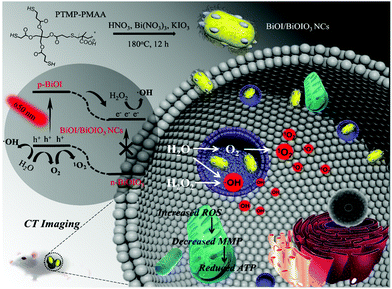
Reductive surfactant-assisted one-step fabrication of a BiOI/BiOIO3 heterojunction biophotocatalyst for enhanced photodynamic theranostics overcoming tumor hypoxia†
Wenyao
Zhen
 ab,
Yang
Liu
bc,
Xiaodan
Jia
a,
Lie
Wu
ab,
Yang
Liu
bc,
Xiaodan
Jia
a,
Lie
Wu
 a,
Chao
Wang
ab and
Xiue
Jiang
a,
Chao
Wang
ab and
Xiue
Jiang
 *ab
*ab
aState Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin 130022, China. E-mail: jiangxiue@ciac.ac.cn
bUniversity of Science and Technology of China, Hefei, Anhui 230026, China
cState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, Jilin, China
First published on 31st January 2019
Abstract
Inorganic biophotocatalysts with suitable size are greatly attractive in photodynamic therapy (PDT) with enhanced permeability and retention effect and the capability to produce reactive oxygen species via type-I reaction for overcoming tumor hypoxia. Herein, we develop a facile one-step hydrothermal method to prepare biocompatible BiOI/BiOIO3 heterostructure nanocomposites (BB NCs) by introducing reductive trithiol-terminated poly-(methacrylic acid) (PTMP-PMAA) as the surfactant, which can reduce partial KIO3 (raw material) into KI, resulting in the generation of BiOI and BiOIO3 in one nanocomposite. Under irradiation with a 650 nm laser, the photogenerated holes/electrons of the agent can effectively generate hydroxyl radicals (˙OH) and singlet oxygen (1O2) through a type I and O2 self-supplying type II PDT process, respectively, under both normoxic and hypoxic environments, which could lead to a reduced mitochondrial membrane potential (MMP) and in turn inhibit the production of ATP to initiate the death of HeLa cells, eventually causing the complete destruction of the tumor at a low dose (0.64 mg kg−1). In addition, the agent also shows extraordinary computed tomography imaging capability due to the high X-ray absorption coefficient (∼41.39 HU L g−1). Our study provides a robust biowindow-responsive hypoxic tumor theranostic agent to break through the limitations of PDT.
Conceptual insightsIn this research, we designed a new reductive surfactant (trithiol-terminated poly-(methacrylic acid), PTMP-PMAA)-assisted one-step method for preparing a BiOI/BiOIO3 (BB NCs) heterojunction biophotocatalyst. The PTMP-PMAA can reduce partially KIO3 (raw material) into KI, leading to the generation of BiOI and BiOIO3 in one nanocomposite with suitable nanosize, aqueous solubility and biocompatibility. Although the use of BiOIO3 as a photocatalyst already exists resulting from its high separation efficiency of photoinduced charges originating from its unique internal polar field, the introduction of BiOI could further enable it to be activated by laser light in the biowindow. The photoinduced holes/electrons of the BB NCs can generate hydroxyl radicals and singlet oxygen by a type I and O2 self-supplying type II photodynamic therapy (PDT) process, respectively. Finally, owing to their high atomic number, BB NCs achieved CT-guided tumor therapy with high-performance. This research provides a biowindow-responsive hypoxic tumor theranostic agent and may shed more light on the application of the biophotocatalyst and opens up a new strategy to prepare heterostructure nanocomposites by using the underlying potential of a suitable surfactant. |
Introduction
As a noninvasive and highly efficient therapeutic modality for cancers, photodynamic therapy (PDT), which employs cytotoxic singlet oxygen (1O2) produced by light-excited organic photosensitizer (PS) molecules and local oxygen through a direct energy transfer (type II PDT), has attracted great attention in the past few decades.1–4 However, the low accumulation and selectivity of PS in tumors and the dependence on oxygen severely limit the practical efficiency of PDT. Conjugation of PS with nanocarriers has dramatically increased the tumor accumulation and relieved tumor hypoxia benefiting from the enhanced permeability and retention (EPR) effect and in situ oxygen production capacity of the nanoparticles.4,5 Nevertheless, the possible leakage of most of the delivered organic PS and the complex construction of the nanocomposites still restrict the clinical applications.6,7Compared to organic PSs, inorganic photocatalysts with enhanced permeability and retention (EPR) effect such as titanium dioxide, zinc oxide and cerium oxide can convert H2O and O2 to cytotoxic reactive oxygen species (ROS) incorporating hydroxyl radicals (˙OH), superoxide radicals (˙O2−) and 1O2via an enhanced type-I PDT process dependent on an electron/hydrogen atom transfer and/or type-II PDT process through energy transfer upon illumination with ultraviolet (UV) light,8–12 making them attractive PDT agents with diminished oxygen dependence in type-II PDT. To solve the limitation of UV light penetration and induce deep PDT, various upconversion-nanoparticle- and ionizing-radiation-based PDT agents have been developed laboriously by multi-step composite processes.13–15 To minimize batch variation, some narrow-band gap photocatalysts with simplified structures have been developed, which can be activated by light in the biowindow,16,17 however, they suffer from reduced photoinduced redox capability. In addition, the quantum efficiency, recombination efficacy of photoderivated electron–hole pairs and separation efficiency of photoinduced carriers also greatly affect the efficiency of the PDT agent in producing ROS.18,19 As a result, a high dose is often used in vivo, which inevitably induces high system toxicity.
BiOIO3 is a superior wide-band gap photocatalyst due to its high separation efficiency of photoinduced charges originating from its unique heterolayered structure and internal polar field.20–22 Fabrication of the BiOI/BiOIO3 heterojunction nanoconstruct could further improve the photocatalytic activity and simultaneously enable it to be activated by visible light. However, its function as a PDT agent for cancer therapy has never been demonstrated, possibly due to the difficulty in the preparation of the BiOI/BiOIO3 heterojunction nanocomposite with suitable nanosize and aqueous solubility.
Herein, for the first time, we develop a facile one-step hydrothermal method to prepare uniform BiOI/BiOIO3 p–n heterostructure nanocomposites (BB NCs) (Scheme 1) as a new agent for efficient type-I and O2 self-supplying type II PDT to overcome tumor hypoxia. The photogenerated electrons (e−) and holes (h+) can be enhanced by the built-in electric field upon the migration of the photoderived electrons (e−) from the conduction band (CB) of BiOI to that of BiOIO3 under the irradiation of a 650 nm laser, which can effectively react with H2O2/H2O to produce ˙OH, and split H2O to produce O2 and subsequently 1O2 under both normoxic and hypoxic conditions. Consequently, the BB NCs can be used as a highly efficient theranostic agent to realize hypoxic tumor destruction at a low dose (0.64 mg kg−1) and excellent computed tomography (CT) imaging as a result of the high X-ray attenuation coefficient of BB NCs (∼41.39 HU L g−1).
Results and discussion
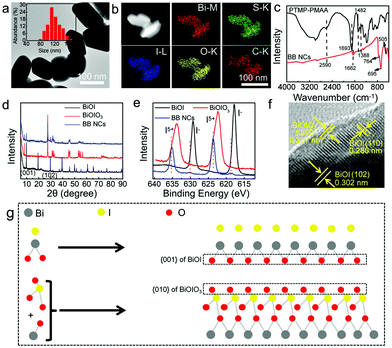
The BB NCs with dimensions suitable for biological applications were fabricated with bismuth(III) nitrate and potassium iodate (KIO3) as the sources of Bi3+ and IO3−, and trithiol-terminated poly-(methacrylic acid) (PTMP-PMAA) (Fig. S1, ESI†) was used as the surfactant to improve the dispersibility and bio-compatibility, in the presence of HNO3 (Scheme 1) without an additional loading process. The growth of the BiONO3 precipitate could be finely suppressed by HNO3, which could determine the size of the BB NCs due to the respective displacement of IO3− and I− (Fig. S2 (ESI†), PTMP-PMAA intrinsically possesses abundant sulfhydryls, and it can react with IO3− to form I−) and the synergetic effect of the stereo-hindrance of PTMP-PMAA23 (Fig. S3, ESI†). Consequently, the as-prepared BB NCs exhibit a short rod-like morphology with a diameter of ∼108 nm (Fig. 1a and Fig. S4, ESI†). We also attempted to use other surfactants to prepare BB NCs, but the size, shape, optical absorbance and catalytic properties were not suitable (Fig. S5, ESI†). Energy-dispersive spectrometry (EDS) indicates the existence of Bi, I, C, S and O elements (Fig. S6, ESI†), which are uniformly distributed in the whole nanocomposites (Fig. 1b), suggesting the successful modification of PTMP-PMAA on the BB NCs as further proved by FTIR spectroscopy measurements (Fig. 1c). The characteristic peak of the symmetric and asymmetric stretching bands of carboxylate (–COO–) at 1388 and 1482 cm−1 could be observed in the FTIR spectra of both BB NCs and PTMP-PMAA molecules, while the peak of the carbonyl group (–CO–) is red-shifted from 1693 cm−1 to 1662 cm−1 after the formation of the NCs, suggesting the modification of PTMP-PMAA by coordination interaction.24,25 Furthermore, the reduction of sulfhydryl could result in the disappearance of the characteristic peak at 2590 cm−1 and the formation of the NCs induced the vibrations of Bi–O (505 cm−1) and I–O (695 and 764 cm−1) bonds, respectively.26The hydrodynamic size of the BB NC aqueous solution is ∼171 nm and the ξ-potential is −29.9 mV and BB NCs showed reasonable stability in saline, phosphate buffer (PBS), DMEM culture medium and 80% FBS (Fig. S7, ESI†) due to the stereo-hindrance effect of PTMP-PMAA.27–29 The nature of the heterojunction was proved by the characterization of X-ray diffraction (XRD) spectroscopy (Fig. 1d and Fig. S8, ESI†). Most of the typical peaks of BB NCs are similar to those of BiOIO3 (ICSD # 262019) except for the appearance of the peaks at 9.658° and 29.645° belonging to the (001) and (102) peaks of BiOI (JCPDS file 10-0445) and the increase in the ratio of (040)/(002) in comparison to that of pure BiOIO3 (Fig. S8, ESI†),26,30 which suggest the coexistence of BiOIO3 and BiOI as further proved by the characteristic peaks of two sets of I 3d for I5+ and I− in the X-ray photoelectron spectroscopy (XPS) spectrum of BB NCs (Fig. 1e and Fig. S9, ESI†). Obvious shifts in their peak positions relative to those of pure BiOIO3 and BiOI suggest the formation of BiOIO3 and BiOI heterojunctions with close interfacial interaction (Fig. S9, ESI†) due to their crystal lattice matching.26,30 The well-resolved lattice fringes with lattice spacing of 0.302 nm, 0.280 nm and 0.287 nm can be indexed to the spacing of the (102) and (110) crystal planes of BiOI and the (002) crystal plane of BiOIO3,11 respectively (Fig. 1f), indicating that the main exposed facets of BiOI and BiOIO3 could be {001} and {010} facets with high reactivity.26,30,31 As shown in Fig. 1g, the similar atom arrangement between the {010} facets of BiOIO3 and the {001} facets of BiOI is important for governing the growth of the {001} facets of BiOI along the {010} facets of BiOIO3. The sharing of oxygen atoms can increase the contacting areas and intimate interface, which greatly enhances the transfer of photogenerated e−–h+ between the BiOI/BiOIO3 heterostructures. We used inductively coupled plasma atomic emission spectroscopy (ICP-AES) to detect the precise content of Bi element and the mass content of Bi is ∼46.8%, and the mole ratio of BiOI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BiOIO3 is about 2.71 (Fig. S10 and S11, ESI†).
BiOIO3 is about 2.71 (Fig. S10 and S11, ESI†).
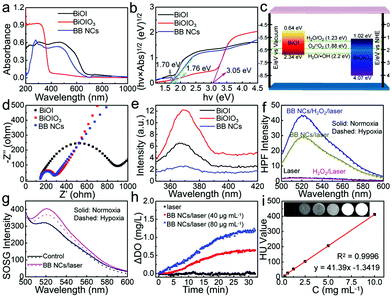
The absorption edge of the as-prepared BB NCs was first evaluated by UV-vis diffuse reflectance spectroscopy (DRS) (Fig. 2a). Relative to the spectrum of pure BiOIO3, the absorption edge of BB NCs has been dramatically extended to the visible region (∼677 nm) as that of pure BiOI. The band gaps of BiOIO3 and BiOI were calculated to be 3.05 and 1.70 eV, respectively (Fig. 2b) with conduction band-edge potentials of 1.02 and 0.64 eV as calculated by the empirical equation (Fig. 2c and Fig. S12, ESI†), which were consistent with their flat-band potentials obtained by Mott–Schottky (M–S) methods (Fig. S12, ESI†). In addition, we can also deduce that the pure BiOI and BiOIO3 belong to p-type and n-type semiconductors, respectively (Fig. S12, ESI†). Therefore, BiOI and BiOIO3 could form a p–n heterojunction with favorable interfacial charge transfer and enhanced carrier separation efficiency as demonstrated by the smaller arc radius in the EIS Nyquist plots of BB NCs (Fig. 2d)32 and its lower photoluminescence (PL) emission peak intensity at 370 nm under the excitation of 245 nm (Fig. 2e).33 Under 650 nm-light irradiation, photoinduced e− in the CB of BiOI could transfer to the CB of BiOIO3, while h+ clustered in the VB of BiOI. Such transfer of electrons in the BB NCs could decrease the recombination rate and further enhances the number of carriers to activate the reactions. We speculated that H2O2 could react with the accumulated photogenerated e− to generate ˙OH,34 and based on their band-edge potential (Fig. 2c), the photogenerated h+ could not only directly react with H2O molecules to form the most toxic ˙OH without the limitation of O2 but also can catalyze H2O to produce O2 and subsequent 1O2, resulting in oxygen-independent type I and O2 self-supplying type II PDT processes. To test the hypothesis, ˙OH probe: hydroxyphenyl fluorescein (HPF) was used. Indeed, the fluorescence intensity of HPF in the aqueous dispersion of BB NCs was significantly strengthened under the irradiation of a 650 nm laser, and was further enhanced by the addition of H2O2 (Fig. 2f), demonstrating that photogenerated (e−)/(h+) can convert H2O2/H2O to ˙OH during the photocatalytic process. Moreover, it was nearly unaffected in hypoxic conditions (Fig. S13, ESI†) as a result of oxygen-independent type I PDT activity of the BB NCs. What's more, the generation of ˙OH was further verified by the degradation of methyl red (MR) and the FL spectra variation of terephthalic acid (Fig. S14, ESI†). Furthermore, 1O2 could also be produced because the fluorescence intensity of singlet oxygen sensor green (SOSG) was obviously increased in the dispersions of BB NCs under irradiation (Fig. 2g), and the amount was not affected in the presence of a superoxide anion scavenger (Fig. S15, ESI†). Notably, in the hypoxic conditions, the 1O2 could be still generated although the amount was reduced partly. This is caused by the O2-evolving capability of BB NCs (Fig. 2h), which results in the O2 self-supplying type II PDT process. More attractively, the high atomic number of the component element of BB NCs (ZI = 53, ZBi = 83) endows it with extraordinary CT imaging ability. The X-ray absorption coefficient of BB NCs can be calculated to be ∼41.39 HU L g−1 based on the concentration-dependent signal enhancement (Fig. 2i), which is dramatically higher than most of the CT agents, such as hollow MoSx (17.94 HU L g−1),35 clinical iopromide (16.4 HU L g−1),36 WS2 quantum dots (37.73 HU L g−1)37 and Au@Pt nanodendrites (28.6 HU L g−1).38 (Table 1, ESI†).
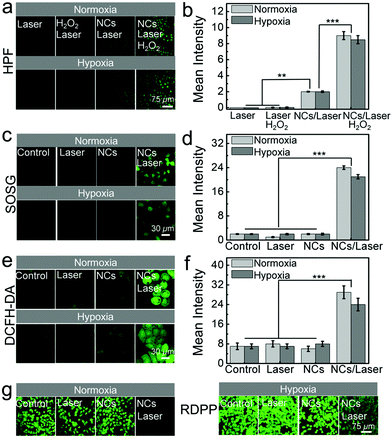
The excellent photocatalytic activity of BB NCs suggests great potential as a PDT agent for cancer treatment. They could produce ˙OH in HeLa cells during photocatalysis processes because the fluorescence of HPF in BB NCs/laser-treated cells was brighter than that in untreated cells, which was further enhanced in BB NCs/H2O2/laser-treated cells, while only H2O2/laser-treated cells showed nearly no fluorescence (Fig. 3a and b). Almost consistent changes in the cellular fluorescence can be found for the identical treatment under hypoxic conditions, suggesting the production of ˙OH independent of oxygen. Meanwhile, the intracellular photocatalysis could also produce 1O2 even in hypoxic cancer cells as revealed by the fluorescence changes of SOSG in BB NCs/laser-treated cells (Fig. 3c and d). Significantly enhanced fluorescence can be observed under both normoxic and hypoxic conditions, which was caused by O2 self-supplying via H2O splitting under irradiation. Besides, ROS probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) was also used to evaluate the total variation of intracellular ROS, and the green fluorescence was greatly enhanced in the HeLa cells pre-incubated with BB NCs and irradiated by a 650 nm laser under normoxic and hypoxic conditions due to the oxidation of the probe (Fig. 3e and f), further proving a strong biophotocatalytic ability of the BB NCs to induce intracellular ROS production. There was no obvious fluorescence distinction under normoxic and hypoxic conditions, indicating that the BB NCs can generate ROS without the limitation of hypoxia in cancer cells. Indeed, when the cells were pre-incubated with BB NCs and irradiated with a 650 nm laser, the fluorescence of [Ru(dpp)3]Cl2 (RDPP, O2 probe) within cells was quenched even in hypoxic conditions (Fig. 3g).
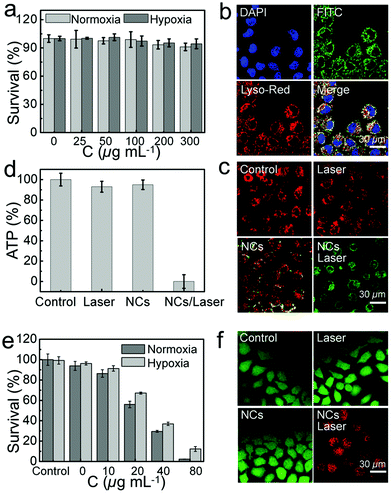
Its biocompatibility was evaluated by standard MTT assay to observe the viability of HeLa cells and L929 cells incubated with BB NCs for 24 h. No significant toxicity of HeLa cells can be found under normoxic or hypoxic conditions even when the concentration of BB NCs was up to 300 μg mL−1 (Fig. 4a), and the survival rate of L929 cells was also nearly unchanged (Fig. S16, ESI†). The hypoxic condition of HeLa cells were realized through incubating the cells in an enclosed incubator, into which was injected a gas mixture with constant ingredients (2% O2, 5% CO2 and 93% N2), and the hypoxic condition can be demonstrated by the increased expression (Fig. S17, ESI†) of hypoxia-inducible factor (HIF-1α). FITC-labeled BB NCs were used to investigate the endocytosis situation,39 DAPI was used to label the cell nucleus,40 and the green fluorescence of FITC was co-localized with lysosome-tracker red, which demonstrates that BB NCs can be internalized by HeLa cells and transported into the lysosome (Fig. 4b). After the BB NCs were delivered to the lysosome, the O2-independent production of a high concentration of ROS could lead to lysosome impairment and may depolarize the mitochondrial membrane potential (MMP) and even in turn initiate apoptosis in HeLa cells.41–43 As shown in Fig. 4c, BB NCs can decrease the MMP tested by 5,5′,6,6′-tetrachloro-1,1′,3,3′ tetraethyl-benzimidazolylcarbocyanine iodide (JC-1), which emits red fluorescence at high MMP and green fluorescence upon collapse of the MMP,4 which will inhibit the production of adenosine 5′-triphosphate (ATP) (Fig. 4d and Fig. S18, ESI†) because the decrease of MMP could uncouple the cascade reactions in the oxidative phosphorylation chain.44,45 All these effects induced almost complete cell death at concentrations of BB NCs up to 80 μg mL−1 under irradiation (Fig. 4e), which resulted in strong red fluorescence signals as stained by propidium iodide (Fig. 4f). These results demonstrate a powerful strategy to treat cancer without the limitation of hypoxia.
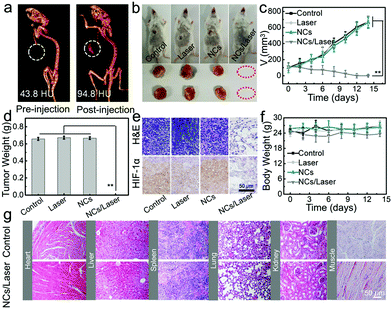
The excellent CT imaging performance in vitro also endows BB NCs with high CT imaging capability in vivo. After intratumoral injection of 0.2 mL of BB NCs (80 μg mL−1), the contrast signal was increased from 43.8 to 96.8 HU (Fig. 5a and Fig. S19, ESI†). To evaluate the therapeutic effect of BB NCs, tumor-bearing mice were divided into four groups: control, laser, BB NCs, and BB NCs/laser. At 24 h post intravenous injection, the tumors were irradiated by a laser (650 nm) for 15 min (0.5 W cm−2), and the body weights and tumor volumes were recorded. It is obvious that the tumors in the BB NCs/laser group were absolutely destroyed (Fig. 5b–d), suggesting an excellent PDT efficiency. Haematoxylin and eosin (H&E) staining (Fig. 5e) was also conducted, and more vacuoles, condensed nuclei, changed shape, and incomplete membrane integrity of the cells were observed in the BB NCs/laser group, suggesting severe destruction.46,47 Also, hypoxia-inducible factor (HIF-1α) staining (Fig. 5e) in BB NCs/laser-treated tumor tissues are largely stained blue, which indicates that BB NCs can produce O2 within tissues under illumination. Notably, the dose of BB NCs we used is lower than that of most of the photocatalytic nanomaterials (Table 2, ESI†), such as BiOI@Bi2S3@BSA (1.6 mg kg−1),15 Ce(III)-doped LiYF4@SiO2@ZnO structure (4 mg kg−1),48 Fe@γ-Fe2O3@H-TiO2 nanocomposites (8 mg kg−1),49 bismuth ferrite nanoparticles (12 mg kg−1),50 and (UCNPs)–platinum(IV) (Pt(IV))–ZnFe2O4 (16 mg kg−1),51 which results in good biocompatibility of the agent. There was no obvious difference in body weight (Fig. 5f) in the different treated groups, pathological changes in the main organs (Fig. 5g) and blood items (Fig. S20, ESI†) although there were evident distributions of BB NCs in the liver and spleen (Fig. S21, ESI†). The content variations of Bi element distributed in the feces and urine at different time points (Fig. S22, ESI†) suggest that the BB NCs were primarily excreted through feces. The blood circulation of Bi was also detected and the blood circulation half-time is about ∼5.61 h (Fig. S23, ESI†).
Conclusions
In summary, we prepared 650 nm-light driven BiOI/BiOIO3 heterostructure nanocomposites through a one-step hydrothermal method realized by the assistance of reductive surfactant: PTMP-PMAA, as the –SH of the used surfactant can react with KIO3 (raw material) to generate KI and then in situ produce BiOI and BiOIO3 in one nanocomposite. More importantly, we found that the main exposed facets of BiOI and BiOIO3 are the {001} and {010} facet, respectively, which can enhance the transfer of photogenerated e−–h+ between the BiOI/BiOIO3 heterostructures and then increase the PDT efficiency by producing 1O2 and ˙OH through O2 self-supplying type II and O2-independent type I PDT processes under visible-light-induced H2O/H2O2 splitting irrespective of hypoxia. After the BB NCs were delivered to the lysosome, a high concentration of ROS induced by biophotocatalysis reduced the MMP and in turn inhibited the production of ATP, which promoted the death of HeLa cells, eventually causing the complete destruction of tumor tissue at a relatively low dose (0.64 mg kg−1). In addition, the new agent also shows extraordinary CT imaging properties (X-ray absorption coefficient is ∼41.39 HU L g−1). The results demonstrate the great potential of BB NCs as hypoxic PDT agents for tumor theranostics.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Joint Sino-German Research Projects (21761132028), the National Natural Science Foundation of China (21675149, 21705146, 21874125, and 21877107), the Key Research Program of Frontier Sciences, CAS (QYZDY-SSW-SLH019), and the Science and Technology Development Program of Jilin Province (20170414037GH, 20170520133JH, 20190201074JC, 20180101015JC), and K. C. Wong Education Foundation.References
- M. Li, J. Xia, R. Tian, J. Wang, J. Fan, J. Du, S. Long., X. Song, J. W. Foley and X. Peng, J. Am. Chem. Soc., 2018, 140, 14851 CrossRef CAS PubMed.
- Z. Sheng, D. Hu, M. Zheng, P. Zhao, H. Liu, D. Gao, P. Gong, G. Gao, P. Zhang, Y. Ma and L. Cai, ACS Nano, 2014, 8, 12310 CrossRef CAS PubMed.
- J. Xu, P. Yang, M. Sun, H. Bi, B. Liu, D. Yang, S. Gai, F. He and J. Lin, ACS Nano, 2017, 11, 4133 CrossRef CAS PubMed.
- H. Chen, Y. Qiu, D. Ding, H. Lin, W. Sun, G. D. Wang, W. Huang, W. Zhang, D. Lee, G. Liu, J. Xie and X. Chen, Adv. Mater., 2018, 30, 1802748 CrossRef PubMed.
- Q. Chen, L. Feng, J. Liu, W. Zhu, Z. Dong, Y. Wu and Z. Liu, Adv. Mater., 2016, 28, 7129 CrossRef CAS PubMed.
- Z. Zhou, J. Song, L. Nie and X. Chen, Chem. Soc. Rev., 2016, 45, 6597 RSC.
- J. Ge, M. Lan, B. Zhou, W. Liu, L. Guo, H. Wang, Q. Jia, G. Niu, X. Huang, H. Zhou, X. Meng, P. Wang, C. Lee, W. Zhang and X. Han, Nat. Commun., 2014, 5, 4596 CrossRef CAS PubMed.
- Z. Hou, Y. Zhang, K. Deng, Y. Chen, X. Li, X. Deng, Z. Cheng, H. Lian, C. Li and J. Lin, ACS Nano, 2015, 9, 2584 CrossRef CAS PubMed.
- Y. Li, W. Zhang, J. Niu and Y. Chen, ACS Nano, 2012, 6, 5164 CrossRef CAS PubMed.
- S. S. Lucky, K. C. Soo and Y. Zhang, Chem. Rev., 2015, 115, 1990 CrossRef CAS PubMed.
- W. Fan, P. Huang and X. Chen, Chem. Soc. Rev., 2016, 45, 6488 RSC.
- C. Yao, W. Wang, P. Wang, M. Zhao, X. Li and F. Zhang, Adv. Mater., 2018, 30, 1704833 CrossRef PubMed.
- S. Cui, D. Yin, Y. Chen, Y. Di, H. Chen, Y. Ma, S. Achilefu and Y. Gu, ACS Nano, 2013, 7, 676 CrossRef CAS PubMed.
- C. Zhang, K. Zhao, W. Bu, D. Ni, Y. Liu, J. Feng and J. Shi, Angew. Chem., Int. Ed., 2015, 54, 1770 CrossRef CAS PubMed.
- Z. Guo, S. Zhu, Y. Yong, X. Zhang, X. Dong, J. Du, J. Xie, Q. Wang, Z. Gu and Y. Zhao, Adv. Mater., 2017, 29, 1704136 CrossRef PubMed.
- W. Zhen, Y. Liu, L. Lin, J. Bai, X. Jia, H. Tian and X. Jiang, Angew. Chem., Int. Ed., 2018, 57, 10309 CrossRef CAS PubMed.
- P. Kalluru, R. Vankayala, C. S. Chiang and K. C. Hwang, Angew. Chem., Int. Ed., 2013, 52, 12332 CrossRef CAS PubMed.
- J. Gupta, J. Mohapatra and D. Bahadur, Dalton Trans., 2017, 46, 685 RSC.
- G. Tian, Y. Chen, W. Zhou, K. Pan, Y. Dong, C. Tian and H. Fu, J. Mater. Chem., 2011, 21, 887 RSC.
- W. Wang, X. Huang, S. Wu, Y. Zhou, L. Wang, H. Shi, Y. Liang and B. Zou, Appl. Catal., B, 2013, 134, 293 CrossRef.
- J. L. Giocondi and G. S. Rohrer, Top. Catal., 2008, 49, 18 CrossRef CAS.
- H. Huang, S. Tu, C. Zeng, T. Zhang, A. H. Reshak and Y. Zhang, Angew. Chem., Int. Ed., 2017, 56, 11860 CrossRef CAS PubMed.
- F. J. R. Hird and J. R. Yates, Biochem. J., 1961, 80, 612 CrossRef CAS PubMed.
- X. Jiang, S. Zhang, F. Ren, L. Chen, J. Zeng, M. Zhu, Z. Cheng, M. Gao and Z. Li, ACS Nano, 2017, 11, 5633 CrossRef CAS PubMed.
- M. I. Majeed, J. Guo, W. Yan and B. Tan, Polymers, 2016, 8, 392–407 CrossRef.
- F. Dong, T. Xiong, Y. Sun, Y. Zhang and Y. Zhou, Chem. Commun., 2015, 51, 8249 RSC.
- M. S. Kandanapitiye, M. Gao, J. Molter, C. A. Flask and S. D. Huang, Inorg. Chem., 2014, 53, 10189 CrossRef CAS PubMed.
- X. M. Sun, J. Wu, Q. F. Li, Q. Z. Liu, Y. F. Qi, L. You, Z. Ji, P. He, P. F. Sheng, J. X. Ren, W. B. Zhang, J. Lu and J. J. Zhang, Appl. Catal., B, 2017, 218, 80 CrossRef CAS.
- X. M. Qi, M. L. Gu, X. Y. Zhu, J. Wu, H. M. Long, K. He and Q. Wu, Chem. Eng. J., 2016, 285, 11 CrossRef CAS.
- H. Huang, K. Xiao, K. Liu, S. Yu and Y. Zhang, Cryst. Growth Des., 2016, 16, 221–228 CrossRef CAS.
- R. Zhou, J. Wu, J. Zhang, H. Tian, P. Liang, T. Zeng, P. Lu, J. Ren, T. Huang, X. Zhou and P. Sheng, Appl. Catal., B, 2017, 204, 465 CrossRef CAS.
- Z. Hosseini, N. Taghavinia, N. Sharifi, M. Chavoshi and M. Rahman, J. Phys. Chem. C, 2008, 112, 18686 CrossRef CAS.
- J. Wu, K. Xu, Q. Liu, Z. Jia, C. Qu, X. Qi, H. Zhang, Y. Guan, P. He and L. Zhu, Appl. Catal., B, 2018, 232, 135 CrossRef CAS.
- S. W. Zhang, J. X. Li, X. K. Wang, Y. S. Huang, M. Y. Zeng and J. Z. Xu, J. Mater. Chem. A, 2015, 3, 10119 RSC.
- J. Wang, L. Liu, Q. You, Y. Song, Q. Sun, Y. Wang, Y. Cheng, F. Tan and N. Li, Theranostics, 2018, 8, 955 CrossRef CAS PubMed.
- Z. Li, J. Liu, Y. Hu, K. A. Howard, Z. Li, X. Fan, M. Chang, Y. Sun, F. Besenbacher, C. Chen and M. Yu, ACS Nano, 2016, 10, 9646 CrossRef CAS PubMed.
- Y. Yong, X. Cheng, T. Bao, M. Zu, L. Yan, W. Yin, C. Ge, D. Wang, Z. Gu and Y. Zhao, ACS Nano, 2015, 9, 12451 CrossRef CAS PubMed.
- X. Liu, X. Zhang, M. Zhu, G. Lin, J. Liu, Z. Zhou, X. Tian and Y. Pan, ACS Appl. Mater. Interfaces, 2017, 9, 279 CrossRef CAS PubMed.
- C. Chu, E. Ren, Y. Zhang, J. Yu, H. Lin, X. Pang, Y. Zhang, H. Liu, Z. Qin, Y. Cheng, X. Wang, W. Li, X. Kong, X. Chen and G. Liu, Angew. Chem., Int. Ed., 2019, 58, 269 CrossRef CAS PubMed.
- P. Zhang, L. Zhang, Z. Qin, S. Hua, Z. Guo, C. Chu, H. Lin, Y. Zhang, W. Li, X. Zhang, X. Chen and G. Liu, Adv. Mater., 2018, 30, 1705350 CrossRef PubMed.
- M. A. Seyed, R. Bhuvaneswari, K. C. Soo and M. Olivo, Int. J. Oncol., 2011, 39, 821 Search PubMed.
- M. Lam, N. L. Oleinick and A. L. J. Nieminen, Biol. Chem., 2001, 276, 47379 CrossRef CAS PubMed.
- K. Berg and J. J. Moan, Photochem. Photobiol., 1997, 65, 403 CrossRef CAS PubMed.
- H. Wang, Z. Gao, X. Liu, P. Agarwal, S. Zhao, D. W. Conroy, G. Ji, J. Yu, C. P. Jaroniec, Z. Liu, X. Lu, X. Li and X. He, Nat. Commun., 2018, 9, 562 CrossRef PubMed.
- X. Peng, F. Li, S. Li and Y. Zhu, Biotechnol. Lett., 2009, 31, 409 CrossRef CAS PubMed.
- G. Lin, Y. Zhang, C. Zhu, C. Chu, Y. Shi, X. Pang, E. Ren, Y. Wu, P. Mi, H. Xia, X. Chen and G. Liu, Biomaterials, 2018, 176, 60 CrossRef CAS PubMed.
- C. Wang, C. Dai, Z. Hu, H. Li, L. Yu, H. Lin, J. Bai and Y. Chen, Nanoscale Horiz., 2019 10.1039/C8NH00299A.
- C. Zhang, K. Zhao, W. Bu, D. Ni, Y. Liu, J. Feng and J. Shi, Angew. Chem., Int. Ed., 2015, 54, 1770 CrossRef CAS PubMed.
- M. Wang, K. Deng, W. Lü, X. Deng, K. Li, Y. Shi, B. Ding, Z. Cheng, B. Xing, G. Han, Z. Hou and J. Lin, Adv. Mater., 2018, 30, 1706747 CrossRef PubMed.
- C. Yang, Y. Chen, W. Guo, Y. Gao, C. Song, Q. Zhang, N. Zheng, X. Han and C. Guo, Adv. Funct. Mater., 2018, 28, 1706827 CrossRef.
- H. Bi, Y. Dai, P. Yang, J. Xu, D. Yang, S. Gai, F. He, B. Liu, C. Zhong, G. An and J. Lin, Small, 2018, 14, 1703809 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8nh00440d |
| This journal is © The Royal Society of Chemistry 2019 |