Step-efficient access to new starburst hole-transport materials with carbazole end-groups for perovskite solar cells via direct C–H/C–Br coupling reactions†
Yu-Chieh
Chang‡
a,
Kun-Mu
Lee‡
 b,
Chang-Chieh
Ting
a and
Ching-Yuan
Liu
b,
Chang-Chieh
Ting
a and
Ching-Yuan
Liu
 *a
*a
aDepartment of Chemical and Materials Engineering, National Central University, Jhongli District, Taoyuan 320, Taiwan, Republic of China. E-mail: cyliu0312@ncu.edu.tw
bDepartment of Chemical and Materials Engineering, Chang Gung University/Department of Pediatrics, Chang Gung Memorial Hospital, Linkou, Taoyuan 333, Taiwan, Republic of China. E-mail: kmlee@mail.cgu.edu.tw
First published on 10th July 2019
Abstract
New starburst small molecules bearing carbazole end-groups are step-economically synthesized in good isolated yields (70–84%) by sequential C–H activation/arylation reactions. Perovskite solar cells employing these molecules (YC05–08) as hole-transporting materials (HTMs) display a promising power conversion efficiency of up to 17.4%.
Introduction
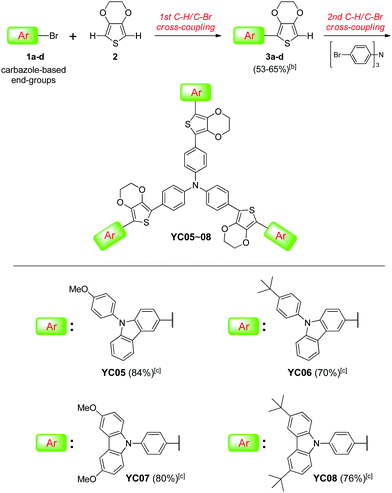
Using appropriate hole-transporting materials (HTMs) puts scientists on the road toward high-efficiency perovskite solar cells (PSCs).1–6 Efficient HTMs in perovskite-based solar cells have been shown to facilitate hole extractions, reduce charge recombinations, and enhance device stabilities.7 Indeed, organic small molecules, such as the well-known spiro-OMeTAD,8 were found to have a noticeable impact on the power conversion efficiency (PCE) of PSCs when they were fabricated in devices as hole-transport layers. Synthetically, however, the starting material for accessing spiro core-based HTMs is fairly expensive (spirobifluorene, ∼185 USD per gram). Preparation of spirobifluorene itself usually requires tedious chemical transformations, involving at least five synthetic steps.9–12 Therefore, small-molecular HTMs incorporating nonspiro-linked building blocks have also been attracting considerable research attention.13 For instance, triphenylamine (TPA) and carbazole (Cbz) are regarded as cost-effective and versatile platforms since their derivatives with extended conjugations can be readily synthesized using Buchwald–Hartwig amination reactions.14 In particular, the TPA moiety has often been used as a core- or an end-group in high-performance HTMs.15 Recently, our group also published a tin-free synthesis shortcut to TPA-based starburst HTMs for perovskite solar cells that exhibited a PCE of up to 17.7%.16 Although 4,4′-dimethoxytriphenylamine is by far the most used terminal unit for small-molecular HTMs, employing carbazole-based derivatives as HTM's end-groups is also becoming increasingly important because of their superior charge-transport ability, chemical stability, and synthetic diversity.17 In contrast to the commonly used hybrid types (Cbz + TPA),17,18 we anticipated that introducing a monotype Cbz to the arms/ends of HTMs would even lower the synthetic cost while exhibiting equally efficient photovoltaic properties. Hence, we report herein a facile synthetic approach to access new starburst HTMs (YC05–08) containing various carbazole derivatives at each end (non-hybrid types) via sequential direct C–H/C–Br cross-coupling reactions19 (Fig. 1).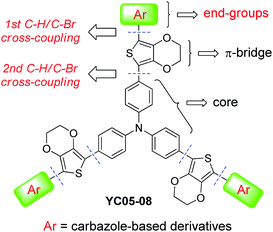 | ||
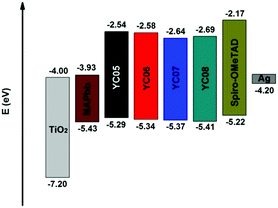
| Fig. 1 New starburst hole-transport materials bearing carbazole derivatives as end-groups for efficient perovskite solar cells. | ||
Results and discussion
The proposed synthesis for the carbazole-ended new HTMs has been carried out and the results are summarized in Scheme 1. First, the direct monoarylation of 3,4-ethylenedioxythiophene (EDOT, 2) with four different carbazole (Cbz) derivatives 1a–d was respectively performed under mild reaction conditions, producing the versatile EDOT-bridged building blocks 3a–d in moderate isolated yields (53–65%). It was worth noting that the 1st C–H/C–Br coupling reaction also generated a significant amount of the symmetrical oligoaryls derived from the diarylation reactions of EDOT. These undesired byproducts can be reduced by adding excess EDOT (3 equiv.) and optimizing the reaction temperature and time (100 °C, 6 h). Next, compounds 3a–d underwent the 2nd C–H bond activation/arylation with the core group (tris(4-bromophenyl)amine) under similar conditions except for an elevated temperature (125–130 °C) and extended reaction time (30 h) in order to make the three-fold C–H/C–Br cross-couplings complete. This step led to the formation of the target starburst HTMs (YC05–08) in good isolated yields (70–84%). Structurally, the end-groups were introduced onto YC05 and YC06 from the 3-position of Cbz whereas the ones for YC07 and YC08 were connected from the N-(4-phenyl)-position of Cbz. More importantly, YC05–08 exhibited moderate to good solubility in chlorinated organic solvents such as dichloromethane, chloroform, and chlorobenzene, which is beneficial to the subsequent solution-processed device fabrication.Prior to the device fabrication of perovskite solar cells, some important properties including UV/vis absorption, electrochemical characteristics, hole mobilities, and thermal stabilities of the obtained HTMs (YC05–08) were investigated (data collected in Table 1). Although YC05–08 are structurally different in terms of carbazole connecting positions and end substituents (–OMe, –tBu), they exhibited similar UV/vis absorption behaviors due to their almost identical conjugation lengths. The λmax values of four HTMs fall approximately in the range of 418–431 nm. The optical bandgap (ΔEg) calculated based on the data of UV/vis absorption and photoluminescence also revealed that the difference in ΔEg between YC07–08 (2.73, 2.72 eV) and YC05–06 (2.75, 2.76 eV) is fairly small. The information regarding the energy level of the highest occupied molecular orbitals (EHOMO) of our HTMs was obtained by performing cyclic voltammetry (CV), showing that YC05–08 exhibited fitted EHOMO (−5.29 to −5.41 eV) which located between the HOMO energies of the photoactive perovskite layer (CH3NH3PbI3, EHOMO = −5.43 eV) and the silver electrode (EHOMO = −4.20 eV). The energy level diagram is demonstrated in Fig. 2.
| HTMs | ΔEga [eV] | E HOMO [eV] | E LUMO [eV] | Hole mobility [cm2 V−1 s−1] | T d [°C] | T g [°C] |
|---|---|---|---|---|---|---|
| a ΔEg was calculated based on the UV/vis absorption and photoluminescence spectra (measured in THF). b E HOMO = −(E1/2 + 0.197 + 4.500 − 0.177) eV (vs. sat. Ag/AgCl and NHE) (the electrochemical measurements performed in THF). c E LUMO = EHOMO + ΔEg. | ||||||
| YC05 | 2.75 | −5.29 | −2.54 | 5.97 × 10−5 | 268 | 197 |
| YC06 | 2.76 | −5.34 | −2.58 | 1.32 × 10−4 | 268 | 219 |
| YC07 | 2.73 | −5.37 | −2.64 | 3.42 × 10−5 | 201 | 201 |
| YC08 | 2.72 | −5.41 | −2.69 | 2.40 × 10−5 | 273 | 251 |
Next, we obtained the hole mobilities (μh) of each HTM molecule by measuring the J–V characteristics in the space-charge limited current (SCLC) region (data listed in Table 1). Compared to YC07–08, YC05–06 had higher μh, which presumably resulted from their better intermolecular packings in the film state. Among the four HTMs, YC06 exhibited the highest μh (1.32 × 10−4 cm2 V−1 s−1) that is comparable to that of spiro-OMeTAD (2.93 × 10−4 cm2 V−1 s−1), thus leading to the superior fill factor (FF) and power conversion efficiency (PCE) of PSCs (see Table 2). Thermal properties of these HTMs were also investigated by means of thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). It was found that our HTMs displayed a lower decomposition temperature (Td = 201–273 °C) than that of the commercial spiro-OMeTAD (Td = 417 °C) while the glass transition temperature (Tg = 197–251 °C) was better than the Tg of spiro-OMeTAD (126 °C).
| HTMs | V oc [V] | J sc [mA cm−2] | FF [%] | PCE [%] | |
|---|---|---|---|---|---|
| a Reverse scans. b The statistical data were calculated based on 8 cells. | |||||
| YC05 | Best | 1.021 | 21.23 | 73.4 | 15.92 |
| Average | 1.013 ± 0.008 | 21.04 ± 0.15 | 71.0 ± 0.02 | 15.16 ± 0.40 | |
| YC06 | Best | 1.021 | 22.32 | 76.5 | 17.44 |
| Average | 1.004 ± 0.007 | 22.15 ± 0.19 | 75.5 ± 0.01 | 16.82 ± 0.37 | |
| YC07 | Best | 1.004 | 22.17 | 66.8 | 14.86 |
| Average | 1.012 ± 0.008 | 20.86 ± 0.70 | 65.4 ± 0.03 | 13.89 ± 0.87 | |
| YC08 | Best | 0.965 | 21.79 | 63.3 | 13.31 |
| Average | 0.966 ± 0.014 | 21.64 ± 0.13 | 61.4 ± 0.01 | 12.86 ± 0.38 | |
| Spiro-OMeTAD | Best | 1.096 | 21.32 | 76.3 | 17.85 |
| Average | 1.080 ± 0.008 | 21.05 ± 0.34 | 75.9 ± 0.01 | 17.29 ± 0.54 | |
In order to further explore the hole-extraction capability of our HTMs, we fabricated devices with the configuration of glass/perovskite/HTMs (YC05–08), respectively, for the measurement of steady-state photoluminescence (PL) and time-resolved photoluminescence (TRPL). The spectra are shown in Fig. 3. Compared to YC07–08, YC05–06-based devices demonstrated a relatively stronger PL quenching effect (Fig. 3a), indicating that YC05–06 are capable of extracting holes more efficiently at the interface between the perovskite layer and HTM. Moreover, the TRPL spectra obtained in Fig. 3b revealed that perovskite/YC05–06 possessed a fitted average lifetime of 35–46 ns that is superior to the 71–128 ns for the devices fabricated with perovskite/YC07–08.
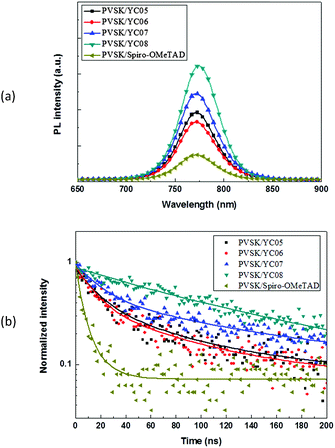 | ||
| Fig. 3 (a) Steady-state PL spectra, and (b) time-resolved PL spectra of the devices fabricated as FTO/perovskite/HTMs. | ||
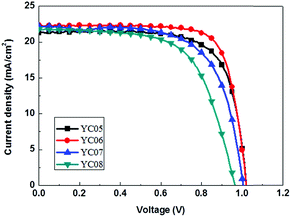
Performance evaluation of the perovskite solar cells employing YC05–08 as hole-transport layers is summarized in Table 2 and the device fabrication procedures are provided in the ESI.† Among the four solar cells, the one with YC06 showed remarkable open-circuit voltage (Voc = 1.021 V) and short-circuit current (Jsc = 22.32 mA cm−2), and a good fill factor (FF = 76.5%), thus leading to the highest power conversion efficiency (PCE) of 17.44% which is comparable to that of the commercially available spiro-OMeTAD-based reference cells (PCE = 17.85%). Compared with YC05, 07 and 08-based devices, the one with YC06 exhibited relatively higher Voc and FF. This might correlate with YC06’s superior PL-quenching, hole-extraction, and hole-transport abilities from the perovskite layer to the hole-transport layer, which is also associated with its higher hole mobility and better HTM surface uniformity. It is interesting that although the HOMO level and μh of YC05 and YC06 are different, they showed similar Voc (∼1.02 V). This implied that the Voc of PSCs was affected not only by the HOMO level and μh of HTMs, but also by the film quality and intermolecular packing in the film state.
It is worth noting that, regarding the hole-transport layer preparation, each HTM solution (in chlorobenzene) was heated to 80–90 °C prior to the spin-coating process. However, YC05 needed to be further heated to 120 °C in chlorobenzene to acquire an entirely clear solution. We reasoned that YC05 possesses only three –OMe groups, thus resulting in a relatively poorer solubility in chlorobenzene. On the other hand, YC07–08 bearing carbazole end-groups from the N-(4-phenyl)-position were shown to exhibit PCE values of 13.31–14.86%, which was attributed to their lower Voc (0.965–1.004 V) and FF (63.3–66.8%). The photovoltaic performance of solar devices with YC05–08 was also demonstrated by a diagram of photocurrent density–voltage (J–V) curves (Fig. 4).
Conclusions
In conclusion, new starburst small molecules with four different carbazole end-groups have been facilely prepared by means of palladium-catalyzed direct C–H activation/arylation reactions. The synthetic strategy shown in this work avoided the employment of toxic organotin reagents required in the most widely used Stille coupling reactions for synthesizing π-conjugated organic materials. Perovskite-based solar devices using YC05–08 as hole-transport layers exhibited moderate to good power conversion efficiencies (13.3–17.4%). Development of convenient and user-friendly synthetic approaches for new organic HTMs is presently underway in our laboratory.Experimental
General procedure A for the synthesis of 3a–d
To a solution of Pd(OAc)2 (0.05 mmol), ligand (0.10 mmol), PivOH (0.30 mmol), and K2CO3 (1.50 mmol) in DMF (3 mL) in a flame-dried Schlenk tube were added the corresponding aryl bromide (1a–d) (1.00 mmol) and ethylenedioxythiophene (EDOT) (2) (3.00 mmol) under N2. The reaction mixture was then heated at 100 °C under N2 for 6 h. After the reaction mixture had cooled to room temperature, water (10 mL) was added. The mixture was extracted with ethyl acetate (2 × 20 mL), and the combined organic layers were washed with brine (50 mL), dried (Na2SO4) and concentrated in vacuo. Purification by flash chromatography yielded the desired products 3a–d.General procedure B for the synthesis of YC05–08
To a solution of Pd(OAc)2 (0.15 mmol), P(adamantyl)2(nBu) (0.30 mmol), PivOH (0.60 mmol), and K2CO3 (3.60 mmol) in DMF (10 mL) in a flame-dried Schlenk flask were added tris(4-bromophenyl)amine (1.00 mmol) and the corresponding end-groups (3a–d) (3.50 mmol) under N2. The reaction mixture was then heated at 125 °C under N2 for 30 h. After the reaction mixture had cooled to room temperature, water (15 mL) was added. The mixture was extracted with dichloromethane (2 × 30 mL), and the combined organic layers were washed with brine (60 mL), dried (Na2SO4) and concentrated in vacuo. Purification by flash chromatography yielded the desired hole-transporting materials YC05–08.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support provided by the Ministry of Science and Technology (MOST), Taiwan (MOST 106-2113-M-008-002 and 106-2218-E-182-005-MY2), National Central University (NCU), Chang Gung University (QZRPD181), and Chang Gung Memorial Hospital, Linkou, Taiwan (CMRPD2G0302 and BMRPF67) is gratefully acknowledged.Notes and references
- S. Ameen, M. A. Rub, S. A. Kosa, K. A. Alamry, M. S. Akhtar, H.-S. Shin, H.-K. Seo, A. M. Asiri and M. K. Nazeeruddin, ChemSusChem, 2016, 9, 10 CrossRef CAS; Z. Yu and L. Sun, Adv. Energy Mater., 2015, 5, 1500213 CrossRef.
- L. Calió, S. Kazim, M. Grätzel and S. Ahmad, Angew. Chem., Int. Ed., 2016, 55, 14522 CrossRef; S. F. Völker, S. Collavini and J. L. Delgado, ChemSusChem, 2015, 8, 3012 CrossRef.
- C. H. Teh, R. Daik, E. L. Lim, C. C. Yap, M. A. Ibrahim, N. A. Ludin, K. Sopian and M. A. M. Teridi, J. Mater. Chem. A, 2016, 4, 15788 RSC.
- C. Rodriguez-Seco, L. Cabau, A. Vidal-Ferran and E. Palomares, Acc. Chem. Res., 2018, 51, 869 CrossRef CAS.
- M. L. Petrus, K. Schutt, M. T. Sirtl, E. M. Hutter, A. C. Closs, J. M. Ball, J. C. Bijleveld, A. Petrozza, T. Bein, T. J. Dingemans, T. J. Savenije, H. Snaith and P. Docampo, Adv. Energy Mater., 2018, 1801605 CrossRef.
- W. Zhou, Z. Wen and P. Gao, Adv. Energy Mater., 2018, 1702512 CrossRef.
- W. Tress, N. Marinova, O. Inganäs, M. K. Nazeeruddin, S. M. Zakeeruddin and M. Grätzel, 2014 IEEE 40th Photovoltaic Specialist Conference (PVSC).
- U. Bach, D. Lupo, P. Comte, J. E. Moser, F. Weissortel, J. Salbeck, H. Spreitzer and M. Grätzel, Nature, 1998, 395, 583 Search PubMed; U. Bach, Y. Tachibana, J. E. Moser, S. A. Haque, J. R. Durrant, M. Grätzel and D. R. Klug, J. Am. Chem. Soc., 1999, 121, 7445 CrossRef CAS; M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395 CrossRef; H. Zhou, Q. Chen, G. Li, S. Luo, T. B. Song, H. S. Duan, Z. Hong, J. You, Y. Liu and Y. Yang, Science, 2014, 345, 542 CrossRef.
- R. G. Clarkson and M. Gomberg, J. Am. Chem. Soc., 1930, 52, 2881 Search PubMed.
- J. M. Tour, R. Wu and J. S. Schumm, J. Am. Chem. Soc., 1990, 112, 5662 CrossRef CAS.
- D. Marsitzky, J. Murray, J. C. Scott and K. R. Carter, Chem. Mater., 2001, 13, 4285 CrossRef CAS.
- F. Thiemann, T. Piehler, D. Haase, W. Saak and A. Lützen, Eur. J. Org. Chem., 2005, 1991 CrossRef CAS.
- D. E. M. Rojas, K. T. Cho, Y. Zhang, M. Urbani, N. Tabet, G. la Torre, M. K. Nazeeruddin and T. Torres, Adv. Energy Mater., 2018, 1800681 CrossRef CAS; A. M. Ontoria, I. Zimmermann, I. G. Benito, P. Gratia, C. R. Carmona, S. Aghazada, M. Grätzel, M. K. Nazeeruddin and N. Martin, Angew. Chem., Int. Ed., 2016, 55, 1 CrossRef; A. Abate, S. Paek, F. Giordano, J. P. C. Baena, M. Saliba, P. Gao, T. Matsui, J. Ko, S. M. Zakeeruddin, K. H. Dahmen, A. Hagfeldt, M. Grätzel and M. K. Nazeeruddin, Energy Environ. Sci., 2015, 8, 2946 RSC; C. Steck, M. Franckevicius, S. M. Zakeeruddin, A. Mishra, P. Bäuerle and M. Grätzel, J. Mater. Chem. A, 2015, 3, 17738 RSC; C. Huang, W. Fu, C.-Z. Li, Z. Zhang, W. Qiu, M. Shi, P. Heremans, A. K.-Y. Jen and H. Chen, J. Am. Chem. Soc., 2016, 138, 2528 CrossRef; Y. Liu, Z. Hong, Q. Chen, H. Chen, W.-H. Chang, M. Yang, T.-B. Song and Y. Yang, Adv. Mater., 2016, 28, 440 CrossRef; K.-M. Lu, K.-M. Lee, C.-H. Lai, C.-C. Ting and C.-Y. Liu, Chem. Commun., 2018, 54, 11495 RSC.
- J. Wang, K. Liu, L. Ma and X. Zhan, Chem. Rev., 2016, 116, 14675 CrossRef CAS; H. Wang, A. D. Sheikh, Q. Feng, F. Li, Y. Chen, W. Yu, E. Alarousu, C. Ma, M. A. Haque, D. Shi, Z.-S. Wang, O. F. Mohammed, O. M. Bakr and T. Wu, ACS Photonics, 2015, 2, 849 CrossRef; S. J. Park, S. Jeon, I. K. Lee, J. Zhang, H. Jeong, J.-Y. Park, J. Bang, T. K. Ahn, H.-W. Shin, B.-G. Kim and H. J. Park, J. Mater. Chem. A, 2017, 5, 13220 RSC.
- H. Choi, H. M. Ko and J. Ko, Dyes Pigm., 2016, 126, 179 CrossRef CAS; H. Choi, S. Park, S. Paek, P. Ekanayake, M. K. Nazeeruddin and J. Ko, J. Mater. Chem. A, 2014, 2, 19136 RSC; H. Choi, S. Paek, N. Lim, Y. H. Lee, M. K. Nazeeruddin and J. Ko, Chem. – Eur. J., 2014, 20, 10894 CrossRef; H. Choi, J. W. Cho, M. S. Kang and J. Ko, Chem. Commun., 2015, 51, 9305 RSC; K. Do, H. Choi, K. Lim, H. Jo, J. W. Cho, M. K. Nazeeruddin and J. Ko, Chem. Commun., 2014, 50, 10971 RSC; K. Rakstys, A. Abate, M. I. Dar, P. Gao, V. Jankauskas, G. Jacopin, E. Kamarauskas, S. Kazim, S. Ahmad, M. Grätzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2015, 137, 16172 CrossRef.
- Y.-C. Chang, K.-M. Lee, C.-H. Lai and C.-Y. Liu, Chem. – Asian J., 2018, 13, 1510 CrossRef CAS.
- B. Xu, E. Sheibani, P. Liu, J. Zhang, H. Tian, N. Vlachopoulos, G. Boschloo, L. Kloo, A. Hagfeldt and L. Sun, Adv. Mater., 2014, 26, 6629 CrossRef CAS; Z. Zhou, Y. Zhao, C. Zhang, D. Zou, Y. Chen, Z. Lin, H. Zhen and Q. Ling, J. Mater. Chem. A, 2017, 5, 6613 RSC; M. Li, Z. Wang, M. Liang, L. Liu, X. Wang, Z. Sun and S. Xue, J. Phys. Chem. C, 2018, 122, 24014 CrossRef; C. Yin, J. Lu, Y. Xu, Y. Yun, K. Wang, J. Li, L. Jiang, J. Sun, A. D. Scully, F. Huang, J. Zhong, J. Wang, Y.-B. Cheng, T. Qin and W. Huang, Adv. Energy Mater., 2018, 1800538 CrossRef.
- C. Lu, I. T. Choi, J. Kim and H. K. Kim, J. Mater. Chem. A, 2017, 5, 20263 RSC; M. S. Kang, S. D. Sung, I. T. Choi, H. Kim, M. P. Hong, J. Kim, W. I. Lee and H. K. Kim, ACS Appl. Mater. Interfaces, 2015, 7, 22213 CrossRef CAS.
- A. Nitti, M. Signorile, M. Boiocchi, G. Bianchi, R. Po and D. Pasini, J. Org. Chem., 2016, 81, 11035 CrossRef CAS; A. Nitti, G. Bianchi, R. Po, T. M. Swager and D. Pasini, J. Am. Chem. Soc., 2017, 139, 8788 CrossRef; D. Pasini and D. Takeuchi, Chem. Rev., 2018, 118, 8983 CrossRef; L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef; K. Murakami, S. Yamada, T. Kaneda and K. Itami, Chem. Rev., 2017, 117, 9302 CrossRef; C. Lu, M. Paramasivam, K. Park, C. H. Kim and H. K. Kim, ACS Appl. Mater. Interfaces, 2019, 11, 14011 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR spectra (1H and 13C) of compounds YC05–08. See DOI: 10.1039/c9qm00309f |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2019 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction conditions for the 1st C–H/C–Br coupling reaction: 1a–d (1.00 mmol), 2 (3.00 mmol), Pd(OAc)2 (0.05 mmol), P(adamantyl)2(
Reaction conditions for the 1st C–H/C–Br coupling reaction: 1a–d (1.00 mmol), 2 (3.00 mmol), Pd(OAc)2 (0.05 mmol), P(adamantyl)2(