Open Access Article
Open Access ArticleTemplate-free hydrothermal synthesis of MgWO4 nanoplates and their application as photocatalysts†
Jie Meng‡
 ,
Tao Chen‡
,
Tao Chen‡ ,
Xiao Wei*,
Jixue Li and
Ze Zhang*
,
Xiao Wei*,
Jixue Li and
Ze Zhang*
Center of Electron Microscopy, State Key Laboratory of Silicon Materials, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027, PR China. E-mail: mseweixiao@zju.edu.cn; zezhang@zju.edu.cn; Fax: +86 571 87952797; Tel: +86 571 87952797
First published on 18th January 2019
Abstract
As a semiconductor, MgWO4 has a potential in photocatalytic applications; however, it has been overlooked in previous studies; in this study, it has been demonstrated that MgWO4 exhibits the ability to drive photocatalytic hydrogen evolution. Compared to nanoparticle structures, MgWO4 nanoplates show an increased photocatalytic ability due to their higher specific surface area. Moreover, the formation mechanism of MgWO4 nanoplates has been discussed. An oriented attachment and ripening process is proposed for the formation of the MgWO4 nanoplates. This study demonstrates that MgWO4 can be considered a valuable photocatalytic material, and future studies should be focused on how to promote its photocatalytic conversion efficiency.
Introduction
Magnesium tungstate (MgWO4), known as a semiconductor with a wide band gap, has been extensively studied in various applications including luminescence, dielectric ceramics, catalysis, solid state lasers and so on.1–4 MgWO4 is currently of interest in the abovementioned fields due to the advantageous combination of light (Mg and O) and heavy (W) elements in its composition and the corresponding special structures.5 As reported in previous studies, the optical band gap of MgWO4 is about 4 eV with UV response.4 Moreover, the bottom of the conduction band (CB) is lower than H+/H2 and the top of the valence band (VB) is higher than O2/H2O; this indicates that the photo-excited hole and electron pairs have enough energy to oxidize and reduce water, respectively.3,6 However, to the best of our knowledge, MgWO4 has remained unexplored in photocatalysis when compared with other compounds of the AWO4 (A = Ca, Sr, Ba) family.7,8Furthermore, as is well-known, the physical and chemical properties of semiconductors highly depend on their microstructures such as sizes, morphologies, crystal structures and so on.9–12 Therefore, the desired properties could be obtained by rational control and design of the abovementioned parameters. In particular, explorations of two-dimensional (2D) nanoplates with novel structures contribute significantly to the evaluation of their possible roles in photocatalysis.13,14 Furthermore, the plate-like or sheet-like structure may not only enhance the specific surface area but also suppress the recombination of photo-induced holes and electrons because these carriers can migrate from the interior to the surface more conveniently; this has been demonstrated in previous work.15 Moreover, it would be beneficial to prepare plate-like structures through template-free and surfactant-free routes with low-cost and controllable synthesis processes. However, a MgWO4 photocatalyst with a nanoplate structure has not yet been reported in previous literatures.
Hence, in this study, the MgWO4 nanoplates were prepared by a facile hydrothermal reaction without any templates or surfactants. The formation process and possible mechanism of the MgWO4 nanoplates have been discussed. Moreover, as a semiconductor, MgWO4 has the ability to drive photocatalytic hydrogen evolution; this has been confirmed in this study. Compared with the nanoparticle structures, MgWO4 nanoplates show an enhanced photocatalytic ability because of the higher specific surface area. This study demonstrates that MgWO4 can be included in the family of photocatalysts, and the morphology of nanoplates provides a reference for the modification of other similar materials.
Results and discussion
Morphology and microstructure
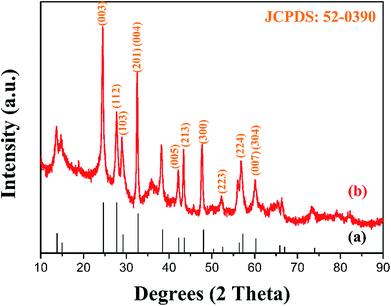
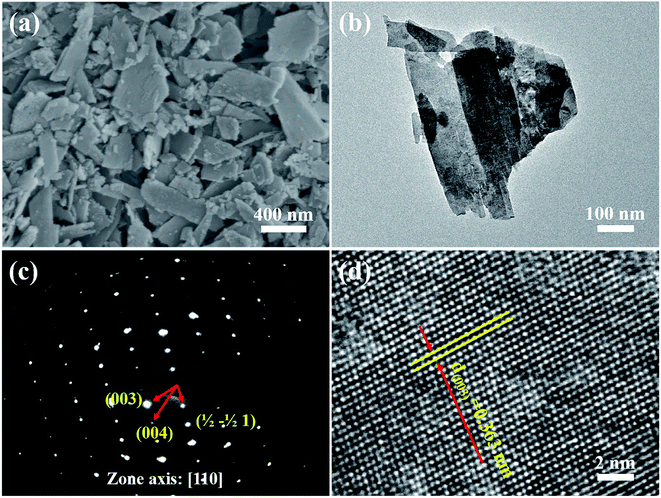
The XRD pattern of the as-prepared MgWO4 nanoplates after hydrothermal reaction for 12 h is displayed in Fig. 1b. All the diffraction peaks can be indexed to the tetragonal structure of MgWO4 with a lattice constant of a = b = 5.63 Å and c = 10.81 Å; this is in agreement with the standard card (JCPDS no. 52-0390). Compared with the main peaks present in the standard pattern (Fig. 1a), the dominant diffraction peak in (003) indicates that the as-obtained samples may show a preferred orientation along the (001) planes; this has been further discussed hereinafter via selected area electron diffraction (SAED) and high-resolution transmission electron microscopy (HRTEM) results.10The SEM image of the as-obtained MgWO4 nanoplates is shown in Fig. 2a. It can be seen that the MgWO4 samples consist of plate-like nanostructures with an irregular shape (the thickness is about 45 nm). These plate-shaped structures were further confirmed by the transmission electron microscopy (TEM) image shown in Fig. 2b. Fig. 2c presents the selected area electron diffraction (SAED) pattern, and the three characteristic spots can be indexed to the diffraction planes of (003), (004) and (1/2 −1/2 0) for the tetragonal MgWO4 phase. The interplanar spacing of about 0.363 nm is in agreement with the (003) facets in the corresponding high-resolution transmission electron microscopy (HRTEM) image (Fig. 2d). According to the SAED and HRTEM analysis, the exposed facets of MgWO4 nanoplates are the (110) planes. The EDS spectrum (Fig. S1 and S2†) indicates that the MgWO4 nanoplates are composed of Mg, W and O elements without other impurity phases, whereas the elements Cu and C result from the micro grid. To further study the surface chemical compositions and states of the MgWO4 nanoplates, X-ray photoelectron spectroscopy (XPS) measurements were carried out, and the full range XPS spectrum and the corresponding high-resolution XPS spectra of Mg 1s, W 4f (4f7/2: 34.5 eV and 4f5/2: 36.6 eV) and O 1s are shown in Fig. S3.† In the Mg 1s spectrum, we can find that the Mg oxidation states are mainly composed of +2, whereas the peak at 1302.9 eV may correspond to the existence of Mg(OH)2.16 The XPS results provide evidence of the existence of Mg2+ and W6+ on the surface of MgWO4 nanoplates.1,2,17
Formation process
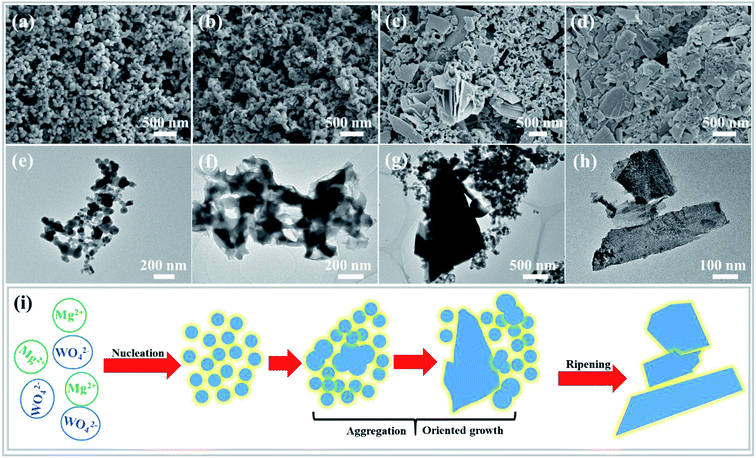
To better understand the formation process of the MgWO4 nanoplates, the samples obtained at different hydrothermal reaction times were examined by TEM, SEM and XRD characterizations (Fig. 3 and S4†). At first, spherical nanoparticles were formed after reaction for 10 min (Fig. 3a). Then, the primary nanoparticles tended to aggregate to produce the precursor of MgWO4 nanoplates when time was prolonged to 30 min (Fig. 3b). When the reaction time reached 1 h, the nanoplates and nanoparticles are observed to co-exist, as shown in Fig. 3c. After hydrothermal treatment for 2 h and corresponding gradual ripening, the MgWO4 nanoplates were constructed, as shown in Fig. 3d.Based on the abovementioned morphology evolution, a rational formation mechanism was speculated, and the corresponding schematic can be seen in Fig. 3i. (i) Under the hydrothermal conditions, the reaction between Mg2+ and WO42− starts in the mixed aqueous solution, and spherical particles are generated as a result of homogeneous nucleation.18 (ii) The primary nanoparticles tend to aggregate to reduce the surface energy, and oriented attachment occurs between conjoined nanoparticles.19,20 (iii) From the thermodynamic perspective, these two adjacent particles tend to share a common crystallographic orientation via rotation or fine-tuning.21 In addition, the main framework of nanoplates is produced, and a crystallization process occurs, as confirmed by the XRD patterns (Fig. S4†). (iv) Finally, the MgWO4 nanoplates are formed at the expense of the rest of the nanoparticles via an Ostwald ripening mechanism (Fig. S5†).22,23
Photocatalytic activity
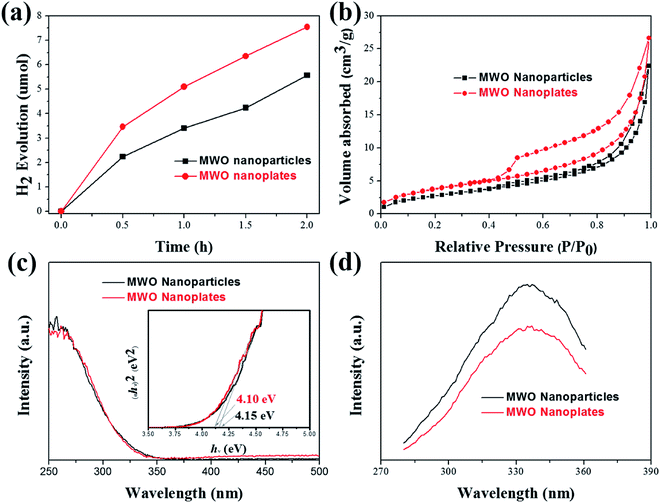
The photocatalytic activities of the as-prepared MgWO4 nanoplates were evaluated for hydrogen evolution under UV light irradiation. For comparison, MgWO4 nanoparticles (Fig. S6 and S7†) were used as photocatalysts under the same experimental conditions. From the XPS valence band spectrum, it can be deduced that the valence band position is located at around 2 eV (Fig. S8†). Because the band gaps of MgWO4 nanoparticles and nanoplates are estimated to 4.15 and 4.10 eV, respectively (Fig. 4c), the conduction band positions are about −2.15 and −2.10 eV, indicating that the photo-excited electrons have enough energy to reduce water (Fig. 5). As shown in Fig. 4a, both MgWO4 samples exhibit photocatalytic activity; this implies that MgWO4 can be a promising photocatalyst. In Fig. S9,† the MgWO4 nanoplates do not show good stability in the recycling tests; this may be related to the photo-corrosion. Moreover, MgWO4 nanoplates exhibited better photocatalytic performance than MgWO4 nanoparticles, and their photocatalytic hydrogen evolution rate was about 1.35 times higher than that of MgWO4 nanoparticles. It is known that the optical absorption range and the separation and migration efficiency are the main reasons impacting the final photocatalytic performance. To effectively study the possible mechanisms contributing to the differences in the photocatalytic rate, related characterizations were carried out, and the corresponding discussion have been provided hereinafter. The typical UV-vis diffuse reflectance spectra (DRS) of the as-prepared samples is displayed in Fig. 4c. The MgWO4 samples with nanoplate shapes show a similar optical absorption ranges or edges with the nanoparticle structures. This indicates that the solar absorption range is not the key factor for the enhanced photocatalytic hydrogen evolution rate of MgWO4 nanoplates. Therefore, photoluminescence (PL) emission spectra were obtained to characterize the recombination of photo-induced carriers. As shown in Fig. 4d, the strong emission peaks centered at about 335 nm could be ascribed to the band–band PL phenomenon of a photo-excited electron–hole; this was consistent with the DRS spectra.24 In other words, the MgWO4 nanoplates show a lower recombination rate of photo-induced carriers when compared with MgWO4 nanoparticles. As observed from the Brunauer–Emmett–Teller (BET) nitrogen adsorption–desorption curves, the specific surface areas of the MgWO4 nanoparticles and nanoplates are 10.46 and 14.05 m2 g−1, respectively (Fig. 4b and Table S1†). The two samples show a typical IV isotherm, suggesting the presence of pores, which is consistent with the TEM analysis. Moreover, the corresponding H2 production rates per surface area of the samples are almost the same (Fig. S10†). Therefore, the increased specific surface areas should be a key factor for the enhanced photocatalytic activities of MgWO4 nanoplates. As is well-known, a plate-like or sheet-like structure with high specific surface area can efficiently suppress the recombination of photo-induced carriers due to the short migration distance from the inner core to the surface; this is demonstrated in that the average particle size of MgWO4 nanoparticles (72 nm) is larger than the average thickness of MgWO4 nanoplates (45 nm). Furthermore, the high specific surface area is also beneficial for increasing the surface optical absorption and redox reaction area. Because of the larger specific surface area and corresponding efficient separation of photo-generated holes and electrons, the MgWO4 nanoplates present a lower PL intensity, thus promoting the photocatalytic activity.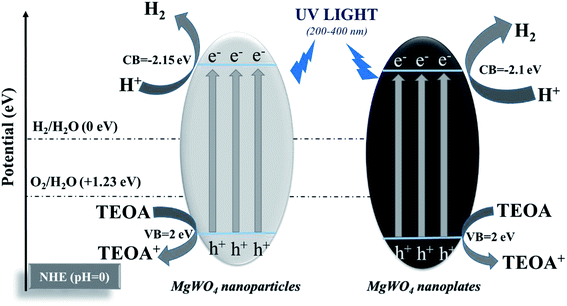 | ||
| Fig. 5 Schematic of photocatalytic hydrogen evolution for MgWO4 nanoparticles and MgWO4 nanoplates (triethanolamine (TEOA) as a sacrificial agent). | ||
Conclusions
In summary, the MgWO4 nanoplates were synthesized by a one-step hydrothermal reaction without any templates or surfactants. An oriented attachment and ripening process are proposed for the formation of the MgWO4 nanoplates. As a semiconductor, MgWO4 has the potential to participate in photocatalytic hydrogen evolution; this has been confirmed in this study. Compared with nanoparticle structures, MgWO4 nanoplates show an increased photocatalytic ability as a result of their higher specific surface areas. This study demonstrates that MgWO4 can be considered a viable photocatalyst, and future studies should be focused on how to promote its photocatalytic conversion efficiency.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number: 11234011, 11327901, 51102208) and the Fundamental Research Funds for the Central Universities (2014QNA4008, 2017QNA4011).Notes and references
- J. Huang, B. Tian, J. Wang, Y. Wang, W. Lu, Q. Li, L. Jin, C. Li and Z. Wang, CrystEngComm, 2018, 20, 608–614 RSC.
- Y. Zu, Y. Zhang, K. Xu and F. Zhao, RSC Adv., 2016, 6, 31046–31052 RSC.
- S. Wannapop, T. Thongtem and S. Thongtem, Appl. Surf. Sci., 2012, 258, 4971–4976 CrossRef CAS.
- P. D. Bhuyan, D. Singh, S. Kansara, P. Yadav, S. K. Gupta, Y. Sonvane, S. K. Rout and E. Sinha, J. Mater. Sci., 2017, 52, 4934–4943 CrossRef CAS.
- F. Danevich, D. Chernyak, A. Dubovik, B. Grinyov, S. Henry, H. Kraus, V. Kudovbenko, V. Mikhailik, L. Nagornaya and R. Podviyanuk, Nucl. Instrum. Methods Phys. Res., Sect. A, 2009, 608, 107–115 CrossRef CAS.
- D. W. Kim, I.-S. Cho, S. S. Shin, S. Lee, T. H. Noh, D. H. Kim, H. S. Jung and K. S. Hong, J. Solid State Chem., 2011, 184, 2103–2107 CrossRef CAS.
- Z. Shan, Y. Wang, H. Ding and F. Huang, J. Mol. Catal. A: Chem., 2009, 302, 54–58 CrossRef CAS.
- C. Shivakumara, R. Saraf, S. Behera, N. Dhananjaya and H. Nagabhushana, Mater. Res. Bull., 2015, 61, 422–432 CrossRef CAS.
- T. Chen, J. Meng, S. Wu, J. Pei, Q. Lin, X. Wei, J. Li and Z. Zhang, J. Alloys Compd., 2018, 754, 184–189 CrossRef CAS.
- G. Xi and J. Ye, Chem. Commun., 2010, 46, 1893–1895 RSC.
- M. Long, W. Cai, Z. Wang and G. Liu, Chem. Phys. Lett., 2006, 420, 71–76 CrossRef CAS.
- J. Meng, X. Fu, K. Du, X. Chen, Q. Lin, X. Wei, J. Li and Z. Zhang, Int. J. Hydrogen Energy, 2018, 43, 9224–9232 CrossRef CAS.
- H. Zhang, J. Yang, D. Li, W. Guo, Q. Qin, L. Zhu and W. Zheng, Appl. Surf. Sci., 2014, 305, 274–280 CrossRef CAS.
- H. Wang, J. Gao, T. Guo, R. Wang, L. Guo, Y. Liu and J. Li, Chem. Commun., 2012, 48, 275–277 RSC.
- S. Xie, Y. Wang, Q. Zhang, W. Deng and Y. Wang, Chem. Commun., 2015, 51, 3430–3433 RSC.
- C. Liu, Y. Xin, X. Tian and P. K. Chu, Thin Solid Films, 2007, 516, 422–427 CrossRef CAS.
- A. Katrib, F. Hemming, P. Wehrer, L. Hilaire and G. Maire, J. Electron Spectrosc. Relat. Phenom., 1995, 76, 195–200 CrossRef CAS.
- Y. Mi, Z. Huang, F. Hu, Y. Li and J. Jiang, J. Phys. Chem. C, 2009, 113, 20795–20799 CrossRef CAS.
- R. L. Penn and J. F. Banfield, Science, 1998, 281, 969–971 CrossRef CAS PubMed.
- J. F. Banfield, S. A. Welch, H. Zhang, T. T. Ebert and R. L. Penn, Science, 2000, 289, 751–754 CrossRef CAS PubMed.
- H. Xu, W. Wang and L. Zhou, Cryst. Growth Des., 2008, 8, 3486–3489 CrossRef CAS.
- W. Yang, F. Gao, G. Wei and L. An, Cryst. Growth Des., 2009, 10, 29–31 CrossRef.
- Y. Sun, B. Mayers and Y. Xia, Nano Lett., 2003, 3, 675–679 CrossRef CAS.
- J. Meng, J. Pei, Z. He, S. Wu, Q. Lin, X. Wei, J. Li and Z. Zhang, RSC Adv., 2017, 7, 24097–24104 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra06671j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |