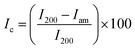New-fangled sources of cellulose extraction: comparative study of the effectiveness of Cissus latifolia and Ficus benghalensis cellulose as a filler
Arunima
Reghunadhan
 *ab,
Nayana G.
Sivan
a,
Shibina
S. K.
a,
Siji K.
Mary
a,
Rekha Rose
Koshy
a,
Janusz
Datta
*ab,
Nayana G.
Sivan
a,
Shibina
S. K.
a,
Siji K.
Mary
a,
Rekha Rose
Koshy
a,
Janusz
Datta
 c and
Sabu
Thomas
c and
Sabu
Thomas
 bd
bd
aResearch and Postgraduate Department of Chemistry, Bishop Moore College, Mavelikara, Alappuzha, India. E-mail: arunimarenjith02@gmail.com
bInternational and Interuniversity Centre for Nanoscience and Nanotechnology, Mahatma Gandhi University, Kottayam-686560, India
cFaculty of Technology, Gdansk University of Technology, Gdansk, Poland
dSchool of Chemical Sciences, Mahatma Gandhi University, Kottayam-686560, India
First published on 20th July 2019
Abstract
Recycled polymers and biopolymers are receiving a great deal of attention these days. If these two can be combined, it will lead to an environment-friendly green material with a great deal of applications. Here the present work is about incorporating bio-based fillers in a recycled polyurethane matrix. Two unusual and extremely novel sources of cellulose have been proposed. The celluloses obtained from Cissus latifolia and Ficus benghalensis were selected as sources. These sources have not been utilized and reported elsewhere to date. The cellulose modified samples of recycled polyurethane were analyzed using FTIR, scanning electron microscopy (SEM) and X-ray diffraction analysis (XRD) to have a preliminary idea about the combination. We found that these were successful as fillers in the matrix. The percentage of crystallinity was decreased in both the composites, which indicates the miscibility. Comparing the two sources, Cissus latifolia based cellulose was more effective in producing interesting morphologies and they had a percentage crystallinity of 75%, which was very high compared to all the reported works.
1. Introduction
Biopolymer based composite materials have received much attention in the past decade. The biodegradability, biocompatibility and ease of availability are major advantages of bio-based materials. These materials are applied in a variety of fields such as biomedical, biocomposite materials and others. Novel sources of biomaterials are always in demand. Bio-based materials are also preferred considering the cost-effectiveness. Cellulose is among the most widely used biomaterials in composite fabrication.1,2 A variety of sources are available for cellulose extraction and selection is made normally depending on the percentage of cellulose available in a particular source.3 Pineapple leaf, coir, kenaf, jute, hemp, rice husk, etc.4,5 have been the most common sources of cellulose. A handful of reports is also available on each of these cellulosic composites. Cellulose having the chemical formula (C6H10O5)n is considered responsible for the strength and mechanical properties of fibers. Most commonly extracted forms of cellulose are microfibers, nanocrystals,6 whiskers, etc. The β glycosidic linkages and the –OH rich surface make them suitable for modification and composite formation. The extraction process involves many chemical methods like bleaching, acid hydrolysis, steam explosion, etc.We have selected two novel sources which were not previously mentioned in any of the available literature. Cissus latifolia and Ficus benghalensis have been selected as materials for cellulose isolation. In nature, they exist as very strong and durable materials. In the case of aerial roots of the banyan tree (Ficus benghalensis), it is well understood that they act as a support for the huge tree. Cissus latifolia is conventionally used as climbing ropes. Considering the strength of these materials the study was meant to derive strong cellulosic materials out of these sources.
Similar to the usage of bio-based materials, recycled materials are also interesting, in particular polymers. A large amount of waste material is being produced every day because of the huge conception of polymeric materials. Polyurethane foam is one among the highly demanded polymers in industry. It is widely used in upholsteries, seating, tires, paneling, etc. Its wide usage normally leads to a huge mass of postconsumer waste, which is often incinerated or landfilled. But this foam waste can be recycled effectively by thermal or chemical recycling methods. Glycolysis, which is the degradation of polyurethanes by glycols, is the simplest way of recycling.7,8 The recovered polyol materials can be further utilized for the production of new polyurethanes. Normally the glycolysis products retain most of the properties and functionalities such as –NH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –COO, etc. Our previous work reported the usage of recycled polyurethanes as a modifier for thermosetting epoxy networks and its use in the microelectronic industry.9 Also, we received good mechanical properties and interesting morphologies with recycled polyurethane.10
O, –COO, etc. Our previous work reported the usage of recycled polyurethanes as a modifier for thermosetting epoxy networks and its use in the microelectronic industry.9 Also, we received good mechanical properties and interesting morphologies with recycled polyurethane.10
Polyurethanes can be modified with cellulose to fabricate biocomposites. These composites will have enhanced mechanical properties and performance. Better dispersion of the fibers is a necessary factor in order to produce desirable properties.11 Composite preparation can be done in a number of ways. Cellulose or its modified forms can be used as the filler in the polyurethane matrix. Wang and coworkers reported the preparation of cellulose/PU composites with shape memory properties.12 Ozgur reported the preparation of cellulose/PU composites with micro to nanoscale fibrillates and studied the effect of size on the properties.13 The liquid suspensions of micro fibrillated cellulose with polyurethanes resulted in transparent composites having high tear strength.14 Quite a large number of scientific reports are available on PU/cellulose-derived systems.
Here the present work is the primary investigation of the possibility of combining recycled and bio-based materials for fabricating nanocomposites. These composites will open up a new class of materials and can be utilized in a variety of fields. In this report, we have selected two novel plant materials for the extraction of cellulose, Cissus latifolia and Ficus benghalensis. Both of these materials are well known for their strength. There is not a single report on cellulose extraction from these sources and also on the concept of using biopolymer–recycled polymer combinations. The materials were characterized using FTIR, electron microscopy and diffraction analysis.
2. Experimental
2.1. Materials
Polyurethane glycolysate was obtained from the Gdansk University of Technology, Poland. The detailed synthetic route of the glycolysis process for obtaining the glycolysate was already reported.15,16 For the extraction of cellulose, we used NaOH, glacial acetic acid, sodium chlorite, and oxalic acid which were procured from Nice Chemicals. Octa phenyl-POSS (CAS No. 5256-79-1) with formula C48H40O12Si8 was purchased from Hybrid Plastics, USA. All the chemicals were used as such without any purification. The raw materials for the cellulose extraction were collected from the local areas of South Kerala. The fibers were isolated from the stem of the banyan tree and Cissus latifolia. The Cissus latifolia stems were very greasy and the outer skin was removed before washing and drying. Copper nanoparticles were synthesized in the laboratory following the procedure explained by Karak et al.172.2. Extraction of cellulose
For the extraction process, the following solutions were used:• Solution A = 27.065 g of NaOH + 78.8 ml glacial acetic acid in 1 liter.
• Solution B = 2% sodium chlorite solution.
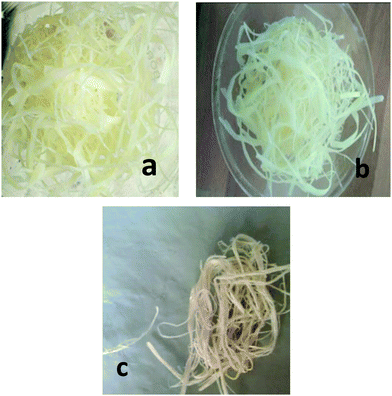
Both the fibers were treated in the same way. Raw fibers (about 10 g) were chopped into short fibers of length 5 cm. They were dried in a hot air oven for the removal of moisture. The dried fibers were then treated with 700 ml of 8% NaOH in an autoclave and kept under 172 kPa pressure and at a temperature of 110 °C for 1 hour. The pressure was released immediately and the fibers were washed with water. Alkali treatment was done to remove the lignin from the fibers. The NaOH-treated fibers were washed several times with distilled water to remove the excess alkali. For bleaching, fibers were mixed with 200 ml of solution A and 200 ml of solution B in a magnetic stirrer at 40 °C for 20 minutes. The bleaching process was repeated four times to obtain white fibers. The white fibers were then treated with 750 ml of 10% H2C2O4 and autoclaved at 172 kPa Psi pressure for 15 minutes. The pressure was released immediately and the process was repeated 7 times. The fibers were taken out, washed, suspended in water and homogenized for 4 hours with a 5 minute interval. The pure cellulose thus obtained was kept in a vacuum oven at 90 °C until it was dried. Microcrystalline cellulose thus obtained was used for the composite preparation.18 The percentage yield of the cellulose materials was 52% for the Cissus latifolia and 48% for the Ficus benghalensis.
2.3. Surface modification of the cellulose to fabricate hybrid fillers
The cellulose obtained was converted into hybrids with two nanomaterials. Cellulose from Cissus latifolia was converted into hybrids by simple mechanical mixing with organic–inorganic filler-octa phenyl POSS (given as cellulose-I) and the banyan tree cellulose was added with copper nanoparticles (given as cellulose-II). Both these fillers were separately added to a polyurethane matrix for composite preparation.2.4. Preparation of composites
A composite of cellulose I and cellulose II with recycled polyurethane in the ratio 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 0.15
20 and 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, respectively was prepared by melt mixing at a temperature of 100 °C and 200 rpm using a magnetic stirrer. The crude mixed samples without casting into the films (samples after complete mixing) were used for the characterization.
20, respectively was prepared by melt mixing at a temperature of 100 °C and 200 rpm using a magnetic stirrer. The crude mixed samples without casting into the films (samples after complete mixing) were used for the characterization.
2.5. Characterization techniques
FTIR spectra with ATR of the individual samples were recorded in a PerkinElmer 400 instrument with spectrum software. A total of 32 scans were done at a resolution of 4 cm−1. Spectra were recorded from 400–4500 cm−1. XRD analysis was performed on a Bruker AXS-8 using CuKα radiation. 2θ values were recorded from 10 to 80°. The SEM analysis of the samples was done using JEOL Model JSM – 6390LV with a tungsten filament. The acceleration voltage applied was 16 V. The samples were platinum coated to avoid electrostatic charge dissipation.3. Results and discussion
The study was meant to find a novel filler for the recycled polyurethane polymer. The successful incorporation of the filler in the polymer matrix was first ensured by FTIR analysis, then the change in crystallinity was examined by XRD and the inclusion of filler was confirmed by the SEM analysis.3.1. Fourier transform infrared spectroscopy
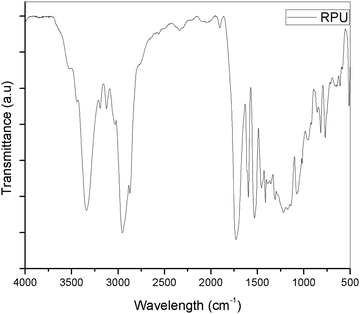
The FTIR-ATR spectra of the polymer, cellulose, O-phenyl POSS (OPOSS) and composite were taken to analyze the change in the spectra. Analysis of the obtained FTIR spectra confirmed the presence (characteristic vibrations) of expected chemical groups, i.e. the carbonyl group in urethane groups and ester-based soft segments (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1726 cm−1), and amine group bending (δN–H) at 1531 cm−1. Bands connected with stretching (νC–O at 1165 and 1138 cm−1) and bending (δC–O at 1078 cm−1) vibrations of C–O–C are also visible in the FTIR spectra, and result from the presence of urethane bonds and ester bonds (introduced by ester-based polyol) in the structure of the prepared poly(ester-urethane)s. The C–H stretching vibrations (ca. 2954 and 2884 cm−1) are also visible.
O at 1726 cm−1), and amine group bending (δN–H) at 1531 cm−1. Bands connected with stretching (νC–O at 1165 and 1138 cm−1) and bending (δC–O at 1078 cm−1) vibrations of C–O–C are also visible in the FTIR spectra, and result from the presence of urethane bonds and ester bonds (introduced by ester-based polyol) in the structure of the prepared poly(ester-urethane)s. The C–H stretching vibrations (ca. 2954 and 2884 cm−1) are also visible.
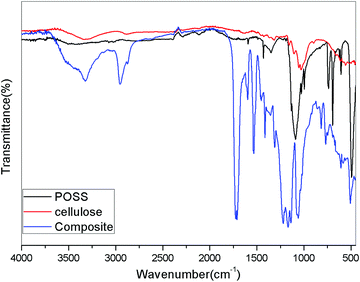
On comparison with the polyurethane spectrum, the composite showed a marked difference. The intensity of the peaks was decreased by the addition of the modified hybrid filler in the polyurethane phase. The –OH peak in the cellulose was noted around 3400 cm−1. On addition to the PU polymer, the peak was combined with the –NH peak of polyurethane and resulted in the broadening of the peak in that region. The aromatic peak remained unaltered because the POSS itself contains a set of aromatic groups in its structure. The region below 2000 cm−1 shows a noticeable variation in the peaks. The methyl peak around 2950 cm−1 became less intense showing weak interaction of the polyurethane groups with the hybrid filler.
The polyurethane contains functional groups which mostly give bending vibrations in that region. The vibrations become more pronounced by the addition of the modified cellulose particles. Hence, it can be undoubtedly said that the cellulose modified with OPOSS can act as an effective modifier to polyurethane. The effectiveness can be further confirmed by the addition of a varying amount of the modifier (Fig. 2–4).
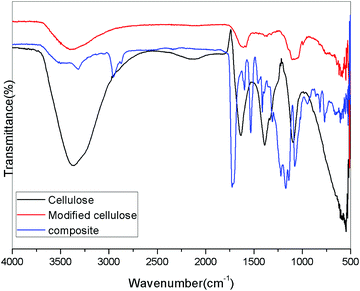
When the cellulose is modified with Cu nanoparticles, the spectra became broad and some peaks were overlapped. The Cu nanoparticles have no direct chemical linkage with the cellulose particles, but they get attached to the surface thereby altering the chemical surface. Also, there is a possibility of the formation of Cu(OH)2 on the surface by combining the –OH groups with Cu particles.19,20 But there was no solid experimental evidence for this kind of compound formation. A noticeable reduction in the intensity of the –OH peaks suggests the interaction with Cu and cellulose. Here we propose that the cellulose surface is decorated with the Cu nanoparticle forming hybrid filler. The modified cellulose can be represented schematically as in Fig. 5. Recent reports suggest that cellulose or its modified form, when decorated with copper nanoparticles, can be used as catalysts.21
3.2. Morphological analysis by scanning electron microscopy
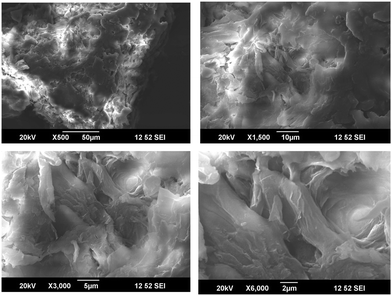
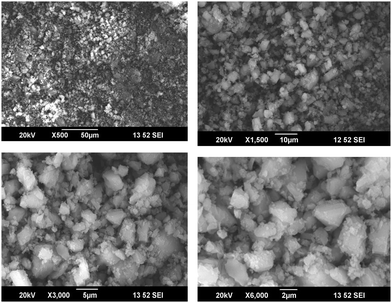
SEM analysis of the cellulose samples at different magnifications are given in Fig. 6. It is clear that the cellulose was in the form of fibers after the chemical treatments.22,23 There was much less impurity and the fibers formed were in the microscale. The individual fibers had a diameter less than 2 μm.When the microcrystalline cellulose was modified with the octa phenyl POSS (O-POSS), there was a noticeable change in the morphology of the cellulose (Fig. 7). The fibrous structure was changed to a very interesting regular flowery pattern. When analyzed at higher magnifications, it was very interesting to note that the O-POSS/cellulose hybrid appeared to be coral-like. The individual particles had an attractive flower shape and the sharp needle like domains suggest that they have highly crystalline nature.
On the addition of the hybrid filler on the polyurethane matrix, we obtained a high degree of miscibility. Most of the fillers were inserted into the polyurethane. The flowery agglomerates were clearly seen in the SEM image (Fig. 8). The large cluster of the hybrid filler was indicated by circles and arrows in the image. Comparing the SEM images of the modified cellulose with the composites suggests that the hybrid filler was compatible with the recycled polymer. The enhanced miscibility of the filler in the polymer matrix can be attributed to the chemical structures of the materials. Cellulose and OPOSS are capable of forming hydrogen-bonded linkages with each other. This filler, when added to polyurethane having so many polar and reactive groups, will interact effectively. So there will be some sort of chemical linkage between the hybrid and the polymer. This will lead to the modification of the properties of polyurethane.
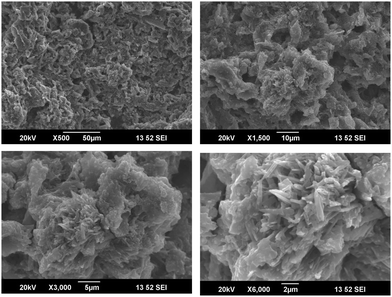
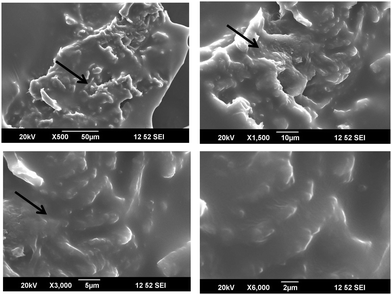
The cellulose derived from the aerial roots of the banyan tree were very fibrous and were in micro scales (Cellulose II). They had a long and thick fibrous structure with some pores on the surface. These pores are suitable for the inclusion of foreign particles. In the first image in Fig. 9(a) we could observe an interesting mesh-like surface. This morphology shows that the cellulose surface is almost regular without impurities. On higher magnification, that is in Fig. 9b and c, the fibrous structure is clear. The rough surface of the fibers is the result of the chemical treatment done during the purification. In Fig. 9d, the interconnected fibers are seen, which suggests good mechanical strength of the fibers. Normally it is known that the aerial roots of the tree are very strong. The strength is retained in the cellulosic fiber. This is confirmed by the particular structure observed in the electron microscopic images at higher magnification.
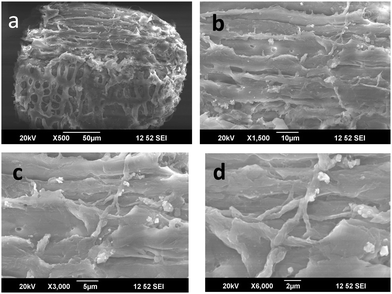
When the cellulose surface was modified using Cu nanoparticles, a very fine particle insertion was clearly seen. As mentioned earlier, the pores in the cellulosic fibers support the Cu nanoparticles and hence they are easily attached to the surface. The Cu particles possess a crystalline nature and hence they appear as white crystals on the surface. On higher magnification, it is seen that the Cu particle insertion was almost uniform, but the particle size distribution is uneven. There were varying sized particles. Some remain in the nanoscale while some combine to form big lumps. However, the microscopic technique was successful in explaining the addition of Cu particles in cellulose. It is also interesting to note that the miscibility was attained by mechanical mixing without the usage of any solvents. Hence, it can be used as a new method for the fabrication of hybrid fillers (Fig. 10 and 11).
The novel hybrid materials were incorporated in the polyurethane matrix by melt mixing. The SEM images at different magnification suggest that the hybrid fillers were inserted into the PU chains. But the miscibility was less because the only possible interacting material was the cellulose with –OH groups. But when cellulose was modified with Cu particles, due to the crystalline nature of the Cu nanoparticles, there was masking of the reactive functionality of cellulose. The images at higher magnification show that the hybrid filler was inserted as agglomerated structures in the polyurethane polymer. We can say that even though there were no direct chemical linkages between the filler and the polymer, the filler modified the surface morphology.
3.3. X-ray diffraction analysis
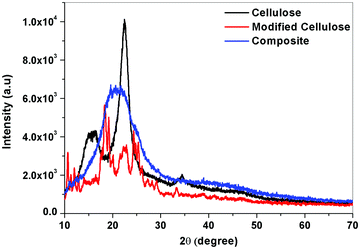
On examining the XRD curve, a visible change in the structure was noticed. The crystalline nature of the cellulose was diminished by the addition to the PU polymer, which is amorphous. This change confirms that the modified cellulose is strongly interacting with the polymer matrix and this will enhance its miscibility (Fig. 12 and 13).The change in crystallinity can be further understood by evaluating the % crystallinity. Ic or percentage crystallinity can be calculated from the XRD peaks of the samples by measuring the intensity at 2θ values of 18° which correspond to the amorphous regions and at 22° corresponding to both amorphous and crystalline regions.
From the above XRD graphs, the percentage crystallinity values can be calculated for cellulose and the variation in the values for modified cellulose and the composite can be understood. It was found that the cellulose obtained from the Cissus latifolia stems was having high crystallinity of about 95%. But the modification of cellulose with o-phenyl POSS decreased the crystallinity. All the crystallinity was lost when it came to forming composites. This value confirms the effectiveness of modified cellulose as a filler for polyurethane. Polyurethane obtained from the glycolysis process has an elastomeric nature. So the material contains more amorphous segments than crystalline. The interaction between the polymer and the filler was strong enough to eliminate the crystallinity of the added filler.
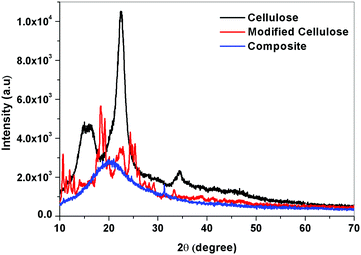
XRD analysis of the cellulose II, modified cellulose II and the composite was considered to ensure the incorporation of cellulose II in the polymer matrix. Major differences in the position and number of peaks can be observed between the X-ray diffraction patterns of the three samples. A high intensity peak in the XRD of cellulose corresponding to the 002 (or 200) crystal plane at 22° suggests that the fibers have a higher cellulosic content. Increase in the intensity of the 002 plane peak for the fibers substantiates the compositional analysis which showed that the fibers had 75% cellulose content. Additional peaks (15, 24°) seen in the graph of the modified cellulose but absent in the neat cellulose should be from the inclusion of copper nanoparticles in the cellulose. Using the peak intensities, the % crystallinity of the fibers and the modified cellulose was calculated. In the case of cellulose from the banyan tree roots, the fibers were not so crystalline and the percentage crystallinity was 58%. As seen from the Fig. 1, the cellulose materials were not so pure and white which suggests that the fibers need more chemical treatment. When the modified cellulose II was added to the polyurethane matrix, the crystalline regions were absent in the XRD similar to the former case. The amorphous polyurethane was strong enough to form a composite with the crystalline cellulose. Hence, the Cu nanoparticle grafted cellulose couldn’t produce a visible change in the diffraction pattern.
4. Conclusions
Cellulose fibers were extracted from two novel sources, Cissus latifolia and Ficus bengalensis. We obtained cellulosic fibers with a high degree of crystallinity of 75% for the former and 58% for the latter. It was well understood that the Cissus latifolia fibers were more crystalline and pure and strong. The obtained fibers can be used as a filler as such or they can be converted into cellulose nanocrystals by homogenization. The homogenized samples were found to be miscible with recycled polyurethane. The Cissus latifolia fibers when modified with octa-phenyl POSS, presented an interesting flowery morphology. Since POSS based materials have high dielectric properties the new fillers are suitable in electronics. The Cu nanoparticle decoration was not so successful in producing any specific morphology. But both the cellulosic materials can be applied as fillers in a polyurethane matrix. The diffraction analysis suggests that the crystallinity of the cellulosic fibers was masked by the polyurethane or they appear as a single phase in the polyurethane matrix. The FTIR results also suggest the successful inclusion of the cellulosic fillers in PU.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to Department of Physics, St. Thomas College, Pala for the XRD analysis, STIC, CUSAT, Kochi for the SEM analysis and School of Chemical Sciences, Mahatma Gandhi University for the FTIR analysis.References
- P. Kandachar and R. Brouwer, Applications of Bio-Composites in Industrial Products, MRS Proc., 2001, 702, U4.1.1., DOI:10.1557/PROC-702-U4.1.1.
- N. V. Gama, B. Soares, C. S. R. Freire, R. Silva, C. P. Neto, A. Barros-timmons and A. Ferreira, Bio-based polyurethane foams toward applications beyond thermal insulation, J. Mater., 2015, 76, 77–85, DOI:10.1016/j.matdes.2015.03.032.
- B. Deepa, E. Abraham, B. M. Cherian, A. Bismarck, J. J. Blaker, L. A. Pothan, A. L. Leao, S. F. de Souza and M. Kottaisamy, Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion, Bioresour. Technol., 2011, 102, 1988–1997, DOI:10.1016/j.biortech.2010.09.030.
- N. Johar, I. Ahmad and A. Dufresne, Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk, Ind. Crops Prod., 2012, 37, 93–99, DOI:10.1016/j.indcrop.2011.12.016.
- E. Abraham, B. Deepa, L. A. Pothen, J. Cintil, S. Thomas, M. J. John, R. Anandjiwala and S. S. Narine, Environmental friendly method for the extraction of coir fibre and isolation of nanofibre, Carbohydr. Polym., 2013, 92, 1477–1483, DOI:10.1016/j.carbpol.2012.10.056.
- A. V. Istomin, T. S. Demina, E. N. Subcheva, T. A. Akopova and A. N. Zelenetskii, Nanocrystalline Cellulose from Flax Stalks: Preparation, Structure, and Use, Fibre Chem., 2016, 48, 199–201, DOI:10.1007/s10692-016-9767-5.
- J. Liszkowska and J. Paciorek-sadowska, Glycolysis of rigid polyurethane-polyisocyanurate foams, Polimery, 2010, 314–319 Search PubMed.
- J. Datta and M. Kacprzyk, Thermal analysis and static strength of polyurethanes obtained from glycolysates, J. Therm. Anal. Calorim., 2008, 93, 753–757, DOI:10.1007/s10973-008-9140-x.
- A. Reghunadhan, J. Datta, M. Jaroszewski, N. Kalarikkal and S. Thomas, Polyurethane glycolysate from industrial waste recycling to develop low dielectric constant, thermally stable materials suitable for the electronics, Arabian J. Chem., 2018 DOI:10.1016/j.arabjc.2018.03.012.
- A. Reghunadhan, J. Datta, N. Kalarikkal, J. T. Haponiuk and S. Thomas, Toughness augmentation by fibrillation and yielding in nanostructured blends with recycled polyurethane as a modifier, Appl. Surf. Sci., 2018, 442, 403–411, DOI:10.1016/j.apsusc.2018.02.128.
- A. Hadjadj, O. Jbara, A. Tara, M. Gilliot, F. Malek, E. M. Maafi and L. Tighzert, Effects of cellulose fiber content on physical properties of polyurethane based composites, Compos. Struct., 2016, 135, 217–223, DOI:10.1016/j.compstruct.2015.09.043.
- Y. Wang, Z. Cheng, Z. Liu, H. Kang and Y. Liu, Cellulose nanofibers/polyurethane shape memory composites with fast water-responsivity, J. Mater. Chem. B, 2018, 6, 1668–1677, 10.1039/c7tb03069j.
- M. Özgür Seydibeyoǧlu and K. Oksman, Novel nanocomposites based on polyurethane and micro fibrillated cellulose, Compos. Sci. Technol., 2008, 68, 908–914, DOI:10.1016/j.compscitech.2007.08.008.
- N. E. Marcovich, M. L. Auad, N. E. Bellesi, S. R. Nutt and M. I. Aranguren, Cellulose micro/nanocrystals reinforced polyurethane, J. Mater. Res., 2006, 21, 870–881, DOI:10.1557/jmr.2006.0105.
- J. Datta and M. Rohn, Glycolysis of polyurethane wastes. Part I. Glycolysis agents and catalysts, Polimery, 2007, 579 CrossRef CAS.
- J. Datta and M. Rohn, Glycolysis of polyurethane wastes, Part II. Purification and use of glycolysis products, Polimery, 2007, 627 CrossRef CAS.
- S. Barua, G. Das, L. Aidew, A. K. Buragohain and N. Karak, Copper–copper oxide coated nanofibrillar cellulose: a promising material, RSC Adv., 2013, 14997–15004, 10.1039/c3ra42209g.
- C. J. Chirayil, J. Joy, L. Mathew, M. Mozetic, J. Koetz and S. Thomas, Isolation and characterization of cellulose nanofibrils from Helicteres isora plant, Ind. Crops Prod., 2014, 59, 27–34, DOI:10.1016/j.indcrop.2014.04.020.
- U. Vainio, K. Pirkkalainen, K. Kisko, G. Goerigk, N. E. Kotelnikova and R. Serimaa, Copper and copper oxide nanoparticles in a cellulose support studied using anomalous small-angle X-ray scattering, Eur. Phys. J. D, 2007, 42, 93–101, DOI:10.1140/epjd/e2007-00015-y.
- A. Musa, M. B. Ahmad, M. Z. Hussein, S. Mohd Izham, K. Shameli and H. A. Sani, Synthesis of Nanocrystalline Cellulose Stabilized Copper Nanoparticles, J. Nanomater., 2016, 2016, 2490906 Search PubMed.
- A. Maleki, V. Eskandarpour, J. Rahimi and N. Hamidi, Cellulose matrix embedded copper decorated magnetic bionanocomposite as a green catalyst in the synthesis of dihydropyridines and polyhydroquinolines, Carbohydr. Polym., 2019, 208, 251–260 CrossRef CAS PubMed.
- P. Krishnamachari, R. Hashaikeh and M. Tiner, Modified cellulose morphologies and its composites; SEM and TEM analysis, Micron, 2011, 42(8), 751–761 CrossRef CAS PubMed.
- F. A. Syamani, S. Sukardi and A. Suryani, Changes in Oil Palm Frond Fiber Morphology, Cellulose Crystallinity and Chemical Functional Groups during Cellulose Extraction Phases, J. Chem. Mater. Res., 2015, 7(3), 105–113 Search PubMed.
| This journal is © the Partner Organisations 2019 |