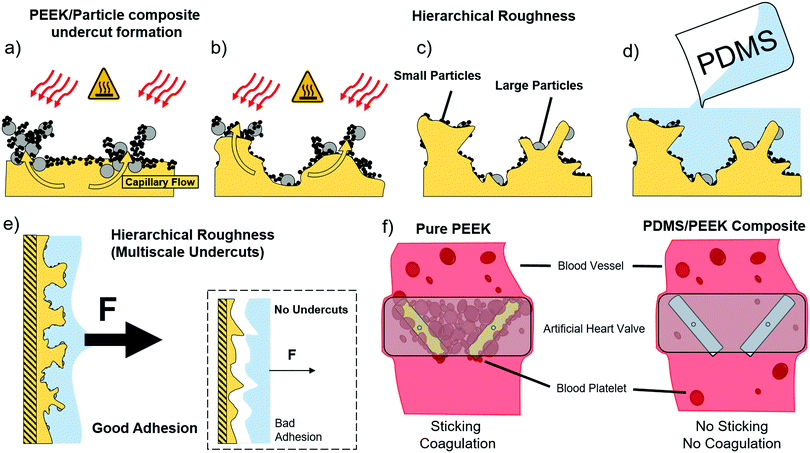
Perfect polymer interlocking by spherical particles: capillary force shapes hierarchical composite undercuts†
Leonard
Siebert
 *a,
Tim
Schaller
b,
Fabian
Schütt
a,
Sören
Kaps
a,
Jürgen
Carstensen
a,
Sindu
Shree
a,
Jörg
Bahr
a,
Yogendra Kumar
Mishra
*a,
Tim
Schaller
b,
Fabian
Schütt
a,
Sören
Kaps
a,
Jürgen
Carstensen
a,
Sindu
Shree
a,
Jörg
Bahr
a,
Yogendra Kumar
Mishra
 *a,
Hans-Hinrich
Sievers
b and
Rainer
Adelung
*a
*a,
Hans-Hinrich
Sievers
b and
Rainer
Adelung
*a
aFunctional Nanomaterials, Institute for Materials Science, Kiel University, Kaiserstr. 2, D-24143, Kiel, Germany. E-mail: lesi@tf.uni-kiel.de; ykm@tf.uni-kiel.de; ra@tf.uni-kiel.de
bDepartment of Cardiac and Thoracic Vascular Surgery, University of Lübeck, Ratzeburger Allee 160, D-23538, Lübeck, Germany
First published on 27th February 2019
Abstract
Polymers often do not provide all necessary properties for certain applications. In this case polymer composites can be used to combine beneficial qualities of each single component. In many cases, especially for surface–bulk-composites, adhesion is an issue because polymers with complementary properties are necessary. If the chemical binding of the pristine polymers does not allow for good adhesion, advanced strategies have to be employed which most of the time lead to chemical alteration of the surface, its destruction or else low adhesion. While roughening of the surface leads to an increase in the interface area, it will not provide a stable bond, if the adhesion between the two polymers is low in the first place. By selforganized introduction of special undercuts onto one of the polymer surfaces and by applying the second polymer in a liquid form, a mechanical interlocking composite can be achieved. In these composites adhesion can be so strong that only cohesive failure in one of the polymers will occur. In this work we evaluated simple fabrication techniques for the design of simple and complex undercuts and the adhesion between the exemplary composite PEEK and PDMS. We find that by utilizing the capillary effect, spherical standard particles can be used to create a surface structure for mechanical interlocking. Additionally, we obtain a 5.6 times higher adhesion between PEEK and PDMS. We come to the conclusion that a multi-scale undercut is necessary to obtain a strong adhesion between soft polymers like PDMS and stiff polymers like PEEK by looking at the detachment mechanism for these different undercut systems. Lastly, the composite is evaluated by blood contact tests to verify the intactness of the blood repellant effects of the PDMS layer.
Conceptual insightsPolymer composites can be used to realize a combination of the special individual features of polymers, e.g. low adhesion of PDMS and high mechanical strength of PEEK. Especially in laminate like composites, a good adhesion between the polymers is important. Modifications of the interface chemistry might lead to undesired alteration of the surfaces in touch, their destruction or else low adhesion. Micro and nano scale mechanical interlocking is an effective alternative only if hook like undercut structures can be provided. However, the notched shape of interlocking particles complicates the fabrication and embedding process. Counterintuitively, the presented strategy is based on simple spherical particles self-organizing into hook like undercuts on the polymer surface by capillary action. By applying the second, liquefied polymer on top, a mechanical interlocking composite laminates where perfect adhesion is provided, i.e. only cohesive failure will occur as failing mechanism. Thus, we obtain 5.6 times higher adhesion strength for exemplary PDMS/PEEK composites and conclude that hierarchical undercuts are necessary to obtain strongest adhesion. Lastly, the composite is evaluated by blood contact tests to verify that the blood repellant feature of the PDMS layer is still intact while the laminate exhibits the mechanical strength of PEEK. |
Introduction
Multi-polymer based composite materials are key components in several state-of-art technological applications because of their simultaneously accessible cumulative properties.1 Individual properties of different polymers play very unique roles and hence it becomes necessary to combine several polymers together in an appropriate composite form, often a requirement for many applications.2 For general applications polymers based composites come in the form of interpenetrating phase composites but in some specific applications,3 the polymer layers are often adhered together using various bonding methods.4–6 In many cases the adhesion of the desired polymers is difficult, simply because materials with complementary properties are used.7 If the pristine chemical bonds do not allow for good adhesion, techniques like plasma treatment,8,9 ion beam treatment,10 chemical etching11 or chemical grafting12 are applied to form reactive bonds. Although these processes are often accompanied by a reorganization13 or a serious chemical alteration14 of the polymer surface, the adhesion strength may still be limited to values one order of magnitude below the cohesive strength of the weakest composite partner.15Additionally, the storage time after the surface treatment prior to the adhesion step is limited for most common practices such as plasma modification. In these cases the treated surfaces revert back to their pristine states because reactive groups react with the environment or the reactive bonds diffuse into the bulk and thus the adhesion lowers again.16,17 Purely mechanical surface treatments (e.g. grinding, grit-blasting) are standard methods to increase the adhesion without changing the chemical nature of the polymers by only increasing the interfacial area or additionally producing undercuts.9,18
While the increase in the surface area just enhances the adhesion by providing more bonding sites (larger contact area), an undercut structure allows for mechanical interlocking19 thus replacing the adhesive bonding strength between the polymers by the cohesive bonding strength of the individual polymers. This process makes the adhesion independent of chemistry and the quality of the interlocking structures is the decisive factor for the strength of the interface.20
Complex shaped nanostructures have been used by Jin et al., where zinc oxide tetrapods (ZnO-T) were utilized to join the Teflon (PTFE) and silicone (PDMS) together which do not adhere directly because of their very low surface energies.20 The interlocking mechanism was investigated and it has been reported that the 3D complex shape indeed plays a very important role. Using a tetrapods based interlocking approach, a peel strength of around 2 N cm−1 between PTFE and PDMS layers was reported, however for certain applications, an even higher adhesion is needed. In this study no cohesive failure was found, meaning a further improvement of the core idea might raise the adhesion even further. Additionally, the necessity of complex shaped particles limits the practicability.
Here we demonstrate a simple fabrication procedure for complex shaped surfaces to promote the adhesion of two typically non-adhering materials. We utilize standard spherical nano and micro particles without any intrinsic possibility for polymer/particle undercut formation by using the capillary effect. We also assess what makes a good undercut structure by the example of joining PDMS, a low surface energy, chemically inert and soft polymer to PEEK, a high strength, high temperature, chemically inert polymer and show that hierarchical undercuts on multiple length scales are most efficient adhesion promoters allowing for only cohesive failure of the individual polymer and therefore giving the strongest possible adhesion. Different fabrication approaches and different detachment mechanisms are evaluated to obtain insight into the most promising type of undercuts and surface modifications.
For the given composite additional platelet adhesion tests have been carried out on differently prepared PDMS surfaces in order to check the hemocompatibility of the as prepared polymer composites. The results indicate that a perfectly flat silicone surface can achieve the highest blood adhesion resistances while still keeping the hemocompatibility of pristine PDMS. This indicates the viability of the utilized fabrication methods with respect to an application as biomedical implants.
Particle interlocking approach
The model for the fabrication of a surface with interlocking sites on multiple scales is depicted in Fig. 1. In the first step the polar polymer substrate is coated with a dry powder mixture of particles. The mixture can consist of more than one different particle type (e.g. ceramic, metal) of two different sizes, preferably polar nanoparticles of 100 nm and smaller and a micron sized particle type. The layer thickness ranges from 100 μm to 500 μm and contains particle agglomerates, where the particle–particle distance is smaller than in the surrounding powder. After coating the surface with a particle layer, different regimes of varying density are present due to these agglomerates (Fig. 1a). This particle film is then heated (e.g. 500–600 °C for PEEK) from the top in order to melt the polar polymer beneath.Dipole–(induced) dipole interactions between the polar particles and the polar polymer lead to a combination of Keesom and Debye attractive forces between these two components. Molten polymer is thereby flowing into the particle network above, preferentially into the particle agglomerates in which the medium distance between the particles is lower than in other places.
More generally, the agglomerated powder layer can be assumed as the series of dimensionally distributed interconnected capillaries. A regime of higher packing density can be regarded as a network of capillaries with low diameter, whereas the surrounding powder can be regarded as a network of high diameter capillaries. According to the laws for capillary action, sufficient wetting of the particles by the polymer is primarily given through a low contact angle of θ < 90°. Additionally, the surface energy γ of polymers at the liquid–air-interface is usually high, reducing the tendency to wet capillaries where the particle–particle-distance is too high. The wetting becomes energetically more favorable however, when the area of the polymer–gas interface is reduced. Thus, the energy barrier Ew for an agglomerate to be wetted completely scales with the medium particle distance squared:
| Ew ∝ r2 |
Only if the microscopic particle–particle-distance is low enough, a sufficient capillary force can be generated, enabling the molten polymer to flow easily. Thus, the denser packed agglomerates are wetted by the polymer (Fig. 1b), which leads to the formation of pillars of particle–polymer composite. Due to the different sizes of the particles and the selective embedding process, a hierarchical roughness with many polymer/particle composite undercuts is produced (Fig. 1c). The heat source is removed and the polymer cools down, solidifying the particle–polymer pillars and the surrounding polymer. The particle sites which could not draw up the polymer or are only partially-embedded are rinsed away by thorough brushing under running water.
A surface of randomly distributed, self-organized interlocking sites is obtained at the polymer surface. When another liquefied polymer is coated onto this surface, these interlocking sites will be covered completely (Fig. 1c), so that only the surface of the composite consists of the second polymer. By solidifying the second polymer a mechanical interlocking between the two polymers is achieved, creating a strong polymer composite sandwich structure.
Since the substrate polymer shows many undercuts on multiple length scales a much stronger adhesion (e.g., here shown 5.6 times stronger adhesion between PDMS and PEEK) than a surface roughened by standard techniques can be expected (inset Fig. 1e). Pulling forces can be distributed much better around the inner walls of a hierarchical undercut than on a flat surface.
By employing this novel concept we fabricated and analyzed a composite of polyether ether ketone (PEEK, a high strength, high temperature thermoplastic) and polydimethylsiloxane (PDMS, a low surface energy elastomer). The properties of both are respectively utilized in a manifold of applications such as surgical implants and many others.
Results and discussions
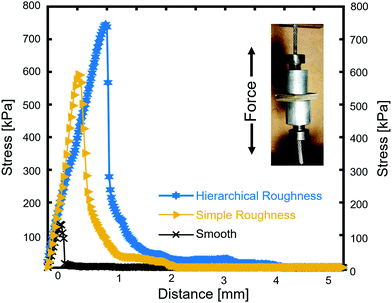
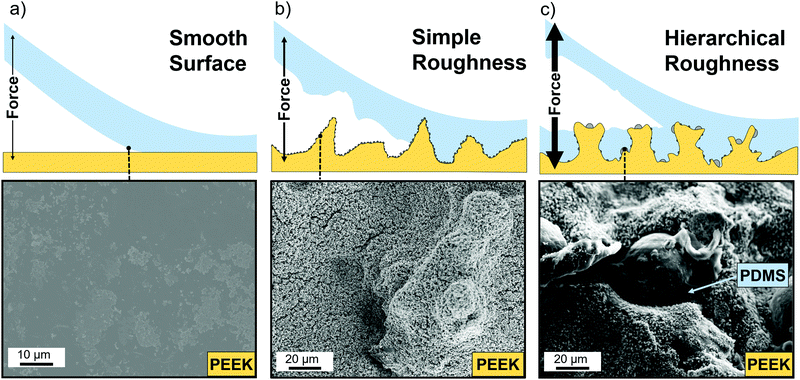
The adhesive properties of the fabricated composites were evaluated in terms of the three different heating approaches (described briefly in Experimental section, ESI†). Fig. 2 shows the three representative curves, one for each treatment, where the inset shows the testing geometry with the two aluminum rods and the PDMS coated PEEK samples in between them. The control sample was a pristine PEEK sample coated with around 1 mm of PDMS. The black curve in Fig. 2 shows the stress–distance-diagram for this sample, where the maximum adhesive strength is 133 kPa. The curve has a linear regime corresponding to the elastic deformation of the PDMS and a steep drop after the maximum indicating a sudden detachment from the PEEK. PDMS was only found on one side of the sandwiched sample, meaning the failure mechanism is adhesive and not cohesive. In comparison, the yellow curve representing a sample which was structured with ZnO nanoparticles only. These particles have been chosen to be big enough to rule out the reinforcement mechanisms of very small nanoparticles. The structured sample shows a factor of 4.5 stronger adhesive strength with a value of 592 kPa. Apart from the increase in adhesive strength, the curve has the same shape as the untreated sample, with a linear elastic range and a sudden decline after the maximum value. Also the failure is adhesive, since only one side of the sample was covered with PDMS, while the other side showed the structured surface. The blue curve represents a surface structured by the Al–ZnO-mixture, with a maximum adhesive strength of 745 kPa, 5.6 times the value of the unstructured sample. While the curve's shape is the same, both parts of the sandwiched sample exhibited PDMS and some parts are even covered in the same area of the sample indicating that the failure mechanism is cohesive. The shape of the curve is important, since it represents a homogeneous structuring of the surface. Inhomogeneously structured samples would exhibit parts of higher and parts of lower adhesive strength and would therefore show additional plateaus in the linear ranges, meaning that some parts of the sample already detached while others remain intact. The absence of these plateaus hints about an uniform surface with all three types of samples. The approach to sandwich the samples works well, since the adhesion test can only affect the weakest parts of the interface between PDMS and PEEK. Cohesive failure is only possible, where the PDMS is strongly attached on both sides. For elastic materials such as PDMS the mechanical adhesion is typically low, because of their ability to change the shape when stress is applied.Understanding the involved rupture mechanisms is of key-importance for transferring the structuring approach to other polymers. The differences in the ultimate bonding strength as a result of the surface treatments is directly related to the complexity of the surface structuring. The detachment mechanisms as well as the structure's corresponding scanning electron microscopy (SEM) images are shown in Fig. 3. An unstructured sample is smooth and the attachment works by van-der-Waals bonds, hydrogen bonds and other dipole–dipole interaction. Because of the non-polar nature of PDMS it only exhibits van-der-Waals bonding which is very weak and the smooth interface can therefore easily detach (Fig. 3a). The ZnO structured sample however exhibits undercuts in the size regime of the ZnO (a few hundred nanometers) (Fig. 3b) as well as the micropillars formed by the mechanism explained in the Experimental section (ESI†). The small undercuts are not sufficient to hold the PDMS in place and the larger structures only rarely exhibit larger undercuts lowering the adhesion of the PDMS compared to the ideal case. EDX of detached PDMS confirmed that some zinc oxide nanoparticles were embedded by the PDMS and came off of the PEEK surface during the pull-off test (Fig. S2, ESI†). This process is usually expected when roundish particles are placed at an interface. A spherical particle cannot provide undercuts to both sides and the detachment of one side from the particles is due to the lack of chemical bonding (compare Fig. S2 and S3, ESI†).
In contrast to that, the Al–ZnO structured sample however exhibited many undercuts on multiple length scales and even showed remaining PDMS in these areas, which failed cohesively (Fig. 3c). Due to the hierarchical roughness of the sample and its complexity, necking of the PDMS is not immediately accompanied by a detachment since the force is distributed among many attachment centers inside the big undercut. With only one undercut length scale, the PDMS at the undercut would show necking and therefore shrink, which would make a detachment inside the undercut easy. When compared to the approach by Jin et al., where complex shaped linkers were used, a significant increase in the adhesive strength can be seen by the fact that cohesive failure occurred. In the former study an increased adhesion but no cohesive failure was found, indicating that the hierarchical roughness is indeed a very important feature of a mechanical interlock structure.
Since not all of the polymer has failed cohesively, however, there is potential for optimization. The undercuts have to be distributed over the whole interface, where the two polymers are supposed to be in contact. The principles investigated here therefore have to be transferred to larger area treatments. Understanding the underlying phenomena of wetting and adhesion leads to a better design of the polymer–polymer interface with respect to the nature of the undercuts. The simplicity of the approach and the manifold of particles available ensure versatile applications of the concept, not requiring difficult or expensive experimental setups but only basic equipment and chemicals.
Contact angle measurements have been performed on PEEK/PDMS samples with different coating thicknesses of PDMS (Fig. S4, ESI†). Structured PEEK without PDMS showed a contact angle of 0°, indicating superhydrophilic behavior. When a diluted PDMS solution was used, the contact angle showed to be superhydrophobic (>120°) even after just one coating step. With a higher concentration of PDMS solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 parts by PDMS/thinner fluid by weights) a thicker coating of PDMS can be observed and the contact angle decreases to the pristine contact angle of PDMS of 105°.21 This means that the contact angle can be tailored to meet the specific application. For the blood contact tests, thickly coated PDMS/PEEK composites were used.
5 parts by PDMS/thinner fluid by weights) a thicker coating of PDMS can be observed and the contact angle decreases to the pristine contact angle of PDMS of 105°.21 This means that the contact angle can be tailored to meet the specific application. For the blood contact tests, thickly coated PDMS/PEEK composites were used.
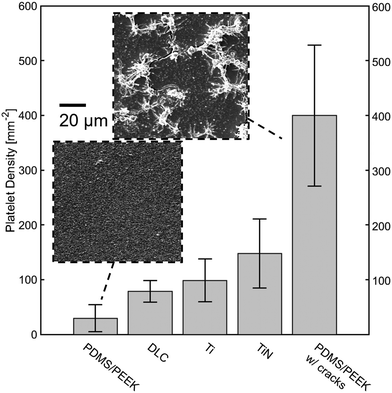
To show the unaltered properties of the PDMS layer on top, blood contact tests have been carried out on samples prepared with two different coating techniques. The fabrication of the coating allowed for different surface architectures, resulting on the one hand in a smooth surface, on the other hand in a crack containing surface (Fig. 4). Both of the surfaces have been tested with human blood and revealed different platelet adhesion behaviors. On average the crack containing surface has 311.4 single thrombocytes cells, 1.6 small cell heaps and 1.8 big cell heaps. Six visual fields had so many cells in more than one layer and without a clear boundary so that the number of cells could not be counted. On average the smooth surface showed 27.8 single cells, 1.3 small and 0.5 big cell heaps, indicating that the thrombogenic activity was severely reduced.
When compared with literature values in Fig. 4, the smooth PDMS surface showed the lowest platelet adhesion, even relative to other promising materials, such as diamond-like carbon (DLC). This can be readily seen in the inset of the SEM images taken of this surface after the blood contact test. On the other hand, the high number of attached cells on the cracked surface is clearly related to the introduction of anchor points for the cells by these crevices. These results are directly linked to Park et al. where polished titanium showed highly reduced platelet adhesion compared to normal sandpaper abraded surfaces.22 The cracks on the surface are most likely due to shrinkage upon thinner evaporation and resulting material stresses. It is also not to rule out that the zinc oxide tetrapods in the smooth sample have an influence on the adhesion behavior. Hölken et al. could show a reduced biofouling activity when incorporating tetrapodal zinc oxide into polythiourethanes.23,24 Both of these possibilities open up a window for further investigations. Although there are more effective ways to treat the PDMS for reduced platelet adhesion (e.g. by grafting PEG to its surface), the results show the preservation of the inertness of the PDMS towards biological materials as well as the increase in adhesion.
Conclusions
In summary, we report on a heightening of the bond strength between the low surface energy polymer PDMS to the high strength polymer PEEK by a factor of 5.6 times by utilizing mechanical interlocking at the polymer–polymer interface. It could be shown that an increase in adhesive strength is achieved by forming undercut structures with additional hierarchical roughness. Undercuts like these can be formed and tuned through various methods proposed here and can even be influenced by considering particle architecture and surface design. Three simple structuring methods have been proposed and tested in order to verify the quality of the treatments. Additional effort has been made to confirm the strong influence of thin coatings of PDMS on the PEEK by contact angle measurements resulting in hydrophobic surfaces of formerly ultrahydrophilic structures. Both SEM and EDX have been utilized to investigate the surface and obtain a fundamental understanding of the underlying rupture mechanisms found through pull-off testing. Further effort can be made by employing different ways of heating the PEEK surface (e.g. microwave or laser treatment) to obtain an infiltration of PEEK into the particle sponge on its surface. Also a larger variety of particles on multiple length scales might result in even better structuring of the surface.Author contributions
LS, TS, H-HS and RA identified the idea and planned the study. LS, SS and FS synthesized and analyzed the materials. LS and FS carried out studies. LS, SS and SK performed adhesion tests. TS and H-HS performed blood contact tests. LS, YKM, FS, JC, JB and RA analyzed the data and wrote the paper with contributions from other authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. A. gratefully acknowledges partial project funding by the Deutsche Forschungsgemeinschaft (DFG) under the grant number AD183/18-1 as well as parts of the Research Training Group “Materials for Brain” (GRK 2154). Additionally, H.-H. S. gratefully acknowledges project funding by the German Federal Ministry of Education and Research under the grant number 13GW055B.References
- S. Ramakrishna, J. Mayer, E. Wintermantel and K. W. Leong, Compos. Sci. Technol., 2001, 61, 1189 CrossRef CAS.
- K. G. Budinski and M. K. Budinski, Engineering materials: properties and selection, Pearson Education, New Jersey, 2010 Search PubMed.
- D. R. Paul and J. W. Barlow, in Multiphase polymers, ed. S. L. Cooper, American Chemical Society, Washington, DC, 1979, vol. 176, p. 315 Search PubMed.
- A. Wilson, I. Jones, F. Salamat-Zadeh and J. F. Watts, Int. J. Adhes. Adhes., 2015, 62, 69 CrossRef CAS.
- E. T. Kang, K. L. Tan, K. Kato, Y. Uyama and Y. Ikada, Macromolecules, 1996, 29, 6872 CrossRef CAS.
- V. Sunkara, D.-K. Park, H. Hwang, R. Chantiwas, S. A. Soper and Y.-K. Cho, Lab Chip, 2011, 11, 962 RSC.
- J. Y. Lai, Y. Y. Lin, Y. L. Denq, S. S. Shyu and J. K. Chen, J. Adhes. Sci. Technol., 1996, 10, 231 CrossRef CAS.
- M. J. Owen and P. J. Smith, J. Adhes. Sci. Technol., 1994, 8, 1063 CrossRef CAS.
- S. I. Moon and J. Jang, J. Mater. Sci., 1999, 34, 4219 CrossRef CAS.
- Y. Suzuki, Nucl. Instrum. Methods Phys. Res., Sect. B, 2003, 206, 501 CrossRef CAS.
- S. R. Kim, J. Appl. Polym. Sci., 2000, 77, 1913 CrossRef CAS.
- K. Kato, Prog. Polym. Sci., 2003, 28, 209 CrossRef CAS.
- T. Venkatesan, Nucl. Instrum. Methods Phys. Res., Sect. B, 1985, 7-8, 461 CrossRef.
- L. S. Penn and H. Wang, Polym. Adv. Technol., 1994, 5, 809 CrossRef CAS.
- P. Rezai, P. R. Selvaganapathy and G. R. Wohl, J. Micromech. Microeng., 2011, 21, 65024 CrossRef.
- F. Awaja, M. Gilbert, G. Kelly, B. Fox and P. J. Pigram, Prog. Polym. Sci., 2009, 34, 948 CrossRef CAS.
- M. Morra, E. Occhiello, R. Marola, F. Garbassi, P. Humphrey and D. Johnson, J. Colloid Interface Sci., 1990, 137, 11 CrossRef CAS.
- W.-S. Kim, I.-H. Yun, J.-J. Lee and H.-T. Jung, Int. J. Adhes. Adhes., 2010, 30, 408 CrossRef CAS.
- M. Baytekin-Gerngross, M. D. Gerngross, J. Carstensen and R. Adelung, Nanoscale Horiz., 2016, 1, 467 RSC.
- X. Jin, J. Strueben, L. Heepe, A. Kovalev, Y. K. Mishra, R. Adelung, S. N. Gorb and A. Staubitz, Adv. Mater., 2012, 24, 5676 CrossRef CAS.
- B. Ruben, M. Elisa, L. Leandro, M. Victor, G. Gloria, S. Marina, S. Mian, K. R. Pandiyan and L. Nadhira, Micro Nano Lett., 2017, 12, 754 CrossRef CAS.
- J. Y. Park, C. H. Gemmell and J. E. Davies, Biomaterials, 2001, 22, 2671 CrossRef CAS.
- I. Hölken, M. Hoppe, Y. K. Mishra, S. N. Gorb, R. Adelung and M. J. Baum, Phys. Chem. Chem. Phys., 2016, 18, 7114 RSC.
- Y. K. Mishra, S. Kaps, A. Schuchardt, I. Paulowicz, X. Jin, D. Gedamu, S. Freitag, M. Claus, S. Wille, A. Kovalev, S. N. Gorb and R. Adelung, Part. Part. Syst. Charact., 2013, 30, 775 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nh00083f |
| This journal is © The Royal Society of Chemistry 2019 |