Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Extracting structural information of Au colloids at ultra-dilute concentrations: identification of growth during nanoparticle immobilization†
George F.
Tierney
 ab,
Donato
Decarolis
ab,
Norli
Abdullah
ab,
Donato
Decarolis
ab,
Norli
Abdullah
 bcd,
Scott M.
Rogers
bcd,
Scott M.
Rogers
 bd,
Shusaku
Hayama
e,
Martha
Briceno de Gutierrez
f,
Alberto
Villa
bd,
Shusaku
Hayama
e,
Martha
Briceno de Gutierrez
f,
Alberto
Villa
 g,
C. Richard A.
Catlow
bdh,
Paul
Collier
f,
Nikolaos
Dimitratos
g,
C. Richard A.
Catlow
bdh,
Paul
Collier
f,
Nikolaos
Dimitratos
 *i and
Peter P.
Wells
*i and
Peter P.
Wells
 *abe
*abe
aSchool of Chemistry, University of Southampton, Highfield, Southampton, SO17 1BJ, UK. E-mail: P.P.Wells@soton.ac.uk
bUK Catalysis Hub, Research Complex at Harwell, Rutherford Appleton Laboratory, Harwell, Didcot, OX11 0FA, UK
cDepartment of Chemistry, Center for Foundation Science, National Defense University of Malaysia, Sungai Besi Camp, 57000 Kuala Lumpur, Malaysia
dDepartment of Chemistry, University College London, 20 Gordon Street, London, WC1H 0AJ, UK
eDiamond Light Source, Harwell Science and Innovation Campus, Chilton, Didcot, OX11 0DE, UK
fJohnson Matthey Technology Centre, Sonning Common, Reading, RG4 9NH, UK
gDipartimento di Chimica, Universitá degli Studi di Milano, via Golgi 19, 20133 Milano, Italy
hCardiff Catalysis Institute, School of Chemistry, Cardiff University, Cardiff, CF10 3AT, UK
iDipartimento di Chimica Industriale “Toso Montanari”, Alma Mater Studiorum Università di Bologna, Viale Risorgimento 4, 40136 Bologna, Italy. E-mail: nikolaos.dimitratos@unibo.it
First published on 21st May 2019
Abstract
Sol-immobilization is increasingly used to achieve supported metal nanoparticles (NPs) with controllable size and shape; it affords a high degree of control of the metal particle size and yields a narrow particle size distribution. Using state-of-the-art beamlines, we demonstrate how X-ray absorption fine structure (XAFS) techniques are now able to provide accurate structural information on nano-sized colloidal Au solutions at μM concentrations. This study demonstrates: (i) the size of Au colloids can be accurately tuned by adjusting the temperature of reduction, (ii) Au concentration, from 50 μM to 1000 μM, has little influence on the average size of colloidal Au NPs in solution and (iii) the immobilization step is responsible for significant growth in Au particle size, which is further exacerbated at increased Au concentrations. The work presented demonstrates that an increased understanding of the primary steps in sol-immobilization allows improved optimization of materials for catalytic applications.
Introduction
The study of supported metal nanoparticles (NPs) is a cornerstone of heterogeneous catalysis; their reduced size, distribution of surface sites, and interaction with metal oxide supports afford enhanced catalytic properties as well as providing effective use of noble metals.1–5 Within the various routes typically used to produce supported metal NPs, the application of sol-immobilization is becoming increasingly popular; it offers a high degree of tunability through the nature of stabilising or reducing agents,6–11 temperature of synthesis,5,12–16 solvent system,5,16,17 or concentration of the metal precursor salt.18There have been many elegant studies that have assessed the influence of these parameters, primarily relying upon advanced electron microscopy to provide structural information on the final supported catalyst.3,19,20 However, to understand better the preparation of supported NPs through sol-immobilization, further insights into all steps in the process are needed. Au NPs are one of the most intensely studied nano-particulate systems due to the wide range of potential applications, from medicine to catalysis.21 Recent approaches to study the structural properties of colloidal solutions of Au NPs, have utilised high brilliance X-rays, produced by synchrotron radiation, for small angle X-ray scattering (SAXS) and X-ray absorption fine structure (XAFS) investigations. Much work in this area has followed the formation of Au NPs using time-resolved studies;21–26 the intention is to understand the principal steps in the evolution from defined precursors to NP entities. Using XAFS, these studies have assessed the formation of Au NPs from precursor Au solutions with concentrations ranging between 7 and 100 mM,21,25–31 with data acquisition times ranging between minutes and hundreds of ms. They have continually increased their sophistication, moving from simple sample environments, such as a large volume cells, towards precision engineered microfluidic reactors28,29 or acoustic levitation systems.21
Other than XAFS, SAXS has proved successful in studying colloidal Au NP systems.21,24 It allows for very fast measurements, in the order of milliseconds, and is able to provide information regarding the particle size and shape during the reaction procedure.24,32 However, the resolution for SAXS is limited to sizes ≥ 1 nm,33 below which meaningful data cannot be extracted. Moreover, a sufficient concentration, ≥200 μM,23 is needed to achieve the required electronic contrast. As a consequence, the concentrations of Au precursors employed in these XAFS and SAXS studies do not always reflect the typical conditions found in the recent literature for the preparation of Au NPs, e.g. 5–100 μM.16,34–41 At such low concentrations, the application of these forms of characterization becomes challenging.
Furthermore, these studies do not explore the changes to the properties of the NPs once they have been supported. It is well known that there is a special interplay between metal NPs and their supports; the strong metal-support interaction,42 and wettability,43 influence the properties of the NPs once immobilised.43–46 To understand how best to optimise the catalytic performance of supported NPs it is important to separate the contributions of synthesis conditions during the colloidal step and immobilization on the resultant colloidal NP structures.
In this work, XAFS studies (Fig. 1) of colloidal Au solutions prepared at different temperatures (1 °C, 25 °C, 50 °C, 75 °C) and concentrations (50 μM, 100 μM, 1000 μM) have been performed. This study assesses the structural properties of colloidal Au solutions at more relevant concentrations than previously reported and allows for the unique contributions of colloidal Au generation and the subsequent immobilization step to be disentangled.
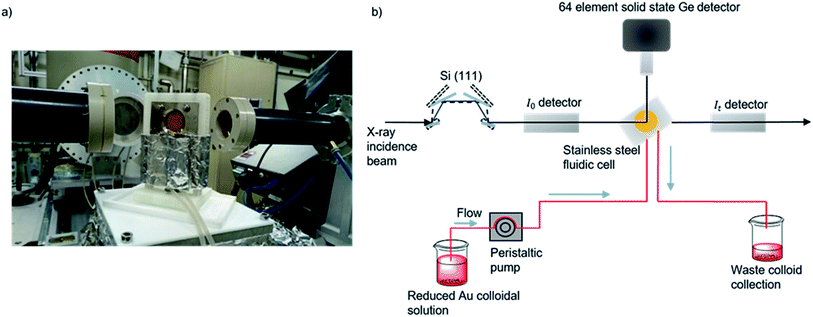 | ||
| Fig. 1 (a) Photograph of the in situ cell on the I20 beamline. (b) Experimental schematic for data acquisition of colloidal Au XAFS at the I20 beamline at the Diamond Light Source, Didcot, UK. A continuous flow of preformed colloid was pumped through PTFE tubing and XAFS data was acquired in fluorescence mode by a solid state Ge detector.48,66 | ||
Experimental section
Materials and methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, 80% hydrolysed, PVA/Au (wt. ratio = 0.65)). Solutions of NaBH4 (0.1 M) >96%, Aldrich, NaBH4/Au (molar ratio = 5) were subsequently added drop-wise over the course of a minute, under continuous stirring evolution of dark red sols was observed. Formation of colloidal Au NPs occurred after a 30 minute time period and studied by UV-vis spectroscopy and XAFS. TiO2 stabilised colloids (0.99 g P25, Degussa), were prepared, with a calculated amount of TiO2 added to give a final metal loading of 1 wt%. The supported Au/TiO2 mixture was acidified to pH 1–2 using H2SO4 before a 60 minute period under vigorous stirring, ensuring full immobilization of the Au NPs on TiO2; the mixture was filtered, washed with distilled water and dried overnight at room temperature. The different temperature and concentrations used in synthesis of the catalysts in this paper are listed in Table 1.
000 g mol−1, 80% hydrolysed, PVA/Au (wt. ratio = 0.65)). Solutions of NaBH4 (0.1 M) >96%, Aldrich, NaBH4/Au (molar ratio = 5) were subsequently added drop-wise over the course of a minute, under continuous stirring evolution of dark red sols was observed. Formation of colloidal Au NPs occurred after a 30 minute time period and studied by UV-vis spectroscopy and XAFS. TiO2 stabilised colloids (0.99 g P25, Degussa), were prepared, with a calculated amount of TiO2 added to give a final metal loading of 1 wt%. The supported Au/TiO2 mixture was acidified to pH 1–2 using H2SO4 before a 60 minute period under vigorous stirring, ensuring full immobilization of the Au NPs on TiO2; the mixture was filtered, washed with distilled water and dried overnight at room temperature. The different temperature and concentrations used in synthesis of the catalysts in this paper are listed in Table 1.
| Sample name | Temperature of preparation (°C) | [Au] (μM) | UV-vis max (nm) | Colloidal Au NPs | Au/TiO2 | Av. EXAFS NP sizea (nm (ref. 59)) | |||
|---|---|---|---|---|---|---|---|---|---|
| Av. TEM NP size (nm) | CNAu–Au | Av. EXAFS NP sizea (nm (ref. 59)) | Av. TEM NP size (nm) | CNAu–Au | |||||
| a The error in the process of calculating particle size from 1st shell coordination number has an intrinsic error of 0.1 nm. CNAu–Au refers to the Au–Au coordination number found by fitting the experimental EXAFS data using the Artemis software package,49 NP EXAFS fits are shown in Fig. S6. The remaining Au first shell fitting parameters for all samples can be found in Table S1. | |||||||||
| A1 | 1 | 100 | 492 | 3.0 ± 0.9 | 9.3 ± 0.6 | 1.6 | 2.7 ± 0.7 | 10 ± 0.7 | 2.0 |
| B | 1 | 50 | 495 | — | 9.2 ± 0.9 | 1.5 | 2.3 ± 0.6 | 9.5 ± 0.8 | 1.6 |
| C | 1 | 1000 | 498 | 4.6 ± 1.5 | 9.4 ± 0.4 | 1.6 | 4.0 ± 1.0 | 10.3 ± 0.5 | 2.3 |
| A2 (ref. 16) | 25 | 100 | 493 | — | 9.8 ± 0.5 | 1.9 | 2.9 ± 0.9 | 8.4 ± 0.3 | 1.4 |
| A3 (ref. 16) | 50 | 100 | 500–510 | — | 10.2 ± 0.7 | 2.2 | 2.8 ± 0.9 | 9.5 ± 0.3 | 1.9 |
| A4 (ref. 16) | 75 | 100 | 538 | — | 10.3 ± 0.7 | 2.3 | 3.3 ± 0.8 | 11.2 ± 0.4 | — |
Results and discussion
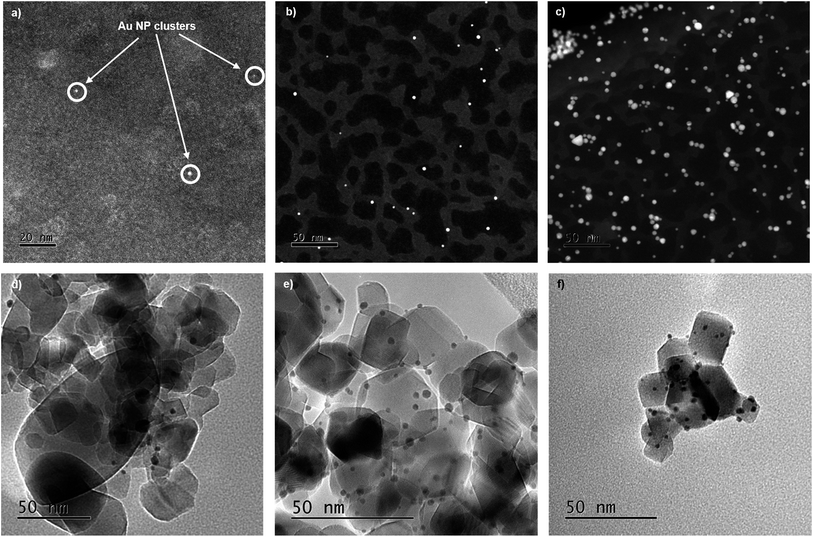
Ultraviolet-visible (UV-vis) spectroscopy has been used to study both the Au precursor solution (reduction rate) and the formation of Au NPs through the formation of surface plasmon resonance (SPR) band before immobilization.51–53 The position of the SPR peak, shown in Fig. S1,†λmax, is related to the particle size and shape of NPs; a shift to higher wavelength is indicative of an increase in Au particle size.51 The intensity of the band can also give a measure of particle size, as larger particles give more intense peaks, however, in this study there is a correlation of parameters with both particle size and concentration contributing towards this. It has been previously reported that the size of Au NPs supported on TiO2 can be tailored by systematic control of the temperature of reduction of Au precursor salts; an increase in reduction temperature has the effect of increasing the particle size of the supported Au NPs.51–53 This observation was repeated in this work (Table 1); as the temperature increases from 25 °C to 75 °C (A1–A4), the position of λmax shifts from 493 nm to 538 nm, indicating a growth in particle size. The first series of Au colloidal solutions were all prepared using an initial Au concentration of 100 μM. However, for the 1 °C reduction temperature two additional concentrations, 50 μM and 1000 μM (B and C), were also investigated. For this variable concentration series, there were no discernible changes to the SPR λmax, the broad nature of these bands makes it difficult to extract any quantifiable assessment of the particle size characteristics.54When analysing the properties of colloidal NPs at concentrations comparable to those employed previously,55 UV-vis and TEM both provide an incomplete description. The broad nature of the Au SPR band and the challenge of measuring colloidal solutions using TEM without causing aggregation of the colloidal NPs (Fig. 2a–c), does not capture sufficient detail to make a reliable comparison between supported and unsupported Au NPs. Recent advances through the increased flux of modern insertion device-based beamlines and multi-element fluorescence detectors, have allowed XAFS to provide a suitable tool for studying both unsupported and supported NP catalysts while avoiding beam damage (Fig. 2, S7 and S8†).
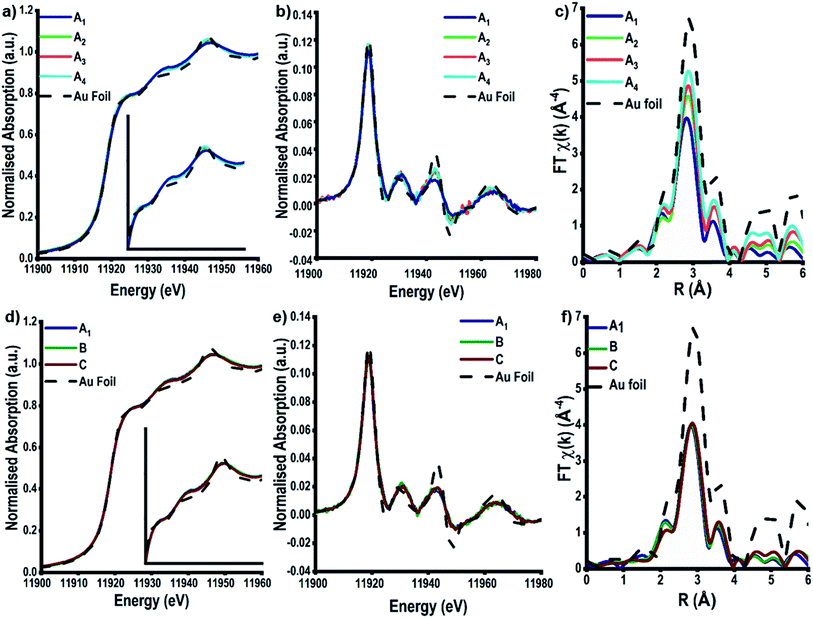
The high energy and stability of the I20-scanning beamline at Diamond Light Source, U.K., has been used to acquire reliable XAFS data, at the Au L3-edge, of our colloidal and TiO2 supported Au NPs (Fig. 3).48 The normalised XANES spectra (Fig. 3a) of colloidal Au prepared at variable temperature (samples A1–4) are characterised by the absence of an intense white line peak at ∼11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 915 eV, which is indicative of Au0 being the only species in solution; the white line arises due to dipole allowed 2p to 5d transitions, as Au0 has a full d-shell there is not an intense main edge transition, as seen in Fig. S2.†29,31,56 On assessing the colloidal solutions as a function of temperature, we observe that the feature at ∼11
915 eV, which is indicative of Au0 being the only species in solution; the white line arises due to dipole allowed 2p to 5d transitions, as Au0 has a full d-shell there is not an intense main edge transition, as seen in Fig. S2.†29,31,56 On assessing the colloidal solutions as a function of temperature, we observe that the feature at ∼11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 946 eV increases in intensity; the change in this feature is more pronounced and easier to observe when plotting the XANES data in its 1st derivative form (Fig. 3b). This continuum feature has been ascribed previously to Au–Au multiple scattering interactions and is linked to the particle size of the Au NPs.29,57,58 The larger the particle size, the higher the amount of multiple scattering from Au–Au interactions, resulting in an increase in the intensity of this feature. Varying the concentration of Au precursors (A1–C), however, does not appear to have an effect on the normalised XANES spectra (Fig. 3d), or the first derivative spectra (Fig. 3e). The absence of the multiple scattering post edge feature suggests that for all three samples, A1–C, the particle size is ≤2 nm.29,57,58
946 eV increases in intensity; the change in this feature is more pronounced and easier to observe when plotting the XANES data in its 1st derivative form (Fig. 3b). This continuum feature has been ascribed previously to Au–Au multiple scattering interactions and is linked to the particle size of the Au NPs.29,57,58 The larger the particle size, the higher the amount of multiple scattering from Au–Au interactions, resulting in an increase in the intensity of this feature. Varying the concentration of Au precursors (A1–C), however, does not appear to have an effect on the normalised XANES spectra (Fig. 3d), or the first derivative spectra (Fig. 3e). The absence of the multiple scattering post edge feature suggests that for all three samples, A1–C, the particle size is ≤2 nm.29,57,58
The EXAFS analysis, however, can be used to obtain quantitative information on the colloidal NP size. Fig. 3c shows the k3-weighted forward Fourier transform EXAFS spectra for samples A1–A4, EXAFS chi data can be found in Fig. S4.† It is evident that there is a change in intensity of the feature located at ∼2.8 Å as the temperature of reduction increases. This feature arises as a consequence of 1st shell Au–Au scattering interactions and the intensity of this peak can be correlated to an increase in the Au–Au coordination number (CN), which, in turn, shows the trend in particle size. With smaller Au NPs having a higher surface fraction there is a greater proportion of under coordinated surface Au atoms, leading to decreases in the average Au–Au CN.60
Calculations of the average Au NP sizes, were performed using methods reported by Beale et al.59 XAFS spectra were modelled based on different particle sizes assuming all Au NPs are; (1) spherical in shape, (2) FCC in packing and (3) <3 nm in diameter. A similar approach was used for samples A1, B, and C, where no changes can be seen in the Fourier transform EXAFS spectra, or in the generated fitting parameters.
The particle sizes obtained from the fits confirmed that, within the error of the measurement, adjusting the concentration of the Au, between 50–1000 μM, precursor does not alter the size of the colloidal NPs produced. This is an important observation as it is already known that for sol-immobilization, increasing the concentration of initial precursor solutions manifests itself as an increase of supported Au NP sizes.55 However, we can now confirm that this effect occurs only during the immobilization phase and not in the preformed colloidal NPs.
To assess the effect of immobilization, the corresponding Au/TiO2 materials (from solutions of A1, B, & C) were prepared and XAFS data were acquired (Fig. S6†); as the Au precursor concentration increases from 50–1000 μM, the NPs size increases from 1.6 to 2.3 nm. This in direct contrast with the results obtained for the colloidal Au NPs, where all the particles have approximately the same particle size.
A possible explanation for this behaviour could be attributed to the mobility of NPs on the support surface. As the synthesis temperature reaches the Hüttig (TH = 0.3Tmelting [K]) and Tamman temperatures (TT = 0.5Tmelting [K]), the surface atoms and bulk atoms respectively become mobile,61 constituting a mechanism for particle mobility.62 For NPs ∼2 nm in size, as observed in the colloidal solutions, the Tmelting can be as low as ∼330 °C,63 giving TH ∼ −93 °C and TT ∼ 25 °C.64 We suggest that with a higher concentration of the Au precursor and subsequent increase in surface NP density, coupled with slow migration of the mobile surface atoms yields NP growth through Ostwald ripening.65 Contrary to this, when preparing the colloid with a lower concentration, NPs are dispersed with larger interparticle distances, revealing negligible changes to NP size pre- and post-immobilization.
A comprehensive explanation for this behaviour has yet to be given in literature, but a further plausible reason could be a saturation of anchoring sites on the surface of the support, which, in turn, force the coalescence of the non-anchored nanoparticles. In the case presented the general Au wt% does not change amongst the samples, however, a possible change in local particle density during the immobilization phase could cause a growth in the particles due to a similar phenomenon, as the one shown by changing the metal loading.34 Regardless of the cause for the increase in size, it is clear that the in order to fully design and optimise supported NPs it is crucial to understand the relatively underexplored immobilization process; this is a key point in directing the resultant NP properties.
Conclusions
This work demonstrates that XAFS is a suitable method to extract quantifiable structural data on ultra-dilute solutions of colloidal Au NPs (50 μM), akin to those commonly used in sol-immobilization processes. These studies have revealed several key findings: (i) the particle size of the colloidal Au is dependent on the temperature of reduction, with lower temperatures generating smaller particles, (ii) the concentration of Au precursor does not influence the size of the formed colloidal NPs within the range of 50 to 1000 μM and (iii) the immobilization stage of synthesis influences the Au particle size growth processes. Therefore, controlling and optimising the immobilization step is of paramount importance for the usage of low/high diluted metal colloidal solutions to synthesize size/shape supported metal nanoparticles.Funding sources
All authors acknowledge UK Catalysis Hub Consortium and EPSRC (Grants EP/K014706/1, EP/K014668/1, EP/K014854/1, EP/K014714/1, and EP/I019693/1). D. D. and P.·P.·W. acknowledge further support through the STFC ST/R002754/1.Conflicts of interest
There are no conflicts to declare.Abbreviations
| NP | Nanoparticle |
| μM | Micromolar (10−6 molar) |
| SPR | Surface plasmon resonance |
| CN | Coordination number |
Data access statement
All data supporting this study are openly available from the University of Southampton repository at https://doi.org/10.5258/SOTON/D0921.Acknowledgements
The authors wish to acknowledge the Diamond Light Source for provision of the beamtime (SP17283). The RCaH are also acknowledged for use of facilities and support of their staff. The electron microscopy department of Johnson Matthey Technology Centre, Sonning Common are acknowledged for their support in imaging Au colloids, both in Sonning Common and at the ePSIC facility at Harwell Science and Innovation Centre, Didcot, OX11 0DE, UK. G. Malta for contribution of HAuCl4 XAFS reference data used in ESI.† UK Catalysis Hub is kindly thanked for resources and support provided via our membership of the UK Catalysis Hub Consortium and funded by EPSRC grant: EP/K014706/2, EP/K014668/1, EP/K014854/1, EP/K014714/1 or EP/M013219/1. The authors wish to thank the STFC GCRF START project for funding the position of Donato Decarolis. N. Abdullah would like to acknowledge the Ministry of Education, Malaysia (MOE) for their financial support through post-doctoral funding.Notes and references
- A. R. Tao, S. Habas and P. Yang, Small, 2008, 4, 310–325 CrossRef CAS.
- T. Teranishi and M. Miyake, Chem. Mater., 1998, 10, 594–600 CrossRef CAS.
- Y. Sun and Y. Xia, Science, 2002, 298, 2176–2179 CrossRef CAS PubMed.
- T. Ahmadi and Z. Wang, Science, 1996, 272, 1994–1996 CrossRef.
- S. M. Rogers, C. R. A. Catlow, C. E. Chan-Thaw, A. Chutia, N. Jian, R. E. Palmer, M. Perdjon, A. Thetford, N. Dimitratos, A. Villa and P. P. Wells, ACS Catal., 2017, 7, 2266–2274 CrossRef CAS.
- Y. Zhang, J. Yu, H. Niu and H. Liu, J. Colloid Interface Sci., 2007, 313, 503–510 CrossRef CAS PubMed.
- O. Rac, P. Suchorska-Wozniak, M. Fiedot and H. Teterycz, Beilstein J. Nanotechnol., 2014, 5, 2192–2201 CrossRef CAS PubMed.
- A. Villa, D. Wang, G. M. Veith, F. Vindigni and L. Prati, Catal. Sci. Technol., 2013, 3, 3036–3041 RSC.
- L. Prati and A. Villa, Acc. Chem. Res., 2014, 47, 855–863 CrossRef CAS PubMed.
- P. K. Khanna, P. V. More, J. P. Jawalkar and B. G. Bharate, Mater. Lett., 2009, 63, 1384–1386 CrossRef CAS.
- S. Pillai and P. V. Kamat, J. Mater. Chem. B, 2004, 108, 945–951 Search PubMed.
- S. Link and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 4212–4217 CrossRef CAS.
- N. Moloto, N. Revaprasadu, P. . Musetha and M. Moloto, J. Nanosci. Nanotechnol., 2009, 9, 4760–4766 CrossRef CAS PubMed.
- L. Prati and A. Villa, Catalysts, 2011, 2, 24–37 CrossRef.
- L. Prati and A. Villa, in Gold Catalysis: Preparation, Characterization, and Applications, CRC Press, 2016, pp. 59–63 Search PubMed.
- S. M. Rogers, C. R. A. Catlow, C. E. Chan-thaw, D. Gianolio, E. K. Gibson, A. L. Gould, N. Jian, A. J. Logsdail, R. E. Palmer, L. Prati, N. Dimitratos, A. Villa and P. P. Wells, ACS Catal., 2015, 5, 4377–4384 CrossRef CAS.
- T. Teranishi and M. Miyake, Chem. Mater., 1998, 4756, 594–600 CrossRef.
- J. Kimling, M. Maier, B. Okenve, V. Kotaidis, H. Ballot and A. Plech, J. Phys. Chem. B, 2006, 110, 15700–15707 CrossRef CAS.
- H. Borchert, E. V. Shevchenko, A. Robert, I. Mekis, A. Kornowski, G. Grübel and H. Weller, Langmuir, 2005, 21, 1931–1936 CrossRef CAS PubMed.
- A. Gniewek, A. M. Trzeciak, J. J. Ziółkowski, L. Kȩpiński, J. Wrzyszcz and W. Tylus, J. Catal., 2005, 229, 332–343 CrossRef CAS.
- J. Polte, T. T. Ahner, F. Delissen, S. Sokolov, F. Emmerling, A. F. Thünemann and R. Kraehnert, J. Am. Chem. Soc., 2010, 132, 1296–1301 CrossRef CAS PubMed.
- J. Ohyama, K. Teramura, T. Shishido, Y. Hitomi, K. Kato, H. Tanida, T. Uruga and T. Tanaka, Chem. Phys. Lett., 2011, 507, 105–110 CrossRef CAS.
- T. Sakai and P. Alexandridis, J. Phys. Chem. B, 2005, 109, 7766–7777 CrossRef CAS.
- B. Abécassis, F. Testard, Q. Kong, B. Francois and O. Spalla, Langmuir, 2010, 26, 13847–13854 CrossRef PubMed.
- L. Yang, H. Cheng, Y. Jiang, T. Huang, J. Bao, Z. Sun, Z. Jiang, J. Ma, F. Sun, Q. Liu, T. Yao, H. Deng, S. Wang, M. Zhu and S. Wei, Nanoscale, 2015, 7, 14452–14459 RSC.
- M. Harada and S. Kizaki, Cryst. Growth Des., 2016, 16, 1200–1212 CrossRef CAS.
- T. Shibata, H. Tostmann, B. Bunker, A. Henglein, D. Meisel, S. Cheong and M. Boyanov, J. Synchrotron Radiat., 2004, 8, 545–547 CrossRef.
- J. Ohyama, K. Teramura, Y. Higuchi, T. Shishido, Y. Hitomi, K. Kato, H. Tanida, T. Uruga and T. Tanaka, ChemPhysChem, 2010, 12, 127–131 CrossRef PubMed.
- T. Yao, Z. Sun, Y. Li, Z. Pan, H. Wei, Y. Xie, M. Nomura, Y. Niwa, W. Yan, Z. Wu, Y. Jiang, Q. Liu and S. Wei, J. Am. Chem. Soc., 2010, 132, 7696–7701 CrossRef CAS PubMed.
- M. Harada, K. Saijo and N. Sakamoto, Colloids Surf., A, 2009, 349, 176–188 CrossRef CAS.
- M. Giorgetti, G. Aquilanti, B. Ballarin, M. Berrettoni, M. C. Cassani, S. Fazzini, D. Nanni and D. Tonelli, Anal. Chem., 2016, 88, 6873–6880 CrossRef CAS PubMed.
- F. Hubert, F. Testard, A. Thill, Q. Kong, O. Tache and O. Spalla, Cryst. Growth Des., 2012, 12, 1548–1555 CrossRef CAS.
- S. Skou, R. E. Gillilan and N. Ando, Nat. Protoc., 2014, 9, 1727 CrossRef CAS.
- J. Kennedy, W. Jones, D. J. Morgan, M. Bowker, L. Lu, C. J. Kiely, P. P. Wells and N. Dimitratos, Catal., Struct. React., 2015, 1, 35–43 CrossRef.
- B. G. Trewyn, I. I. Slowing, S. Giri, H. Chen and V. S. Lin, Acc. Chem. Res., 2007, 40(9), 846–853 CrossRef CAS PubMed.
- S. Ayyappan, R. S. Gopalan, G. N. Subbanna and C. N. R. Rao, J. Mater. Res., 1997, 12, 398–401 CrossRef CAS.
- J. A. Trindell, J. Clausmeyer and R. M. Crooks, J. Am. Chem. Soc., 2017, 139, 16161–16167 CrossRef CAS PubMed.
- M. Bowker, C. Morton, J. Kennedy, H. Bahruji, J. Greves, W. Jones, P. R. Davies, C. Brookes, P. P. Wells and N. Dimitratos, J. Catal., 2014, 310, 10–15 CrossRef CAS.
- M. M. Maye, J. Luo, I.-I. S. Lim, N. N. Kariuki, D. Rabinovich and C.-J. Zhong, J. Am. Chem. Soc., 2003, 125, 9906–9907 CrossRef CAS PubMed.
- K. R. Brown and M. J. Natan, Langmuir, 1998, 14, 726–728 CrossRef CAS.
- X.-M. Li, M. R. de Jong, K. Inoue, S. Shinkai, J. Huskens and D. N. Reinhoudt, J. Mater. Chem., 2001, 11, 1919–1923 RSC.
- M. Boronat and A. Corma, Langmuir, 2010, 26, 16607–16614 CrossRef CAS PubMed.
- B. S. Lee, Y. S. Chi, J. K. Lee, I. S. Choi, C. E. Song, S. K. Namgoong and S. Lee, J. Am. Chem. Soc., 2004, 126, 480–481 CrossRef CAS PubMed.
- B. Roldan Cuenya, M. Alcántara Ortigoza, L. K. Ono, F. Behafarid, S. Mostafa, J. R. Croy, K. Paredis, G. Shafai, T. S. Rahman, L. Li, Z. Zhang and J. C. Yang, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 1–14 CrossRef.
- M. Comotti, W. Li, B. Spliethoff and F. Schu, J. Am. Chem. Soc., 2006, 917–924 CrossRef CAS PubMed.
- B. R. Cuenya, Thin Solid Films, 2010, 518, 3127–3150 CrossRef CAS.
- J. G. Mesu, A. M. Beale, F. M. F. De Groot and B. M. Weckhuysen, J. Phys. Chem. B, 2006, 110, 17671–17677 CrossRef CAS PubMed.
- S. Hayama, G. Duller, J. P. Sutter, M. Amboage, R. Boada, A. Freeman, L. Keenan, B. Nutter, L. Cahill, P. Leicester, B. Kemp, N. Rubies and S. Diaz-moreno, J. Synchrotron Radiat., 2018, 25, 1556–1564 CrossRef CAS PubMed.
- M. Newville, J. Synchrotron Radiat., 2001, 8, 96–100 CrossRef CAS PubMed.
- B. Ravel, in X-ray Absorption and X-ray Emission Spectroscopy. Theory and Applications, ed. J. A. van Bokhoven and C. Lamerbiti, Wiley, 2016, p. 283 Search PubMed.
- V. Amendola, R. Pilot, M. Frasconi, O. M. Maragò and A. M. Iatì, J. Phys.: Condens. Matter, 2017, 29, 203002 CrossRef PubMed.
- X. Zhou, G. Liu, J. Yu and W. Fan, J. Mater. Chem., 2012, 22, 21337 RSC.
- L. Xu, J. Peng, C. Srinivasakannan, L. Zhang, D. Zhang, C. Liu, S. Wang and A. Q. Shen, RSC Adv., 2014, 4, 25155 RSC.
- X. Huang and M. A. El-Sayed, J. Adv. Res., 2010, 1, 13–28 CrossRef.
- N. Dimitratos, A. Villa, L. Prati, C. Hammond, C. E. Chan-Thaw, J. Cookson and P. T. Bishop, Appl. Catal., A, 2016, 514, 267–275 CrossRef.
- Z. Song, J. P. L. Kenney, J. B. Fein and B. A. Bunker, Geochim. Cosmochim. Acta, 2012, 86, 103–117 CrossRef CAS.
- M. Harada and H. Einaga, Langmuir, 2007, 23, 6536–6543 CrossRef CAS PubMed.
- Y. M. Yiu, P. Zhang and T. K. Sham, Nanotechnology, 2003, 3, 183–186 Search PubMed.
- A. M. Beale and B. M. Weckhuysen, Phys. Chem. Chem. Phys., 2010, 12, 5562–5574 RSC.
- W. Szczerba, H. Riesemeier and A. F. Thünemann, Anal. Bioanal. Chem., 2010, 398, 1967–1972 CrossRef CAS PubMed.
- J. A. Moulijn, A. E. van Diepen and F. Kapteijn, Appl. Catal., A, 2001, 212, 3–16 CrossRef CAS.
- T. W. Hansen, A. T. DeLaRiva, S. R. Challa and A. K. Datye, Acc. Chem. Res., 2013, 46, 1720–1730 CrossRef CAS PubMed.
- G. Guenther and O. Guillon, J. Mater. Sci., 2014, 49, 7915–7932 CrossRef CAS.
- D. James Martin, D. Decarolis, Y. I. Odarchenko, J. J. Herbert, T. Arnold, J. Rawle, C. Nicklin, H.-G. Boyen and A. M. Beale, Chem. Commun., 2017, 53, 5159–5162 RSC.
- W. Z. Ostwald, Z. Phys. Chem., 1900, 34, 495–503 Search PubMed.
- S. Diaz-Moreno, M. Amboage, M. Basham, R. Boada, N. E. Bricknell, G. Cibin, T. M. Cobb, J. Filik, A. Freeman, K. Geraki, D. Gianolio, S. Hayama, K. Ignatyev, L. Keenan, I. Mikulska, J. Frederick, W. Mosselmans, J. J. Mudd and S. A. Parry, J. Synchrotron Radiat., 2018, 1–12 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9na00159j |
| This journal is © The Royal Society of Chemistry 2019 |