Open Access Article
Open Access ArticleImprovement of high-glucose and insulin resistance of chromium malate in 3T3-L1 adipocytes by glucose uptake and insulin sensitivity signaling pathways and its mechanism†
Weiwei Fenga,
Yongchao Liua,
Fan Feia,
Yao Chena,
Yangyang Dinga,
Mengjiao Yana,
Yun Fengb,
Ting Zhaoc,
Guanghua Maoa,
Liuqing Yang *c and
Xiangyang Wu*a
*c and
Xiangyang Wu*a
aInstitute of Environmental Health and Ecological Security, School of the Environment and Safety Engineering, Jiangsu University, 301 Xuefu Rd., 212013 Zhenjiang, Jiangsu, China. E-mail: wuxy@ujs.edu.cn; Fax: +86 511 88791200; Tel: +86 511 88791200
bSchool of Medical Science and Laboratory Medicine, Jiangsu University, 301 Xuefu Rd., 212013 Zhenjiang, Jiangsu, China
cSchool of Chemistry and Chemical Engineering, Jiangsu University, 301 Xuefu Rd., 212013 Zhenjiang, Jiangsu, China. E-mail: yangliuqing@ujs.edu.cn; Fax: +86 511 88791800; Tel: +86 511 88791800
First published on 21st December 2018
Abstract
Previous study has revealed that chromium malate could improve insulin resistance and the regulation of fasting blood glucose in type 2 diabetic rats. This study was designed to investigate the effect of chromium malate on hypoglycemic and improve insulin resistance activities in 3T3-L1 adipocytes with insulin resistance and investigate the acting mechanism. The result indicated that chromium malate exhibited direct hypoglycemic activity in vitro. Compared with the model group, chromium malate could significantly promote the expression levels of GLUT-4, Akt, Irs-1, PPARγ, PI3K and p38-MAPK and their mRNA, increase p-AKT/AKT level, AKT and AMPKβ1 phosphorylation and reduce Irs-1 phosphorylation and p-Irs-1/Irs-1 level in 3T3-L1 adipocytes (p < 0.05). Chromium malate is more effective in regulating the proteins and mRNA expressions than those of chromium trichloride and chromium picolinate. Compared to the model group, pretreatment with the specific p38-MAPK inhibitor completely inhibited the GLUT-4 and Irs-1 proteins and mRNA expressions induced by the chromium malate. In conclusion, chromium malate had a beneficial influence on improvement of controlling glucose levels and insulin resistance in 3T3-L1 adipocytes with insulin resistance by regulating proteins productions and genes expressions in glucose uptake and insulin sensitivity signaling pathways.
Introduction
Diabetes is a kind of metabolic disorders and the prevalence of diabetes in adolescents and adults show a fast growth in the world.1,2 According to the International Diabetes Federation, from 2013 to 2035, the number of human with diabetes has increased from 382 million to 592 million.3 Type 2 diabetes mellitus (T2DM), which accounts for more than 90% of diabetes cases, has become a serious public health issue.4 Scientific communities have suggested that obesity, air pollution and inheritance could increase the risk of T2DM.5–7 The high fasting blood glucose levels and insulin resistance, as the most important factors in the occurrence and development of T2DM, have been commonly found in patients with T2DM.Chromium (Cr, III) is a beneficial micronutrient. Our previous studies have shown that organic chromium could improve glycometabolism and insulin resistance in T2DM, as well as Brooks et al., Yang et al. and Liu et al. reported.8–11 Previous studies suggested that the functionary mechanisms of the anti-hyperglycemic property of organic chromium complexes might be induced by improving glucose uptake and insulin resistance.12–15 However, little research has been reported on systematic studies on the signaling pathways of glucose uptake and insulin sensitivity by organic chromium complex in 3T3-L1 adipocytes with insulin resistance.
In our previous study, chromium malate has been discovered as a safe supplemental or alternative functional food supplement for patients with T2DM.16,17 And the anti-hyperglycemic property and the improving insulin resistance of chromium malate had been found in type 2 diabetic rats, however there were no systematic studies on the mechanism of glucose uptake and insulin sensitivity. Therefore, this study was to discover the possible anti-hyperglycemic and improving insulin resistance mechanisms of chromium malate in 3T3-L1 adipocytes with insulin resistance. The study would be helpful to explore the application value of organic chromium complexes – chromium malate, which could develop a novel functional food or drug.
Methods
Materials and chemicals
The chromium malate was synthesized according to the method described by Wu et al.18 Its chemical formula and molecular weight were Cr2C12H22O20 [or Cr2(C4H4O5)3·5H2O] and 590.18 g mol−1, respectively. The Cr content is 17.45%. Chromium malate can dissolve in water completely. Standard proteins were purchased from Cell Signaling Technology, Inc (America). Ultra-pure water was used throughout the whole experiments.Cell culture
3T3-L1 adipocytes were obtained from Institute of Cell Biology, Chinese Academy Sciences (Shanghai, China). Cells were cultured in 95% air–5% CO2 atmosphere in DMEM medium supplemented with 10% FBS at 37 °C. The differentiated cells were obtained after treating with insulin (10 μg mL−1) and IBMX (0.5 mM) for 2 days, insulin (10 μg mL−1) for 2 days, 10% FBS-DMEM for 5 days.19,20Models of insulin resistance
The differentiated cells (2 × 105 cells per mL) were placed in 96-well plates and incubated at 37 °C for 24 h in 10% FBS-DMEM. The cells were incubated with 1 × 10−6 mol L−1 insulin for 24 h. 24 h later, 3T3-L1 adipocytes were washed by DMEM and cultured by 10% FBS-DMEM, and then the glucose content of supernatant was measured by Glucose oxidase-peroxidase kit. Western blot was used to assay the Irs-1 and p-Irs-1 productions. The glucose content in 3T3-L1 adipocytes was exhibited significantly higher level than that of normal cells, accompanied with significantly reducing Irs-1 and significantly increasing p-Irs-1 was taken as successful induction of insulin resistance in 3T3-L1 adipocytes.Cytotoxicity of chromium malate on 3T3-L1 adipocytes
The differentiated cells (4 × 104 cells per mL) with insulin resistance were placed in 96-well plates. The various concentrations of chromium malate (5.0, 10.0 and 20.0 μg Cr per mL, low, middle and high dose groups), chromium picolinate (20.0 μg Cr per mL) and chromium trichloride (20.0 μg Cr per mL) were exposed for 24 h. The MTT Kit was used to assay the cytotoxicity of chromium malate. The absorbance was measured in Multimode Reader (SYNERGY H4, BioTek, USA) at 570 nm.Effect of chromium malate on glucose uptake of 3T3-L1 adipocytes
The differentiated cells (2 × 105 cells per mL) with insulin resistance were placed in 96-well plates. The various concentrations of chromium malate (5.0, 10.0 and 20.0 μg Cr per mL, low, middle and high dose groups), chromium picolinate and chromium trichloride (20.0 μg Cr per mL) were exposed for 24 h. The 3T3-L1 adipocytes without insulin resistance were exposed to the normal saline and chromium malate (20.0 μg Cr per mL) for 24 h as control groups. 3T3-L1 adipocytes were washed by DMEM and cultured by 10% FBS-DMEM, and then the glucose contents of supernatant were measured by Glucose oxidase-peroxidase kit.Assay of glucose uptake signaling pathway proteins
The differentiated cells (2 × 105 cells per mL) with insulin resistance were placed in 6-well plates. The experiment design and the doses of chromium malate, chromium picolinate and chromium trichloride were as same as above described. After incubating for 24 h, the cells were collected. The total protein was extracted and measured. Western blot was used to assay the GLUT-4, p-AMPKβ1 and Akt productions.21,22 The blots were visualized and the bands were photographed and analyzed with the Quantity One software.Assay of insulin sensitivity signaling pathway proteins
The 3T3-L1 adipocytes cultured, experiment design and the doses of chromium malate, chromium picolinate and chromium trichloride were same as above described. Western blot was used to assay the Irs-1, p-Irs-1, PPARγ, PI3K and p38-MAPK productions.Effect of gene expression on glucose uptake and insulin sensitivity signaling pathways
The 3T3-L1 adipocytes cultured, experiment design and the doses of chromium malate, chromium picolinate and chromium trichloride were as same as above described. After incubating for 24 h, the cells were washed with cold PBS and collected. Real-time PCR (RT-PCR) was used to assay the GLUT-4, AMPKα2, Akt2, Irs-1, PPARγ, PI3K and p38-MAPK mRNA production.23,24 The primers sequence used in this experiment were shown in Table 1.| Gene | Forward primer (5′ to 3′) | Reverse primer (3′ to 5′) |
|---|---|---|
| GAPDH | TGGTGGACCTCATGGCCTAC | CAGCAACTGAGGGCCTCTCT |
| GLUT-4 | GGGCTGTGAGTGAGTGCTTTC | CAGCGAGGCAAGGCTAGA |
| AMPKα2 | GGTGTTATCCTGTATGCCCTTCT | TGTCTTTGATAGTTGCTCGCTTC |
| Akt2 | ATGTAGACTCTCCAGATGAG | TGAGATAATCGAAGTCATTCA |
| Irs-1 | TGTGCCAAGCAACAAGAAAG | ACGGTTTCAGAGCAGAGGAA |
| PPARγ | CTTTACCACGGTTGATTTCTC | CAGGCTCTACTTTGATCGCA |
| PI3K | ACTGGAGGAAGACTTGAAG | CGTTTCCCAACCATTCGTT |
| p38-MAPK | CGCTGCTGCCGCTGGAAGAT | TTTGGCAATGATGGACT |
Effect of receptor protein and gene expression after inhibiting p38MAPK, PI3K and PPARγ expression
The 3T3-L1 adipocytes cultured and the doses of chromium malate were as same as above described. The 3T3-L1 adipocytes with insulin resistance were exposed to the chromium malate, chromium picolinate, chromium trichloride and GW5074 or LY294002 or GW9662 (3 μM) for 24 h. After incubating for 24 h, the cells were collected. The extraction and measure methods of total protein and RNA were as same as above described. Western blot and RT-PCR were used to assay the GLUT-4, Irs-1 and their genes expressions, respectively.Statistical analysis
Statistical analyses were carried out using the program SPSS 16.0 (SPSS Inc, Chicago, USA). Averages and standard error were expressed as mean ± SEM. The one-way analysis of variance (ANOVA) and Tukey test were performed to data analysis and determine the significant differences. A value of P < 0.05 was considered significantly different statistically.Results and discussion
Models of insulin resistance
The glucose contents of insulin induced 3T3-L1 adipocytes were 22.57 ± 0.64 mmol L−1. The glucose contents in 3T3-L1 adipocytes with insulin resistance were significantly higher than that of normal cells (18.62 ± 0.59 mmol L−1) (p < 0.05). Compared to the normal cells, insulin induced 3T3-L1 adipocytes exhibited significant high levels of glucose (p < 0.05). And the Irs-1, AKT, p-AKT and p-AKT/AKT was significantly reduced, p-Irs-1 and p-Irs-1/Irs-1, was significantly increased in insulin induced 3T3-L1 adipocytes (p < 0.05) (ESI Fig. 1†). Consistent with our results, Wang and Yao have also reported that 1 × 10−6 mol L−1 insulin with 3T3-L1 adipocytes exhibit insulin resistance.12 The glucose contents in 3T3-L1 adipocytes were exhibited significant higher level than that of the normal cells, accompanied with Irs-1, AKT, p-AKT and p-AKT/AKT reduction and p-Irs-1 and p-Irs-1/Irs-1 increase, which were taken as a successful induction of insulin resistance in 3T3-L1 adipocytes. Therefore, the results confirmed that the 3T3-L1 adipocyte model of insulin resistance is successful.Cytotoxicity of chromium malate on 3T3-L1 adipocytes
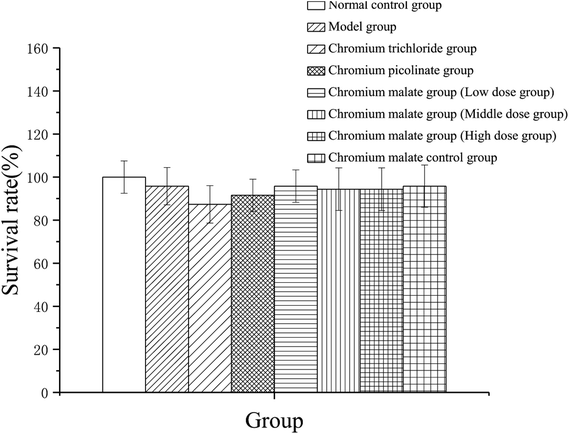
To assess the cytotoxicity of chromium malate in insulin resistance cells, the 3T3-L1 adipocytes with insulin resistance were incubated with various concentrations of chromium malate (5.0, 10.0 and 20.0 μg Cr per mL, low, middle and high dose groups), chromium trichloride and chromium picolinate (20.0 μg Cr per mL), respectively. As shown in Fig. 1, compared to the normal control, model and chromium malate control groups, the survival rate of cells did not change significantly in chromium malate (low, middle and high dose groups), chromium trichloride and chromium picolinate groups. High dose of chromium malate did not significantly increase/reduce the survival rate in 3T3-L1 adipocytes with insulin resistance when compared with chromium trichloride and chromium picolinate. The earlier studies reported that 150 μM inorganic chromium complex chromium trichloride (7.8 μg Cr per mL) could cause supercoiled DNA and linearized DNA molecules in Jurkat cells.25 Chromium picolinate is one of the most commonly used organic chromium complexes. However, studies show that 1.5 mM chromium picolinate (78 μg Cr per mL) could induce 51% cells undergoing apoptosis and damaged 86% of the mitochondria.26 It can be seen that the cytotoxicity of organic chromium complexes is lesser than those of inorganic chromium complexes. The result suggests that chromium malate had no obvious effect on cytotoxicity in 3T3-L1 adipocytes with insulin resistance at the dose of 0–20.0 μg Cr per mL.Effect of chromium malate on glucose uptake of 3T3-L1 adipocytes
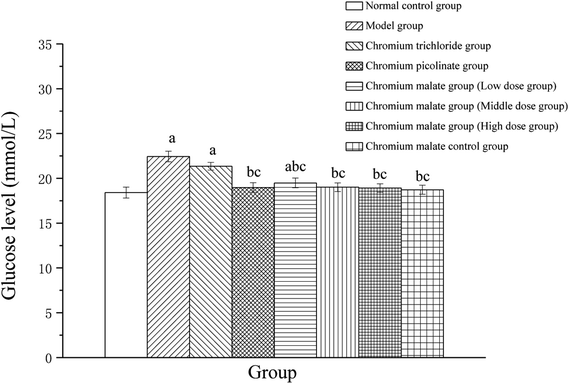
The glucose level changes of 3T3-L1 adipocytes with insulin resistance treated with chromium malate were shown in Fig. 2. After exposure to 24 h, the glucose levels of chromium malate in the middle and high dose groups and chromium picolinate group had declined and closed to normal levels (18.41 ± 0.61 mmol L−1). The glucose levels of the chromium malate groups and model group (22.44 ± 0.59 mmol L−1) were significantly different (p < 0.05). The hypoglycemic activity of chromium malate exhibited a dose dependency. The glucose levels of 3T3-L1 adipocytes with insulin resistance in chromium malate and chromium picolinate groups were decreased significantly when compared with the chromium trichloride group (21.35 ± 0.43 mmol L−1) (p < 0.05). Consistent with our results, Wang and Yao have reported that CrPic could induce glucose uptake in insulin resistance 3T3-L1 adipocytes.12 The results confirmed that chromium malate could contribute to glucose uptake and decrease the glucose levels. And the hypoglycemic activity of chromium malate as indicated by glucose levels was superior to that of chromium trichloride. Moreover, exposure of chromium malate had no obvious effect on glucose level of 3T3-L1 adipocytes without insulin resistance.Assay of glucose uptake signaling pathway proteins
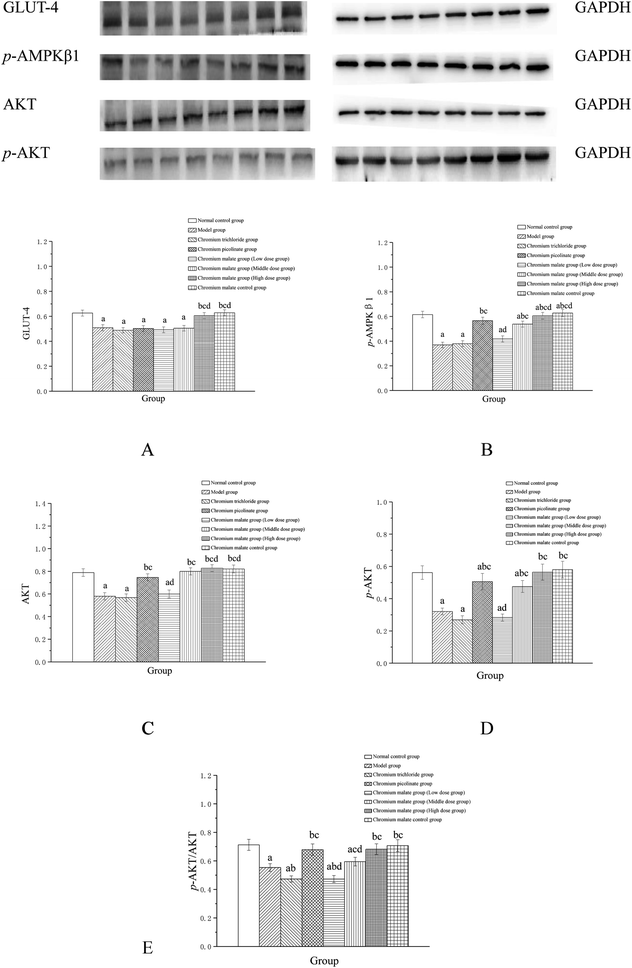
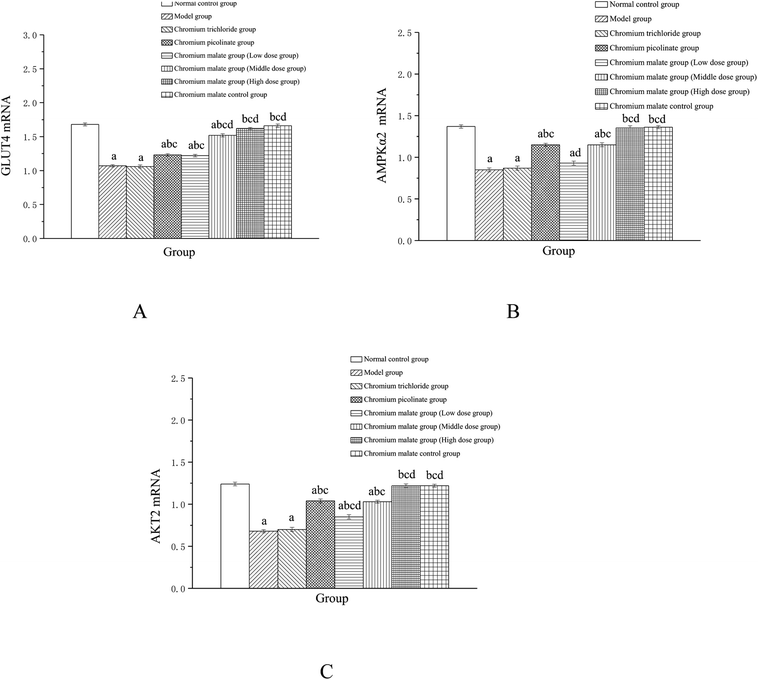
The possibility of the specific blood glucose accumulation that in turn may negatively affect glucose uptake is represented by high fasting blood glucose (FBG) levels in patients with T2DM. Previous studies have shown that GLUT-4 and AKT could increase the uptake of glucose to enhance the utilization of glucose and reduce the FBG levels.27,28 Evidence has suggested that AMPK play a key role in the control of FBG levels in T2DM.29 Once activated, AMPK can improve glucose uptake and inhibit glucose production.30,31 The abnormal proteins and genes expressions of GLUT-4, AMPK and AKT in the glucose uptake signaling pathway can result in the increasing FBG levels. Therefore, we examined the effects of chromium malate on GLUT-4, p-AMPKβ1, AKT, p-AKT and p-AKT/AKT (E) levels in insulin resistance 3T3-L1 adipocytes (Fig. 3). Chromium malate could enhance the GLUT-4, p-AMPKβ1, AKT, p-AKT and p-AKT/AKT levels in 3T3-L1 adipocytes with insulin resistance. Compared with the model group, a significant increase in GLUT-4 and p-AKT/AKT levels was observed in chromium malate high dose group and the levels of p-AMPKβ1, AKT and p-AKT in chromium malate middle and high dose groups and chromium picolinate group were significantly increased (p < 0.05). However, the levels of GLUT-4, p-AMPKβ1, AKT, p-AKT and p-AKT/AKT in chromium trichloride group had no significant change compared to the model group. Compared with the model group, the p-AMPKβ1, Akt, p-AKT and p-AKT/AKT levels of 3T3-L1 adipocytes in chromium picolinate group with insulin resistance increased significantly (p < 0.05). The levels of GLUT-4, p-AMPKβ1, Akt, p-AKT and p-AKT/AKT in chromium malate high dose group were significantly increased compared to the chromium trichloride group (p < 0.05). The chromium malate control group could not significantly enhance the levels of GLUT-4, p-AMPKβ1, AKT, p-AKT and p-AKT/AKT when compared with the normal control group. Therefore, chromium malate had no obvious effect on the GLUT-4, p-AMPKβ1, Akt, p-AKT and p-AKT/AKT levels of 3T3-L1 adipocytes without insulin resistance. Jovanovic et al. reported that Co-Factor III Cr Yeast could provoke a significant increase of the GLUT-4 and AMPK expressions and AKT phosphorylation in Holstein calves.32 Peng et al. reported that chromium phenylalanine could sensitize signaling pathway in cells with insulin resistance via the activation of Akt and AMPK.33 The literatures shown that the organic chromium complexes could improve glucose uptake in model organism. These results suggested that chromium malate could exhibit its glucose uptake activity by regulating glucose uptake signaling pathways proteins productions through up-regulation of GLUT-4 and Akt, promoting AMPKβ1 and AKT phosphorylation and p-AKT/AKT ratio. And chromium malate is more effective in regulating GLUT-4, p-AMPKβ1, Akt and p-AKT than those of chromium trichloride and chromium picolinate.Assay of insulin sensitivity signaling pathway proteins
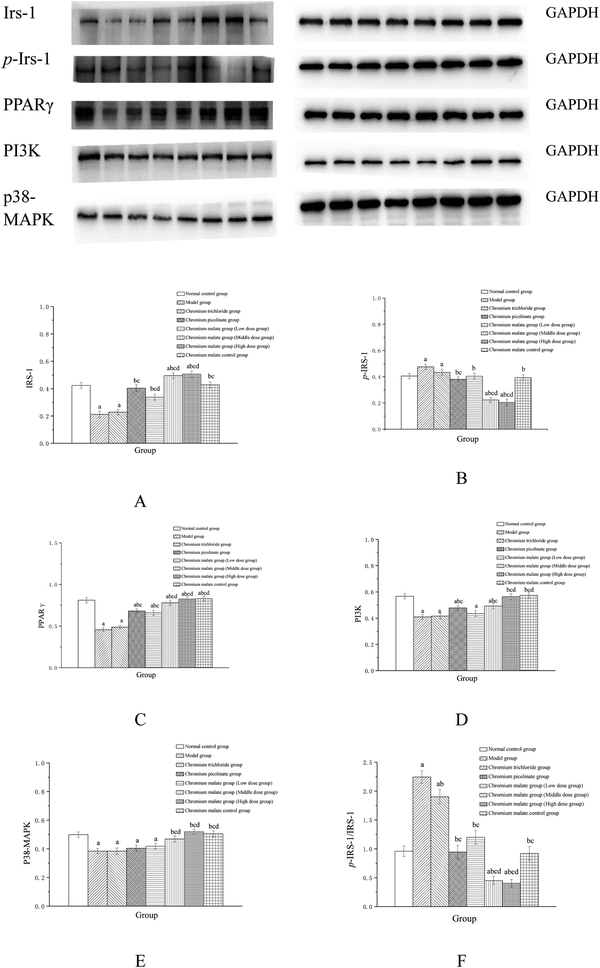
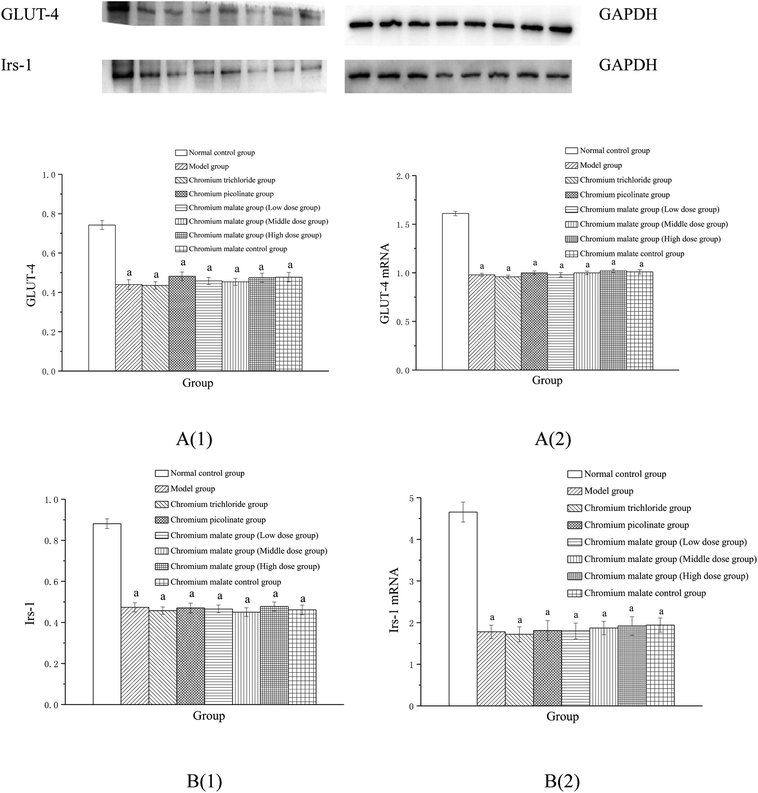
The insulin sensitivity signaling pathway can modulate insulin resistance in T2DM, and its impairment is one of the main molecular mechanisms of T2DM.34 It is involved in Irs-1, PPARγ, PI3K and p38-MAPK genes and proteins expressions. Previous studies have suggested that IRS-1 and PI3K are key molecules in insulin sensitivity signaling pathway.35,36 The lower levels of IRS-1 and PI3K in insulin signal transduction pathway with defects were found in T2DM.37 Peroxisome proliferator-activated receptor gamma (PPAR gamma) can enhance insulin sensitivity in muscle and adipose tissues, and some of exogenous potent PPARγ ligands are used as medicines to T2DM.38 p38-MAPK can regulate glucose metabolism. However, the activation of the p38-MAPK can result in phosphorylation of the insulin receptor and IRS proteins, leading to impaired insulin signaling.39 Therefore, we examined the effects of chromium malate on Irs-1, p-Irs-1, p-Irs-1/Irs-1, PPARγ, PI3K and p38-MAPK levels in insulin resistance 3T3-L1 adipocytes. Effects of chromium malate on Irs-1, p-Irs-1, p-Irs-1/Irs-1, PPARγ, PI3K and p38-MAPK levels in 3T3-L1 adipocytes with insulin resistance were presented in Fig. 4. Chromium malate could enhance the levels of Irs-1, PPARγ, PI3K and p38-MAPK, and reduce p-Irs-1 and p-Irs-1/Irs-1 levels in 3T3-L1 adipocytes with insulin resistance. Compared with the model group, chromium malate showed significant increase in the Irs-1 and PPARγ levels, significant reduction in the p-Irs-1 and p-Irs-1/Irs-1 levels in low, middle and high dose groups and significant increase in the PI3K and p38-MAPK levels in middle and high dose groups (p < 0.05). Chromium malate increased the productions of Irs-1, PPARγ, PI3K and p38-MAPK, and decreased the Irs-1 phosphorylation and p-Irs-1/Irs-1 in dose-independent manners. The group exposed to chromium picolinate showed significant increase in Irs-1, PPARγ and PI3K levels, and significant decrease in p-Irs-1 and p-Irs-1/Irs-1 levels except for p38-MAPK production, when compared with the model group (p < 0.05). The results of the chromium trichloride group showed that no significant changes were observed in Irs-1, p-Irs-1, PPARγ, PI3K and p38-MAPK productions. Comparing the levels of the Irs-1, p-Irs-1, p-Irs-1/Irs-1, PPARγ, PI3K and p38-MAPK with the chromium picolinate and chromium trichloride groups, the results showed that a significant change occurred in chromium malate high dose group (p < 0.05). The chromium malate could not significantly change the levels of Irs-1, p-Irs-1, p-Irs-1/Irs-1, PPARγ, PI3K and p38-MAPK in chromium malate control group when compared with the normal control group. Therefore, chromium malate had no obvious effect on the Irs-1, p-Irs-1, p-Irs-1/Irs-1, PPARγ, PI3K and p38-MAPK levels of 3T3-L1 adipocytes without insulin resistance. These results suggested that chromium malate could improve insulin resistance by regulating insulin sensitivity signaling pathway proteins production through up-regulation of Irs-1, p-Irs-1, PPARγ, PI3K and p38-MAPK, and down-regulation of Irs-1 phosphorylation and p-Irs-1/Irs-1. And chromium malate is more effective in regulating Irs-1, p-Irs-1, p-Irs-1/Irs-1, PPARγ, PI3K and p38-MAPK levels than those of chromium trichloride and chromium picolinate.Effect of gene expression on glucose uptake signaling pathway
Effects of chromium malate on the GLUT-4, AMPKα2 and AKT2 mRNA expression levels of 3T3-L1 adipocytes with insulin resistance were showed in Fig. 5. Chromium malate could enhance the mRNA expressions of GLUT-4, AMPKα2 and Akt2 in 3T3-L1 adipocytes with insulin resistance. Compared to the model group, the mRNA expression levels of GLUT-4 and AKT2 in chromium malate low, middle and high dose groups and the mRNA expression levels of AMPKα2 in chromium malate middle and high dose groups were significantly increased (p < 0.05). Chromium malate increased the mRNA expressions of GLUT-4, AMPKα2 and Akt2 in a dose dependency manner. The mRNA expression levels of GLUT-4, AMPKα2 and Akt2 of 3T3-L1 adipocytes with insulin resistance in chromium picolinate group were increased significantly when compared with the model group (p < 0.05). However, the GLUT-4, AMPKα2 and Akt2 mRNA expression in chromium trichloride group had no significant change. Compared with the chromium picolinate and chromium trichloride groups, the mRNA expressions of GLUT-4, AMPKα2 and Akt2 in chromium malate high dose group were increased significantly (p < 0.05). The mRNA expressions of GLUT-4, AMPKα2 and Akt2 had no significant change between chromium malate control group and normal control group. Therefore, chromium malate had no obvious effect on the GLUT-4, AMPKα2 and Akt2 mRNA expressions of 3T3-L1 adipocytes without insulin resistance. Consistent with our results, Hao et al. have reported that oligomannuronate chromium complex could enhance the mRNA expression of GLUT-4 in C2C12 skeletal muscle cells with insulin resistance.13 These results revealed that chromium malate could exhibit its glucose uptake activity by regulating gene expressions of receptor proteins on glucose uptake signaling pathway through up-regulation of GLUT-4, AMPKα2 and Akt2 mRNA. And chromium malate is more effective in regulating GLUT-4, AMPKα2 and Akt2 mRNA expressions than those of chromium trichloride and chromium picolinate.Effect of gene expression on insulin sensitivity signaling pathway
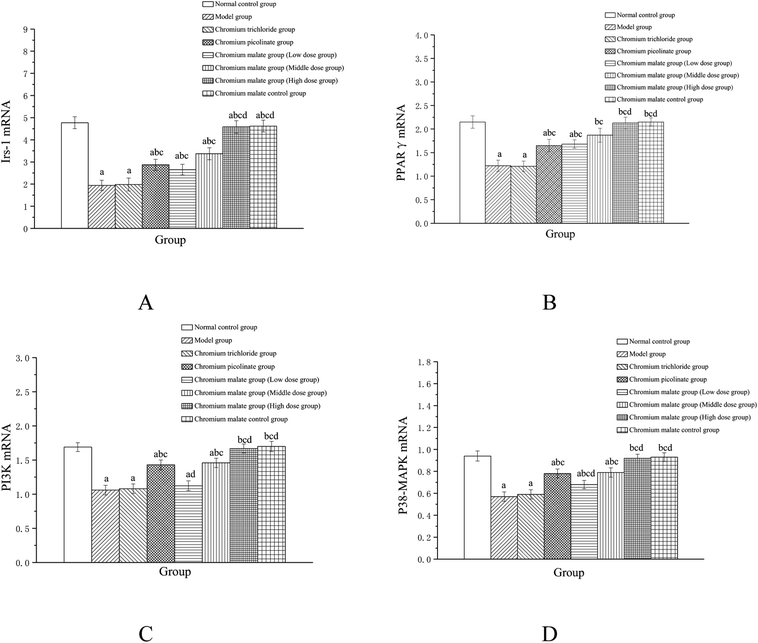
Effects of chromium malate on the Irs-1, PPARγ, PI3K and p38-MAPK mRNA expression levels of 3T3-L1 adipocytes with insulin resistance were presented in Fig. 6. Chromium malate could enhance the mRNA expressions of Irs-1, PPARγ, PI3K and p38-MAPK in 3T3-L1 adipocytes with insulin resistance. Compared with the model group, chromium malate showed significant increase in the mRNA expressions levels of Irs-1, PPARγ and p38-MAPK in low, middle and high dose groups and significant increase in the mRNA expression levels of PI3K in middle and high dose groups (p < 0.05). Chromium malate increased the mRNA expressions of Irs-1, PPARγ, PI3K and p38-MAPK in dose-dependent manners. Chromium picolinate group showed significant increase in the mRNA expressions of Irs-1, PPARγ, PI3K and p38-MAPK when compared with the model group (p < 0.05). However, chromium trichloride group showed no significant change in the mRNA expressions of Irs-1, PPARγ, PI3K and p38-MAPK. Comparing the mRNA expression levels of the Irs-1, PPARγ, PI3K and p38-MAPK in the chromium picolinate and chromium trichloride groups, the results showed that a significant increase was observed in chromium malate high dose group (p < 0.05). The mRNA expression levels of Irs-1, PPARγ, PI3K and p38-MAPK had no significant change between chromium malate control group and normal control group. Therefore, chromium malate had no obvious effect on the Irs-1, PPARγ, PI3K and p38-MAPK mRNA expressions of 3T3-L1 adipocytes without insulin resistance. Liu et al. reported that chromium yeast could improve glucose metabolism in insulin-resistant 3T3-L1 adipocytes via increase Irs-1 mRNA.11 A similar expression trend was observed for PPARγ mRNA in 3T3-L1 adipocytes treatment with Cr(III)-citrate.40 These results suggested that chromium malate could improve insulin resistance by regulating gene expressions of receptor proteins on insulin sensitivity signaling pathway through up-regulation of Irs-1, PPARγ, PI3K and p38-MAPK mRNA. And chromium malate is more effective in regulating Irs-1, PPARγ, PI3K and p38-MAPK mRNA expressions than those of chromium trichloride and chromium picolinate.Models of inhibiting p38 MAPK, PI3K and PPARγ expression
Compared to the 3T3-L1 adipocytes with insulin resistance, GW5074 or LY294002 or GW9662 induced significantly low levels of p38 MAPK, PI3K and PPARγ in 3T3-L1 adipocytes (p < 0.05) (ESI Fig. 2†). The p38 MAPK, PI3K and PPARγ levels in 3T3-L1 adipocytes with insulin resistance exhibited significant inhibitions than that of the insulin resistance cells without corresponding inhibitors, which were taken as successful inhibited p38 MAPK, PI3K and PPARγ expression. Therefore, the results confirmed that the models of inhibiting p38 MAPK, PI3K and PPARγ expression are successful.Effect of receptor protein and gene expression after inhibiting p38MAPK expression
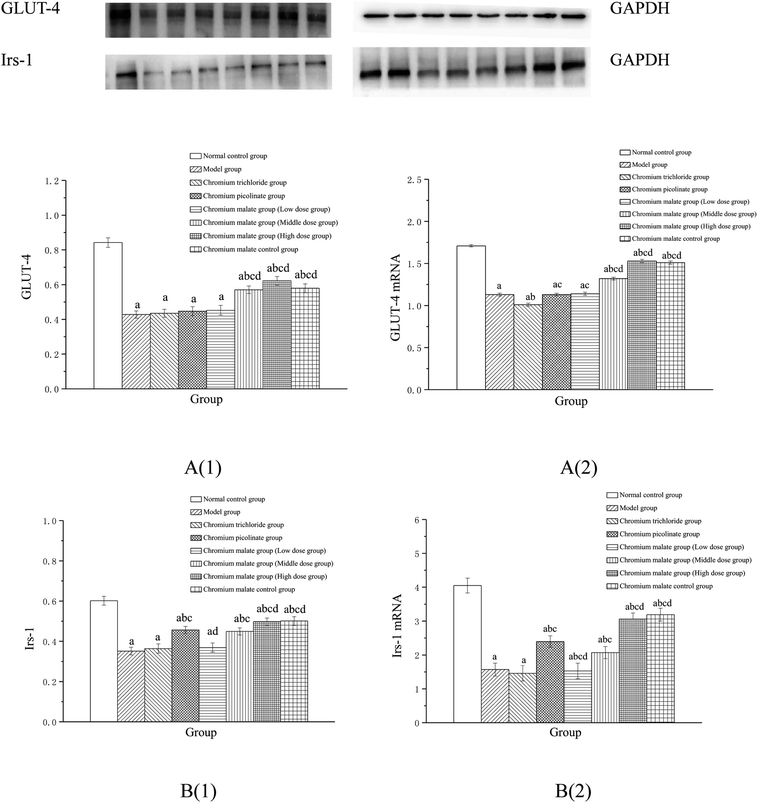
These results suggested that chromium malate had hypoglycemic activity and could improve insulin resistance in 3T3-L1 adipocytes with insulin resistance by regulating p38-MAPK-induced proteins productions and genes expressions. And the hypoglycemic activity and the improvement of insulin resistance of chromium malate were not only through regulating p38-MAPK-induced proteins production and genes expression, but also through regulating other key protein-induced proteins productions and genes expressions.
Effect of receptor protein and gene expression after inhibiting PI3K expression
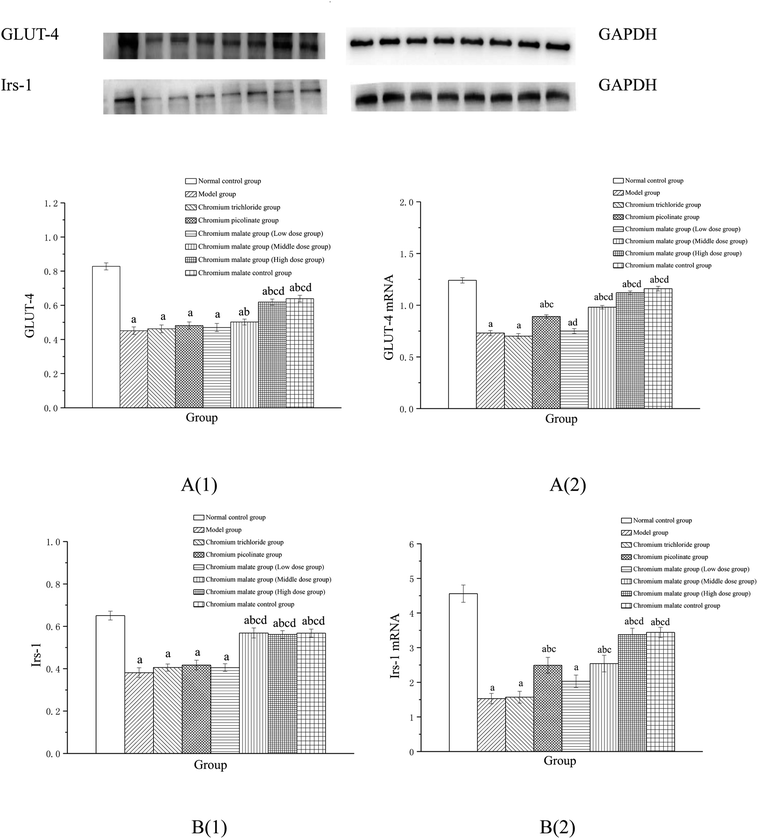
These results suggested that chromium malate could improve high-glucose levels and insulin resistance in 3T3-L1 adipocytes with insulin resistance by regulating PI3K-induced proteins productions and genes expressions. And the hypoglycemic activity and improving insulin resistance of chromium malate were not only through regulating PI3K-induced proteins productions and genes expressions, but also through regulating other key protein-induced proteins productions and genes expressions.
Effect of receptor protein and gene expression after inhibiting PPARγ expression
These results suggested that chromium malate could improve high-glucose levels and insulin resistance in 3T3-L1 adipocytes with insulin resistance by regulating PPARγ-induced proteins productions and genes expressions.
Conclusion
Chromium malate exhibited direct hypoglycemic activity in vitro and greater benefits in treating 3T3-L1 adipocytes with insulin resistance induced high-glucose levels than that of chromium trichloride. Chromium malate could induce 3T3-L1 adipocytes with insulin resistance to express GLUT-4, Akt, Irs-1, PPARγ, PI3K and p38-MAPK proteins and mRNA, increase AMPKβ1 phosphorylation and p-AKT/AKT level and reduce Irs-1 phosphorylation and p-Irs-1/Irs-1 level. The results of pretreatment with the specific p38MAPK/PI3K/PPARγ inhibitor showed that the chromium malate could improve high-glucose levels and insulin resistance in 3T3-L1 adipocytes with insulin resistance by regulating proteins productions and genes expressions in glucose uptake and insulin sensitivity signaling pathways.Conflicts of interest
No.Acknowledgements
This work was supported financially by Specialized Research Fund for the Natural Science Foundation of China (31271850) and Research Foundation for Advanced Talents of Jiangsu University (15JDG146).References
- F. Akhlaghi, K. L. Matson, A. H. Mohammadpour, M. Kelly and A. Karimani, Clin. Pharmacokinet., 2017, 56, 561–571 CrossRef CAS.
- World Health Organization (WHO), http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf, 2016, accessed November 2016.
- L. Guariguata, D. R. Whiting, I. Hambleton, J. Beagley, U. Linnenkamp and J. E. Shaw, Diabetes Res. Clin. Pract., 2014, 13, 137–149 CrossRef PubMed.
- Y. K. Jiao, M. L. Zhang, S. M. Wang and C. Y. Yan, Int. J. Biol. Macromol., 2017, 101, 543–552 CrossRef CAS PubMed.
- T. G. Delgado-Leon, J. M. Salas-Pacheco, F. Vazquez-Alaniz, A. A. Vertiz-Hernandez, O. D. Lopez-Guzman, E. Lozano-Guzman, A. Martinez-Romero, N. Urtiz-Estrada and M. Cervantes-Flores, J. Trace Elem. Med. Biol., 2018, 46, 144–149 CrossRef CAS PubMed.
- S. K. Park, Diabetes, 2017, 66, 1755–1757 CrossRef CAS PubMed.
- A. Ortega, G. Berna, A. Rojas, F. Martin and B. Soria, Int. J. Mol. Sci., 2017, 18 DOI:10.3390/ijms18061188.
- W. W. Feng, T. Zhao, G. H. Mao, W. Wang, Y. Feng, F. Li, D. H. Zheng, H. Y. Wu, D. Jin, L. Q. Yang and X. Y. Wu, PLoS One, 2015, 10, e0125952 CrossRef.
- M. A. Brooks, J. L. Grimes, K. E. Lloyd, K. Krafka, A. Lamptey and J. W. Spears, Poult. Sci., 2016, 95, 1096–1104 CrossRef CAS.
- X. P. Yang, K. Palanichamy, A. C. Ontko, M. N. A. Rao, C. X. Fang, J. Ren and N. Sreejayan, FEBS Lett., 2005, 579, 1458–1464 CrossRef CAS.
- L. Liu, W. M. Cui, S. W. Zhang, F. H. Kong, M. A. Pedersen, Y. Wen and J. P. Lv, RSC Adv., 2015, 5, 3482–3490 RSC.
- Y. Q. Wang and M. H. Yao, J. Nutr. Biochem., 2009, 20, 982–991 CrossRef CAS PubMed.
- C. Hao, J. J. Hao, W. Wang, Z. R. Han, G. S. Li, L. J. Zhang, X. Zhao and G. L. Yu, PLoS One, 2011, 6, e24598 CrossRef CAS PubMed.
- W. Qiao, Z. L. Peng, Z. S. Wang, J. Wei and A. Zhou, Biol. Trace Elem. Res., 2009, 131, 133–142 CrossRef CAS.
- P. Zhao, J. Y. Wang, H. Ma, Y. Xiao, L. L. He, C. Tong, Z. H. Wang, Q. S. Zheng, E. K. Dolence, S. Nair, J. Ren and J. Li, Biochem. Pharmacol., 2009, 77, 1002–1010 CrossRef CAS PubMed.
- W. W. Feng, H. Y. Wu, Q. Li, Z. X. Zhou, Y. Chen, T. Zhao, Y. Feng, G. H. Mao, F. Li, L. Q. Yang and X. Y. Wu, Biol. Trace Elem. Res., 2015, 168, 181–195 CrossRef CAS PubMed.
- W. W. Feng, W. J. Zhang, T. Zhao, G. H. Mao, W. Wang, X. S. Wu, Z. X. Zhou, J. Huang, Y. T. Bao, L. Q. Yang and X. Y. Wu, Biol. Trace Elem. Res., 2015, 168, 150–168 CrossRef CAS PubMed.
- X. Y. Wu, F. Li, W. D. Xu, J. L. Zhao, T. Zhao, L. H. Liang and L. Q. Yang, Biol. Trace Elem. Res., 2011, 143, 1031–1043 CrossRef CAS PubMed.
- J. J. Guo, Y. Cao, C. T. Ho, S. K. Jin and Q. R. Huang, J. Funct. Foods, 2017, 34, 297–303 CrossRef CAS.
- P. J. Park, J. Y. Cho and E. G. Cho, Eur. J. Cell Biol., 2017, 96, 301–311 CrossRef CAS.
- G. H. Mao, Y. Ren, W. W. Feng, Q. Li, H. Y. Wu, D. Jin, T. Zhao, C. Q. Xu, L. Q. Yang and X. Y. Wu, Carbohydr. Polym., 2015, 134, 406–412 CrossRef CAS PubMed.
- X. Y. Xia, X. Xiang, F. H. Huang, M. M. Zheng, R. H. Cong, L. Han and Z. Zhang, RSC Adv., 2018, 43, 24338–24345 RSC.
- A. F. Stattermayer, S. Traussnigg, E. Aigner, C. Kienbacher, U. Huber-Schonauer, P. Steindl-Munda, A. Stadlmayr, F. Wrba, M. Trauner, C. Datz and P. Ferenci, J. Trace Elem. Med. Biol., 2017, 39, 100–107 CrossRef.
- M. Diaz, C. Garcia, G. Sebastiani, F. de Zegher, A. Lopez-Bermejo and L. Ibanez, Diabetes, 2017, 66, 779–784 CrossRef CAS.
- Z. J. Fang, M. Zhao, H. Zhen, L. F. Chen, P. Shi and Z. W. Huang, PLoS One, 2014, 9, 1–9 Search PubMed.
- K. R. Manygoats, M. Yazzie and D. M. Stearns, J. Inorg. Chem., 2002, 7, 791–798 CAS.
- L. Wang, Z. B. Liu, X. F. Liu and Y. T. Wu, RSC Adv., 2016, 93, 90777–90785 RSC.
- M. Kim, K. Song and Y. S. Kim, Front. Pharmacol., 2017, 8 DOI:10.3389/fphar.2017.00405.
- R. Costa, I. Rodrigues, L. Guardao, S. Rocha-Rodrigues, C. Silva, J. Magalhaes, M. Ferreira-de-Almeida, R. Negrao and R. Soares, J. Nutr. Biochem., 2017, 45, 39–47 CrossRef CAS.
- R. Dhanya, A. D. Arya, P. Nisha and P. Jayamurthy, Front. Pharmacol., 2017, 8 DOI:10.3389/fphar.2017.00336.
- S. Y. Lee, F. Y. Lai, L. S. Shi, Y. C. Chou, I. C. Yen and T. C. Chang, Phytomedicine, 2015, 22, 477–486 CrossRef CAS PubMed.
- L. Jovanovic, M. Pantelic, R. Prodanovic, I. Vujanac, M. Duric, S. Tepavcevic, S. Vranjes-Duric, G. Koricanac and D. Kirovski, Biol. Trace Elem. Res., 2017, 180, 223–232 CrossRef CAS.
- M. Peng and X. P. Yang, J. Inorg. Chem., 2015, 146, 97–103 CAS.
- F. M. Fauzi, C. M. John, A. Karunanidhi, H. Y. Mussa, R. Ramasamy, A. Adam and A. Bender, J. Ethnopharmacol., 2017, 197, 61–72 CrossRef PubMed.
- P. H. Fang, M. Yu, L. Zhang, D. Wan, M. Y. Shi, Y. Zhu, P. Bo and Z. W. Zhang, Mol. Cell. Endocrinol., 2017, 448, 77–86 CrossRef CAS.
- Y. Q. Wang and M. H. Yao, J. Nutr. Biochem., 2009, 20, 982–991 CrossRef CAS.
- N. Yu, X. Fang, D. D. Zhao, Q. Q. Mu, J. C. Zuo, Y. Ma, Y. Zhang, F. F. Mo, D. W. Zhang and G. J. Jiang, PLoS One, 2017, 12 DOI:10.1371/journal.pone.0168980.
- E. K. Song, Y. R. Lee, Y. R. Kim, J. H. Yeom, C. H. Yoo, H. K. Kim, H. M. Park, H. S. Kang, J. S. Kim, U. H. Kim and M. K. Han, Cell Rep., 2012, 1607–1619 CrossRef CAS PubMed.
- Y. X. Liu, A. Song, S. S. Zang, C. Wang, G. Y. Song, X. L. Li, Y. J. Zhu, X. Yu, L. Li, Y. Wang and L. Y. Duan, J. Ethnopharmacol., 2015, 162, 244–252 CrossRef PubMed.
- O. Tsave, M. P. Yavropoulou, M. Kafantari, C. Gabriela, J. G. Yovos and A. Salifoglou, J. Inorg. Biochem., 2016, 163, 323–331 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra07470d |
| This journal is © The Royal Society of Chemistry 2019 |