huh, J. W. Wiench, J.-C. Yoo, M. Pruski and V. S. Y. Lin, Chem. Mater., 2003, 15, 4247–4256 CrossRef CAS.
- B. V. V. S. P. Kumar, K. Salikolimi and M. Eswaramoorthy, Langmuir, 2014, 30, 4540–4544 CrossRef CAS PubMed.
- J. D. Fisk, R. Batten, G. Jones, J. P. O'Reill and A. M. Shaw, J. Phys. Chem. B, 2005, 109, 14475–14480 CrossRef CAS PubMed.
- V. Manzo, O. Navarro, L. Honda, K. Sánchez, M. Inés Toral and P. Richter, Talanta, 2013, 106, 305–308 CrossRef CAS PubMed.
- L. Heinen, T. Heuser, A. Steinschulte and A. Walther, Nano Lett., 2017, 17, 4989–4995 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8na00414e |
| This journal is © The Royal Society of Chemistry 2019 |