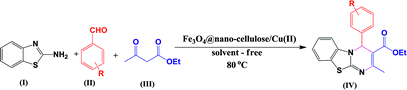Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fe3O4@nano-cellulose/Cu(II): a bio-based and magnetically recoverable nano-catalyst for the synthesis of 4H-pyrimido[2,1-b]benzothiazole derivatives†
Nasrin Safajooa,
Bi Bi Fatemah Mirjalili *a and
Abdolhamid Bamoniri
*a and
Abdolhamid Bamoniri b
b
aDepartment of Chemistry, College of Science, Yazd University, Yazd, P. O. Box 89195-741, Islamic Republic of Iran. E-mail: fmirjalili@yazd.ac.ir; Fax: +98 3538210644; Tel: +98 3531232672
bDepartment of Organic Chemistry, Faculty of Chemistry, University of Kashan, Kashan, Islamic Republic of Iran
First published on 11th January 2019
Abstract
Fe3O4@nano-cellulose/Cu(II) as a green bio-based magnetic catalyst was prepared through in situ co-precipitation of Fe2+ and Fe3+ ions in an aqueous suspension of nano-cellulose. The mentioned magnetically heterogeneous catalyst was characterized by FT-IR, XRD, VSM, FESEM, TEM, XRF, EDS and TGA. In this research, the synthesis of 4H-pyrimido[2,1-b]benzothiazole derivatives was developed via a three component reaction of aromatic aldehyde, 2-aminobenzothiazole and ethyl acetoacetate using Fe3O4@nano-cellulose/Cu(II) under solvent-free condition at 80 °C. Some advantages of this protocol are good yields, environmentally benign, easy work-up and moderate reusability of the catalyst. The product structures were confirmed by FT-IR, 1H NMR, and 13C NMR spectra.
Introduction
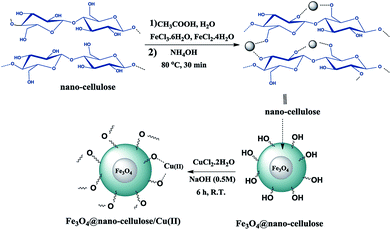
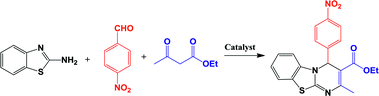
Fused heterocyclic compounds containing nitrogen and sulfur are important compounds because of their pharmacological properties.1 Among these compounds benzothiazoles and pyrimido[2,1-b]benzothiazoles have attracted considerable interest. Some of these compounds have various biological activities such as antiviral,2,3 antitumor,4–6 antiinflammatory,7,8 antiallergic,9 antimicrobial,10,11 anticonvulsant,12 antiproliferative13 and antifungal activities.14 Pyrimido[2,1-b]benzothiazole derivatives were synthesized through multicomponent reaction between 2-amino benzothiazole, aromatic aldehydes and β-ketoesters.15–18 Previously, this protocol has been catalyzed by iron fluoride,19 pyridine,11 acetic acid,20 1,1,3,3-N,N,N′,N′-tetramethylguanidinium trifluoroacetate (TMGT),16 tetrabutylammonium hydrogen sulfate (TBAHS),17 N-sulfonic acid modified poly(styrene-maleic anhydride) (SMI-SO3H),21 chitosan,18 aluminum trichloride22 and Fe3O4@nano-cellulose/TiCl.23 Some of the reported protocols have harsh conditions and long reaction times. Thus, in this work, a new simple protocol for the synthesis of these compounds is reported.Biopolymers, especially cellulose and its derivatives, have some unparalleled properties, which make them attractive alternatives for ordinary organic or inorganic supports for catalytic applications.24 Cellulose is the most abundant natural material in the world and it can play an important role as a biocompatible, renewable resource and biodegradable polymer containing OH groups.25 Cotton is a natural, cheap, and readily available source of cellulose. Fe3O4 nanoparticles are coated with various materials such as surfactants,26 polymers,27,28 silica,29 cellulose23 and carbon30 to form core–shell structures. Magnetic nanoparticles as heterogeneous supports have many advantages such as high dispersion in reaction media and easy recovery by an external magnet.31–38 Cu(II) as a safe and ecofriendly cation is a good Lewis acid and can activate the carbonyl group for nucleophilic addition reactions. Thus, the main purpose of the present work is the preparation of Fe3O4@nano-cellulose/Cu(II) as a new and bio-based magnetic nanocatalyst for one-pot synthesis of pyrimido[2,1-b]benzothiazoles via condensation of aromatic aldehydes, ethyl acetoacetate and 2-aminobenzothiazole.
Results and discussion
Fe3O4@nano-cellulose/Cu(II) was prepared in a two-step process. First, Fe3O4@nano-cellulose was synthesized by co-precipitation of Fe2+ and Fe3+ ions in the presence of nano-cellulose and then it was used as a magnetic support for loading CuCl2 onto the cellulose section of it (Scheme 1). The magnetically heterogeneous catalyst named Fe3O4@nano-cellulose/Cu(II), is characterized by Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), vibrating sample magnetometer (VSM), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), X-ray fluorescence (XRF), energy-dispersive X-ray spectroscopy (EDS), and thermo-gravimetric analysis (TGA).The FT-IR spectra of nano-cellulose, Fe3O4@nano-cellulose and Fe3O4@nano-cellulose/Cu(II) are shown in Fig. 1.
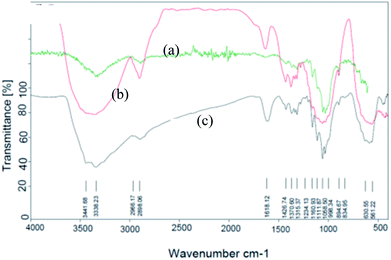 | ||
| Fig. 1 FT-IR spectra of (a) nano-cellulose, (b) Fe3O4 @ nano-cellulose and (c) Fe3O4@nano-cellulose/Cu(II). | ||
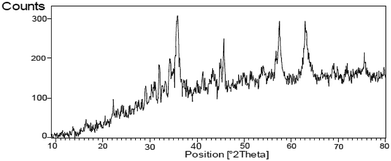
The FT-IR spectrum of nano-cellulose has shown a broad band at 3338 cm−1 which corresponds to the stretching vibrations of OH groups. The absorption bands at1058 and 1108 cm−1 display the stretching vibrations of the C–O bonds. For Fe3O4@nano-cellulose, in addition to the cellulose absorptions bands, stretching vibrations of Fe/O groups at 586 and 634 cm−1 are appeared which is indicated that the magnetic Fe3O4 nano particles are coated by nano-cellulose. The FT-IR spectrum of Fe3O4@nano-cellulose/Cu(II) has shown a characteristic absorption band under 500 cm−1 that may be attributed to Cu–O band for Cu bonded to cellulose. X-ray diffraction (XRD) pattern of Fe3O4@nano-cellulose/Cu(II) is shown in Fig. 2. Fe3O4 has shown diffraction peaks at 2θ = 35.79°, 43.42°, 53.94°, 57.51° and 63.08° with FWHM equal to 0.39, 0.78, 0.94, 0.31 and 0.96 respectively, which are quite matched with the cubic spinel structure of pure Fe3O4. A diffraction peaks at 2θ = 16.45° and 22.18° with FWHM equal to 0.23 and 0.47, respectively, has shown the existence of cellulose. Other signals in 2θ = 13.68, 29.10, 32.01, 34.25 and 45.71 probably reveal the existence of cellulose and bonding of Cu(II) to cellulosic shell (Table 1).
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Pos. [°2θ] | 13.6815 | 16.4566 | 22.1853 | 29.1068 | 32.0094 | 34.2572 |
| FWHM [°2θ] | 0.6298 | 0.4723 | 0.2362 | 0.2362 | 0.3149 | 0.3149 |
| No. | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|
| Pos. [°2θ] | 35.7935 | 43.4246 | 45.7114 | 53.9425 | 57.5101 | 63.0810 |
| FWHM [°2θ] | 0.3936 | 0.7872 | 0.3149 | 0.9446 | 0.3149 | 0.9600 |
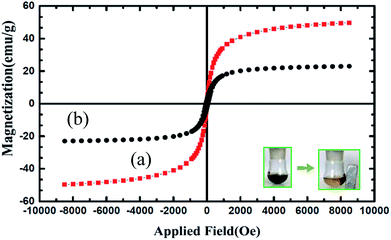
The magnetic properties of Fe3O4 and Fe3O4@nano-cellulose/Cu(II) were characterized at RT (300 K) by a vibrating sample magnetometer (VSM) and their hysteresis curves are presented in Fig. 3. The zero coercivity and remanence of the hysteresis loops of these magnetic nanoparticles confirm superparamagnetic property of them at room temperature. The amount of specific saturation magnetization (Ms) for Fe3O4 nanoparticles was about 50 emu g−1, which decreased to 25 emu g−1 after the bonding of Cu(II) on the surface of Fe3O4@nano-cellulose. Despite this significant decrease, the saturated magnetization of these magnetic nanoparticles is sufficient for magnetic separation.
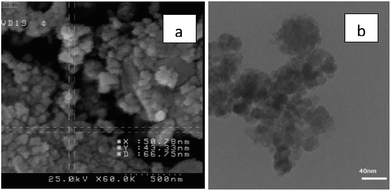
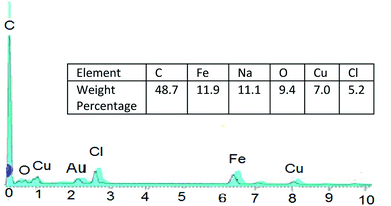
The particles size of Fe3O4@nano-cellulose/Cu(II) were investigated by field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM) in which the dimensions of them were achieved below 70 nm (Fig. 4). The chemical composition of catalyst has been measured using X-ray fluorescence (XRF) analysis (Table 2). In order to obtain the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl ratio in Fe3O4@nano-cellulose/Cu(II) by XRF analysis, Kilo Counts Per Seconds (KCPS) values of elements in catalyst were compared with KCPS values of the same elements in pure samples, NaCl and CuSO4. By this comparison, the amount of Cu and Cl were obtained 1.38 g (0.02 mol) and 0.12 g (0.003 mol), respectively. Thus, the ratio of Cu
Cl ratio in Fe3O4@nano-cellulose/Cu(II) by XRF analysis, Kilo Counts Per Seconds (KCPS) values of elements in catalyst were compared with KCPS values of the same elements in pure samples, NaCl and CuSO4. By this comparison, the amount of Cu and Cl were obtained 1.38 g (0.02 mol) and 0.12 g (0.003 mol), respectively. Thus, the ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl in catalyst is approximately 6
Cl in catalyst is approximately 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
| Elemental component | Fe3O4@nano-cellulose/Cu(II) | CuSO4 | NaCl | |||
|---|---|---|---|---|---|---|
| KCPS | wt% | KCPS | wt% | KCPS | wt% | |
| CO2 | 1.5 | 74.8 | 0.2 | 13.3 | ||
| Fe2O3 | 1118.9 | 21.7 | 0.6 | 0.0174 | ||
| CuO | 19.5 | 1.45 | 563.9 | 41.2 | ||
| SiO2 | 2.8 | 0.796 | 0.1 | 0.0403 | ||
| Na2O | 0.9 | 0.573 | 0.2 | 0.218 | ||
| CaO | 4.3 | 0.190 | 0.2 | 0.00479 | ||
| I | 2.0 | 0.0799 | 1.2 | 0.145 | ||
| Cl | 1.0 | 0.0681 | 516.5 | 62 | ||
| Sb2O3 | 1.7 | 0.0606 | 0.7 | 0.0746 | ||
| Al2O3 | 0.2 | 0.0565 | ||||
| SO3 | 0.4 | 0.0523 | 184.8 | 43.7 | ||
| MnO | 2.2 | 0.0482 | ||||
| MgO | 0.2 | 0.0432 | 2.2 | 1.02 | ||
| SnO2 | 1.2 | 0.0340 | 0.7 | 0.0546 | ||
| Re | 0.7 | 0.0333 | 0.5 | 0.0585 | ||
| CoO | 2.2 | 0.0285 | ||||
| Cr2O3 | 0.4 | 0.0100 | ||||
| Pd | 0.1 | 0.00860 | ||||
| TiO2 | 0.3 | 0.00860 | ||||
| Rh | 0.1 | 0.00747 | ||||
| K2O | 0.2 | 0.00734 | ||||
| SrO | 0.5 | 0.00340 | ||||
| Ho2O3 | 0.4 | 0.0446 | ||||
| HfO2 | 1.8 | 0.0381 | ||||
| P2O5 | 0.1 | 0.0346 | ||||
| Rh | 0.1 | 0.0213 | ||||
| Total | 100 | 100 | ||||
And so, existence of Cu and Cl in catalyst was confirmed by EDS analysis data (Fig. 5).
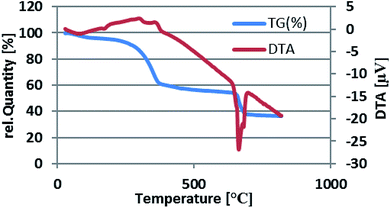
The thermal stability of Fe3O4@nano-cellulose/Cu(II) was investigated by thermo-gravimetric analysis (TGA) in the temperature range of 30–800 °C (Fig. 6).
The TGA curve illustrates four mass-loss steps. Firstly, a very small weight loss (2.53%) from 50 to 100 °C is corresponded to remove of catalyst moisture. Subsequently, the main weight loss step in the temperature ranges 200–370 °C (33%) is attributed to the decomposition of cellulose units through the formation of levoglucosan and other volatile compounds. Finally, there are two weight loss steps in the temperature ranges 400–600 and 650–690 °C (5 and 16%, respectively). According to the TG–DTA diagram of Fe3O4@nano-cellulose/Cu(II), it was revealed that this catalyst is suitable for the promotion of organic reactions below 200 °C.
Catalyst efficiency for synthesis of 4H-pyrimido[2,1-b]benzothiazole derivatives
After characterization of Fe3O4@nano-cellulose/Cu(II), the activity of catalyst was evaluated for the synthesis of 4H-pyrimido[2,1-b]benzothiazole derivatives.For optimization of the reaction conditions, the reaction of 2-aminobenzothiazole, 4-nitrobenzaldehyde and ethyl acetoacetate as a model reaction was investigated (Table 3). As shown in Table 3, entry 14, it was found that 0.03 g of Fe3O4@nano-cellulose/Cu(II) under solvent-free condition at 80 °C is the best reaction condition. In order to compare the efficiency of present nano-catalyst with other catalysts, the model reaction was also performed using the reported catalysts for the synthesis of 4H-pyrimido[2,1-b]benzothiazole derivatives. As Table 4 indicates, in comparison with other reported catalysts, we have found that Fe3O4@nano-cellulose/Cu(II) promoted reaction has shorter reaction time, higher yields of products, green reaction conditions and simpler workup. Finally, the above optimized reaction conditions were explored for the synthesis of 4H-pyrimido[2,1-b]benzothiazole derivatives and the results are summarized in Table 5. The reusability of the catalyst was also investigated on the model reaction. The magnetic nature of the catalyst allowed its facile recovery by simple separation by an external magnet, washing with ethanol and drying at room temperature to provide an opportunity for recycling experiments. The separated nano-catalyst was reused in the above-mentioned reaction for the synthesis of IVb for four times without considerable loss of its catalytic activity (Table 3). Partial loss of activity may be due to blockage of catalyst active sites and/or partial leaching of Cu from the catalyst.
| Entry | Solvent | Catalyst (g) | Condition | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
a The amount ratio of 2-aminobenzothiazole (mmol), 4-nitrobenzaldehyde (mmol) and ethyl acetoacetate (mmol) are equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.b Isolated yield.c Fe3O4@ nano-cellulose/Cu(II). 1.b Isolated yield.c Fe3O4@ nano-cellulose/Cu(II). |
|||||
| 1 | — | — | 80 °C | 7 h | 30 |
| 2 | — | CuCl2 | 80 °C | 3h | 69 |
| 3 | — | Fe3O4 | 70 °C | 3h | 37 |
| 4 | — | Fe3O4@nano-cellulose | 80 °C | 4 h | 41 |
| 5 | C2H5OH | — | R. T | 7 h | — |
| 6 | C2H5OH | Catalyst (0.04)c | R. T | 3 h | 35 |
| 7 | C2H5OH | Catalyst (0.04)c | Reflux | 3 h | 57 |
| 8 | H2O | Catalyst (0.04)c | Reflux | 3 h | 42 |
| 9 | CH3OH | Catalyst (0.04)c | Reflux | 3 h | 51 |
| 10 | — | Catalyst (0.04)c | R. T | 3 h | 43 |
| 11 | — | Catalyst (0.04)c | 70 °C | 1 h | 85 |
| 12 | — | Catalyst (0.05)c | 80 °C | 0.5 | 93 |
| 13 | — | Catalyst (0.04)c | 80 °C | 0.5 | 97 |
| 14 | — | Catalyst (0.03)c | 80 °C | 0.5 | 97 |
| 15 | — | Catalyst (0.02)c | 80 °C | 0.5 | 84 |
| 16 | — | Catalyst (0.03), 2thrunc | 80 °C | 0.5 | 93 |
| 17 | — | Catalyst (0.03), 3rdrunc | 80 °C | 0.5 | 88 |
| 18 | — | Catalyst (0.03), 4thrunc | 80 °C | 0.5 | 83 |
| Ent. | Solvent | Catal. | Tem. (°C) | Time (h) | Yielda (%) | Ref. |
|---|---|---|---|---|---|---|
| a Isolated yield.b Tetrabutylammonium hydrogen sulfate.c 1,1,3,3-N,N,N′,N′-Tetramethylguanidinium trifluoroacetate. | ||||||
| 1 | CH3OH | Acetic acid (20 mol%) | 65 | 18 | 62 | 20 |
| 2 | EG | TBAHS (30 mol%)b | 120 | 2 | 72 | 17 |
| 3 | HOAc | Chitosan (0.080 g) | 70 | 1.6 | 93 | 18 |
| 4 | — | TMGT (0.080 g)c | 100 | 5 | 53 | 16 |
| 5 | — | AlCl3 (10 mol%) | 65 | 1.2 | 97 | 22 |
| 6 | — | Fe3O4@NCs/TiCl (0.03 g) | 70 | 0.6 | 96 | 23 |
| 7 | — | Fe3O4@ nano-cellulose/Cu(II) (0.03 g) | 80 | 0.5 | 97 | This work |
| Ent. | R | Prod. | Time (min) | Yieldb (%) | M. P. | Ref. | |
|---|---|---|---|---|---|---|---|
| Found | Report | ||||||
a I (mmol)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) II (mmol) II (mmol)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) III (mmol) III (mmol)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3O4@ nano-cellulose/Cu(II) (g) is equal to 1 Fe3O4@ nano-cellulose/Cu(II) (g) is equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03.b Isolated yield. 0.03.b Isolated yield. |
|||||||
| 1 | H– | IVa | 45 | 84 | 178–180 | 177–179 | 17 |
| 2 | 4-NO2– | IVb | 30 | 97 | 171–173 | 170–172 | 22 |
| 3 | 4-Cl– | IVc | 30 | 95 | 87–89 | 86–88 | 21 |
| 4 | 4-Br– | IVd | 30 | 97 | 110–114 | 110–114 | 16 |
| 5 | 4-OH– | IVe | 60 | 82 | 210–212 | 210–212 | 22 |
| 6 | 2-NO2– | IVf | 45 | 88 | 122–125 | 122–125 | 23 |
| 7 | 2-Cl– | IVg | 40 | 87 | 124–126 | 125–127 | 17 |
| 8 | 2-EtO– | IVh | 60 | 75 | 171–175 | 171–175 | 23 |
| 9 | 3-NO2– | IVi | 35 | 93 | 222–224 | 222–224 | 21 |
| 10 | 3-OH– | IVj | 65 | 79 | 260–263 | 260–263 | 23 |
| 11 | 2,4-(Cl)2– | IVk | 45 | 85 | 133–135 | 133–135 | 17 |
| 12 | 2,4-(MeO)2– | IVl | 75 | 74 | 164–166 | 164–166 | 23 |
| 13 | 3,4-(OH)2– | IVm | 70 | 71 | 225–227 | 225–227 | 23 |
Substituents on the aldehyde showed a significant effect in terms of the yield and reaction time under the optimized reaction conditions. The electron-withdrawing groups increase rate and yields of reaction compared to electron-donating groups. Suggested mechanism for the synthesis of 4H-pyrimido[2,1-b]benzothiazole (IV) in presence of Fe3O4@ nano-cellulose/Cu(II) was shown in Scheme 2. Cu(II) activate the carbonyl group of benzaldehyde (II) for Knoevenagel reaction with β-ketoesters (III) to production of intermediate (I). Meanwhile, Cu(II) activate the carbonyl group in intermediate (I) for Michael addition with 2-aminobenzothiazole and then interamolecular cyclization to production of product (IV).
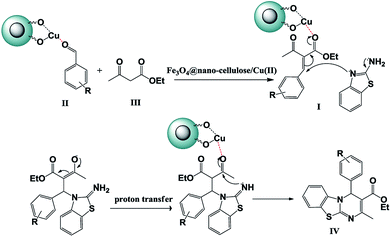 | ||
| Scheme 2 Proposed mechanism for the synthesis of 4H-pyrimido [2,1-b]benzothiazole derivatives IVa–m. | ||
The structures of the products IVa–m were studied by their melting point, IR and 1H NMR spectra. In the FTIR spectra of products, the ester C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration band is appeared at 1690 cm−1 due to conjugation.
O stretching vibration band is appeared at 1690 cm−1 due to conjugation.
Conclusions
We have demonstrated the preparation and characterization of Fe3O4@ nano-cellulose/Cu(II) as a novel magnetite recoverable, eco-friendly, inexpensive and efficient nanocatalyst. The catalytic activity of the prepared catalyst was investigated in the synthesis of 4H-pyrimido[2,1-b] benzothiazole derivatives through one-pot three-component reaction of aldehydes, ethyl acetoacetate, and 2-aminobenzothiazole under solvent-free condition at 80 °C. This protocol includes some important advantages such as mild reaction conditions, short reaction time, excellent yields, easy work-up, high purity of products. And so, magnetic separation and reusability of nanocatalyst is other advantages of this protocol.Experimental
General remarks
All compounds were purchased from Aldrich, Merck, and Fluka chemical companies. Nano-cellulose and Fe3O4@nano-cellulose were synthesized via our previously reported methods.23 FT-IR spectra were run on a Bruker, Equinox 55 spectrometer. A Bruker (DRX-400 Avance) NMR was used to record the 1H NMR and 13C NMR spectra. The X-ray diffraction (XRD) pattern was obtained by a Philips Xpert MPD diffractometer equipped with a Cu Kα anode (k = 1.54 A°) in the 2θ range from 10 to 80°. XRF analysis was done with Bruker, S4 Explorer instrument. VSM measurements were performed by using a vibrating sample magnetometer (Meghnatis Daghigh Kavir Co. Kashan, Iran). Melting points were determined by a Buchi melting point B-540 B.V.CHI apparatus. Field emission scanning electron microscopy (FESEM) image was obtained on a Mira 3-XMU. Transmission electron microscopy (TEM) image was obtained using a Philips CM120 with a LaB6 cathode and accelerating voltage of 120 kV. energy-dispersive X-ray spectroscopy (EDS) of Fe3O4@nano-cellulose/Cu(II) was measured by an EDS instrument and Phenom pro X. Thermal gravimetric analysis (TGA) was conducted using “STA 504” instrument.Preparation of Fe3O4@nano-cellulose/Cu(II)
In a flask containing 50 ml of 0.5 M NaOH, Fe3O4@nano-cellulose (0.5 g) was added with stirring. Then, 75 ml of CuCl2 aqueous solution, 0.04 M, was added. A dark blue solution was obtained immediately that was stirred at room temperature. After 6 h, the magnetically heterogeneous catalyst, Fe3O4@nano-cellulose/Cu(II), removed from solution by an external magnet. The catalyst washed with ethanol and water two times and dried at an oven at 80 °C.General procedure for synthesis of 4H-pyrimido[2,1-b]benzothiazole derivatives
A mixture of 2-aminobenzothiazole (1 mmol), aldehyde (1 mmol), ethyl acetoacetate (1 mmol) and Fe3O4@nano-cellulose/Cu(II) (0.03 g) was heated at 80 °C. After completion of the reaction (monitored by TLC), the reaction mixture was dissolved in hot ethanol (3 ml) and the catalyst was separated by using an external magnet. Subsequently by adding water to the decanted solution, the product was appeared as a pure solid in high yields. The recovered catalyst was washed 3 times with ethanol, dried and reused for subsequent runs under the same reaction conditions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Research Council of Yazd University is gratefully acknowledged for the financial support for this work.Notes and references
- G. P. Ellis, Chemistry of heterocyclic compounds: synthesis of fused heterocycles, Wiley, New York, vol. 47, 2009 Search PubMed.
- A. D. Borthwick, D. E. Davies, P. F. Ertl, A. M. Exall, T. M. Haley, G. J. Hart, D. L. Jackson, N. R. Parry, A. Patikis, N. Trivedi, G. G. Weingarten and J. M. Woolven, J. Med. Chem., 2003, 46, 4428–4449 CrossRef CAS.
- M. A. El-Sherbeny, Arzneim.-Forsch./Drug Res., 2000, 50, 848–853 CAS.
- A. M. Youssef and E. Noaman, Arzneim.-Forsch./Drug Res., 2007, 57, 547–553 CAS.
- A. Y. Hassan, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 2856–2869 CrossRef CAS.
- M. T. Gabr, N. S. El-Gohary, E. R. El-Bendary and M. M. El-Kerdawy, Eur. J. Med. Chem., 2014, 85, 576–592 CrossRef CAS.
- V. K. Deshmukh, P. Raviprasad, P. A. Kulkarni and S. V. Kuberkar, Int. J. Chem. Tech. Res., 2011, 3, 136–142 CAS.
- D. V. Kashinath, Y. M. Rajmani and C. S. Ravindra, J. Pharm. Res., 2013, 6, 574–578 Search PubMed.
- A. Bartovič, D. Ilavský, O. Šimo, L. Zalibera, A. Belicová and M. Seman, Collect. Czech. Chem. Commun., 1995, 60, 583–593 CrossRef.
- K. R. Lanjewar, A. M. Rahatgaonkar, M. S. Chorghade and B. D. Saraf, Indian. J. Chem., Sec. B, 2009, 48, 1732–1737 Search PubMed.
- P. K. Sahu, P. K. Sahu, S. K. Gupta, D. Thavaselvamd and D. D. Agarwal, Eur. J. Med. Chem., 2012, 54, 366–378 CrossRef CAS PubMed.
- M. M. M. Gineinah, Sci. Pharm., 2001, 69, 53–61 CAS.
- I. Ćaleta, M. Grdiša, D. Mrvoš-Sermek, M. Cetina, V. Tralić-Kulenović, K. Pavelić and G. Karminski-Zamola, Il Farmaco, 2004, 59, 297–305 CrossRef.
- S. Maddila, S. Gorle, N. Seshadri, P. Lavanya and S. B. Jonnalagadda, Arabian J. Chem., 2016, 9, 681–687 CrossRef CAS.
- A. A. Pavlenko, K. S. Shikhaliev, A. Y. Potapov and D. V. Krylsky, Chem. Heterocycl. Compd., 2005, 41, 796–797 CrossRef.
- A. Shaabani, A. Rahmati and S. Naderi, Bioorg. Med. Chem. Lett., 2005, 15, 5553–5557 CrossRef CAS PubMed.
- L. Nagarapu, H. K. Gaikwad, J. D. Palem, R. Venkatesh, R. Bantu and B. Sridhar, Synth. Commun., 2013, 43, 93–104 CrossRef CAS.
- P. K. Sahu, P. K. Sahu, S. K. Gupta and D. D. Agarwal, Ind. Eng. Chem. Res., 2014, 53, 2085–2091 CrossRef CAS.
- A. B. Atar, Y. S. Jeong and Y. T. Jeong, Tetrahedron, 2014, 70, 5207–5213 CrossRef CAS.
- P. K. Sahu, P. K. Sahu, Y. Sharma and D. D. Agarwal, J. Heterocycl. Chem., 2014, 51, 1193–1198 CrossRef CAS.
- M. M. Heravi, E. Hashemi, Y. S. Beheshtiha, K. Kamjou, M. Toolabi and N. Hosseintash, J. Mol. Catal. A: Chem., 2014, 392, 173–202 CrossRef CAS.
- P. K. Sahu, P. K. Sahu, J. Lal, D. Thavaselvam and D. D. Agarwal, Med. Chem. Res., 2012, 21, 3826–3834 CrossRef CAS.
- S. Azad and B. B. F. Mirjalili, RSC Adv., 2016, 6, 96928–96934 RSC.
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed.
- A. Shaabani and A. Maleki, Appl. Catal., A, 2007, 331, 149–151 CrossRef CAS.
- Y. Lu, X. Lu, B. T. Mayers, T. Herricks and Y. Xia, J. Solid State Chem., 2008, 181, 1530–1538 CrossRef CAS.
- H. F. Rase, Handbook of Commercial Catalysts: Heterogeneous Catalysts, CRC Press, New York, 2000 Search PubMed.
- A. El Harrak, G. Carrot, J. Oberdisse, C. Eychenne-Baron and F. Boué, Macromolecules, 2004, 37, 6376–6384 CrossRef CAS.
- P. Tartaj and C. J. Serna, J. Am. Chem. Soc., 2003, 125, 15754–15755 CrossRef CAS PubMed.
- Z. Zhang, H. Duan, S. Li and Y. Lin, Langmuir, 2010, 26, 6676–6680 CrossRef CAS PubMed.
- B. Rác, A. Molnar, P. Forgo, M. Mohai and I. Bertóti, J. Mol. Catal. A: Chem., 2006, 244, 46–57 CrossRef.
- Y. Shen, J. Tang, Z. Nie, Y. Wang, Y. Ren and L. Zuo, Sep. Purif. Technol., 2009, 68, 312–319 CrossRef CAS.
- B. Movassagh, A. Takallou and A. Mobaraki, J. Mol. Catal. A: Chem., 2015, 401, 55–76 CrossRef CAS.
- T. Zeng, W. W. Chen, C. M. Cirtiu, A. Moores, G. Song and C. J. Li, Green Chem., 2010, 12, 570–573 RSC.
- M. A. Zolfigol, A. R. Moosavi-Zare, P. Moosavi, V. Khakyzadeh and A. Zare, C. R. Chim., 2013, 16, 962–966 CrossRef CAS.
- M. A. Zolfigol, V. Khakyzadeh, A. R. Moosavi-Zare, A. Rostami, A. Zare, N. Iranpoor, M. H. Beyzavi and R. Luque, Green Chem., 2013, 15, 2132–2151 RSC.
- A. Khazaei, F. Gholami, V. Khakyzadeh, A. R. Moosavi-Zare and J. Afsar, RSC Adv., 2015, 5, 14305–14310 RSC.
- A. Khazaei, A. R. Moosavi-Zare, F. Gholami and V. Khakyzadeh, Appl. Organomet. Chem., 2016, 30, 691–694 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra09203f |
| This journal is © The Royal Society of Chemistry 2019 |