Open Access Article
Open Access ArticleAdsorption capacity of kelp-like electrospun nanofibers immobilized with bayberry tannin for uranium(VI) extraction from seawater†
Jie Mengab,
Xiaoyan Lin *ab,
Haonan Lia,
Yida Zhanga,
Jian Zhou
*ab,
Haonan Lia,
Yida Zhanga,
Jian Zhou b,
Yan Chenab,
Ran Shangc and
Xuegang Luob
b,
Yan Chenab,
Ran Shangc and
Xuegang Luob
aSchool of Materials Science and Engineering, Southwest University of Science and Technology, Mianyang 621010, Sichuan, China. E-mail: lxy20100205@163.com
bEngineering Research Center of Biomass Materials, Ministry of Education, Mianyang 621010, Sichuan, China
cState Key Laboratory of NBC Protection for Civilian, Beijing 102205, China
First published on 12th March 2019
Abstract
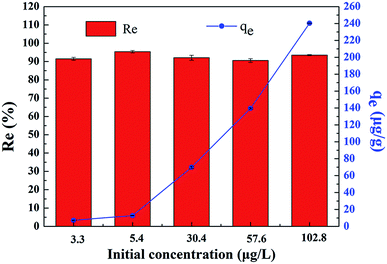
The extraction of uranium(VI) from seawater is of paramount interest to meet the rapid expansion of global energy needs. A novel gelatin/PVA composite nanofiber band loaded with bayberry tannin (GPNB-BT) was used to extract uranium(VI) from simulated seawater in this study. It was fabricated by electrostatic spinning and crosslinking, and characterized by SEM, EDX, FTIR, and XPS. Batch experiments were carried out to investigate the adsorption of uranium(VI) onto GPNB-BT. Simultaneously, the regeneration–reuse process of the GPNB-BT was determined and illustrated here. The GPNB-BT exhibited excellent adsorption and regeneration performance, and a maximum adsorption capacity of 254.8 mg g−1 toward uranium(VI) was obtained at 298.15 K, pH = 5.5. Further, the regeneration rate for uranium did not decrease significantly after five cycles. Moreover, even at an extremely low initial concentration of 3 μg L−1 in the simulated seawater for 24 h, GPNB-BT showed an ultrahigh adsorption rate of more than 90% and adsorption capacity of 7.2 μg g−1 for uranium. The high density of adjacent phenolic hydroxyl groups and the specific surface area of GPNB-BT improved the adsorption ability of GPNB-BT for uranium. Therefore, the GPNB-BT was shown to have an application prospect for the effective extraction of uranium(VI) from seawater.
1. Introduction
Uranium(VI), which plays an important role in the development of the nuclear industry, mainly exists in two forms in nature: deposits in terrestrial ores and in seawater.1,2 Uranium resources from terrestrial ores are limited, so it is desired to extract uranium from seawater, in which the quantity of uranium is around 4.5 billion tons.3 To date, lots of technologies have emerged for the extraction of uranium, such as solvent extraction,4 chemical precipitation,5 ion-exchange,6 and adsorption.7,8 Among these methods, adsorption is considered to be an effective and economic way forward to extract uranium from seawater.9 It is reported that various minerals,10 phosphates,11 poly-resins,12 and microorganisms13 have been used as adsorbents for the recovery of uranium(VI) from wastewater. However, the adsorption capacity of adsorbents to uranium still needs to be improved.It has been reported that plant tannins exhibit specific affinity toward many metal ions.14 Therefore, it may be expected that tannins could be used for the purpose of uranium recovery from aqueous solutions. Bayberry tannin (BT) is a kind of cheap and ubiquitous natural biomass, which has lots of phenolic hydroxyl groups to form a chelate with metal ions. However, tannins cannot be used as an adsorbent directly for the adsorption of uranium because they dissolve easily in solution. To overcome this disadvantage, many techniques have been made to immobilize tannins onto various water-insoluble materials, such as cellulose,15 viscose rayon fiber,16 agarose,17 and other materials containing amino groups, such as albumin, chitosan, and collagen. Collagen has numerous functional groups, such as –NH2, –COOH, and –OH, which can react with uranium.18 However, the small specific surface area and few active sites on collagen limit the amount of tannins immobilized onto collagen and the adsorption capacity for uranium.
The electrostatic spinning and crosslinking of collagen and BT is an effective method to improve the specific surface area and active sites of collagen. A collagen nanofiber membrane with a porous network can been produced by electrospinning, wherein an electric field is used to control the formation and deposition of nanofibers. These are produced with a large surface area and high porosity.19 Gelatin is the main product of collagen hydrolysis, including –NH2, –COOH, and –OH, which can react with tannins well.20 However, the yield of electrospun gelatin fiber is generally low and the mechanical strength of gelatin fiber is poor. Poly vinyl alcohol (PVA) is typically blended with gelatin to increase the mechanical strength and stability of the fibers in the water,21 which can also be an adsorbent with active groups to adsorb uranium(VI). Therefore, it would be promising to produce a novel nanofiber membrane that combines the phenolic hydroxyls of BT and the high specific surface area and porosity of the gelatin/PVA nanofiber band (GPNB).
For all the above reasons, in the present study, such a GPNB-BT was synthesized and assembled from gelatin, PVA, and bayberry tannin by electrospinning, and then crosslinked by glutaraldehyde, which has a strong mechanical strength and hydrophilic property. The effects of pH, dosage, contact time, initial uranium concentrations, temperature, and co-existing anions on the extraction of uranium were studied. The adsorption isotherm and adsorption kinetics were also investigated to understand the adsorption process in detail. Furthermore, the adsorbent before and after adsorption was characterized and the mechanism of the adsorption process of uranium was deduced. It is expected that such a GPNB-BT with a high surface area and porosity and a large amount of tannins immobilized could be widely used for the extraction of uranium from seawater in the future.
2. Materials and methods
2.1. Materials
Gelatin from porcine skin (Type A, Bloom 250) and poly (vinyl alcohol) (PVA, Mw 44.05, 98.0–99.0 mol% alcoholysis degree) were purchased from Aladdin. BT was supplied by Baise Forest Chemical Plant (Guangxi, China) produced from the barks of myrica esculenta by extraction with an acetone–water solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and the tannin content was about 77%. Glacial acetic acid and glutaraldehyde used in the experiment were of analytical grade, and were purchased from Kelong Co. Ltd without further purification. Uranium nitrate hexahydrate (UO2(NO3)2·6H2O) was obtained from HuBei Chushengwei Chemistry Co., Ltd. The uranium stock solution was prepared by dissolving (UO2(NO3)2·6H2O) in ultrapure water.
1, v/v) and the tannin content was about 77%. Glacial acetic acid and glutaraldehyde used in the experiment were of analytical grade, and were purchased from Kelong Co. Ltd without further purification. Uranium nitrate hexahydrate (UO2(NO3)2·6H2O) was obtained from HuBei Chushengwei Chemistry Co., Ltd. The uranium stock solution was prepared by dissolving (UO2(NO3)2·6H2O) in ultrapure water.
2.2. Synthesis of GPNB-BT
The PVA was dissolved in ultrapure water for 2 h at 90 °C to prepare an 8% (m m−1) of PVA sol. Meanwhile, the gelatin was dissolved in glacial acetic acid and heated at 60 °C for 1 h to prepare an 18% (m m−1) gelation sol. Then, the PVA and gelatin sol were mixed at a solution mass ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for 12 h to obtain a spinning solution, which was loaded in a 10 mL syringe and electrospun at a flow rate of 0.3 mL h−1. The voltage between the needle and the collector was 20 kV. The temperature of electrospinning was 35 °C. The nanofiber membrane was collected, dried in a vacuum oven overnight at 60 °C, and then cut into pieces of 2 × 6 cm. The kelp-like nanofiber band, named as GPNB, was finally obtained.
4 for 12 h to obtain a spinning solution, which was loaded in a 10 mL syringe and electrospun at a flow rate of 0.3 mL h−1. The voltage between the needle and the collector was 20 kV. The temperature of electrospinning was 35 °C. The nanofiber membrane was collected, dried in a vacuum oven overnight at 60 °C, and then cut into pieces of 2 × 6 cm. The kelp-like nanofiber band, named as GPNB, was finally obtained.
Meanwhile, 0.3 g of BT was dissolved in 100 mL of ultrapure water completely and mixed with 0.3 g of GPNB at 25 °C for 4 h. After that, the GPNB soaked in tannins was filtered and put into 100 mL of ultrapure water. At the same time, 1.2 mL glutaraldehyde was added into the solution and reacted at 50 °C for 4 h. Subsequently, the product was collected by filtration, thoroughly washed with ultrapure water, and dried in a vacuum oven at 60 °C for 12 h, and finally the GPNB-BT was obtained.
2.3. Batch adsorption experiments
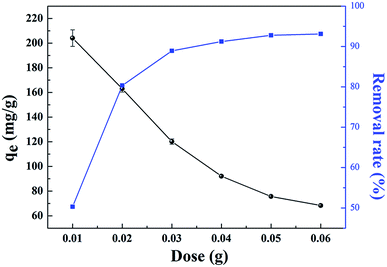
The effects of the solution pH, contact time, initial uranium concentrations, adsorbent dose, and temperature on the removal of uranium(VI) were studied. The adsorption experiments was carried out in a 100 mL conical flask. Typically, 0.02 g of GPNB-BT was added to 50 mL of the uranium(VI) solution with an initial concentration of 80 mg L−1. The pH was adjusted to the desired value with 1 M HCl and 1 M NaOH. All the conical flasks were kept in a thermostated shaker for a specified time, and the experiments were repeated three times. The mixed solutions were filtered after the adsorption of uranium and the concentration of uranium was detected at 650 nm using a U-3900 ultraviolet spectrophotometer (Hitachi U-3900). The equilibrium adsorption capacity (qe) and removal efficiency (Re) of uranium were calculated by the following equations:
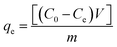 | (1) |
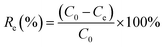 | (2) |
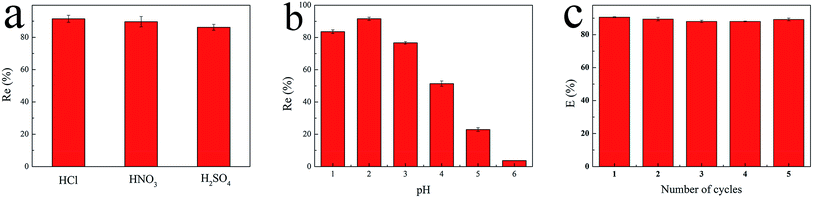
2.4. Regeneration studies
In order to evaluate the reusability of GPNB-BT, the GPNB-BT after adsorption of uranium was regenerated by treating it with 50 mL of HCl solution at pH = 1 to 6 for 24 h. Then, the regenerated GPNB-BT adsorbed uranium. This regeneration–reuse process was repeated for five cycles.The regeneration rate (E) was calculated by the following equation:
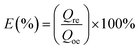 | (3) |
2.5. Characterization of GPNB-BT
The surface morphology of the GPNB-BT was characterized by scanning electron microscopy (SEM, Ultra 55, Zesis Corporation). Then energy dispersive spectrometry (EDX) was employed to understand the elemental composition before and after adsorption. Fourier transform infrared spectrometry (FTIR, Nicolet-6700, Perkin Elmer Instruments Corporation) was used to analyze the molecular structure. X-ray photoelectron spectroscopy (XPS, Thermo Scientific Escalab 250, Thermo Fisher Corporation) was used to detect the binding energies of the GPNB-BT before and after adsorption. The water contact angle was also measured on a dedicated instrument (K100, Germany Klux Corporation).3. Results and discussion
3.1. Adsorption behavior of the GPNB-BT toward uranium(VI)
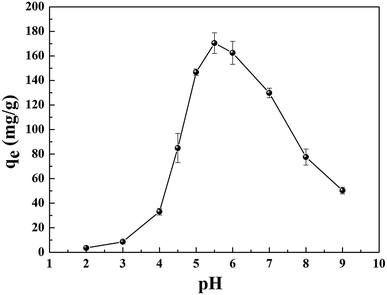
The reasons for this phenomenon was as follows: the concentration of H+ in the solution is high at low pH, which leads more H+ to occupy the active site on the adsorbent. At the same time, the uranyl ion is prevented from approaching the active site of the adsorbent by the electrostatic repulsion of H+.22 The lower the pH, the greater the resistance between H+ from the solution and the uranyl ions. At pH < 5.0, the important polynuclear hydroxo–uranyl complex cations exist as (UO2)2(OH)22+, (UO2)4(OH)7+, (UO2)4(OH)62+, (UO2)4(OH)26+, and uranium mainly takes on the forms of (UO2)3(OH)5+ in the pH region 5.0–6.0.23 Therefore, the adsorption capacity is enhanced due to the degree of protonation being reduced. When the pH exceeds 6.0, the uranyl ion is easily hydrolyzed and it is difficult to bind uranium on GPNB-BT because of the U(VI)-carbonate species (UO2)2(CO3)(OH)3− from pH 6.0 to 9.0.24,25 This indicates that the adsorbent has the maximum capacity at pH 5.5, which was the pH thus used in rest of the further studies.
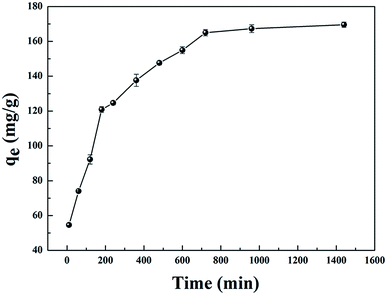 | ||
| Fig. 3 Effect of contact time on the adsorption capacity of GPNB (initial uranium concentration: 80 mg L−1, adsorbent dose: 0.02 g, pH: 5.5, volume of solution: 50 mL, temperature: 298.15 K). | ||
In order to explain the behavior of uranium adsorption by GPNB-BT, the pseudo-first-order kinetic and pseudo-second-order kinetic models were used to investigate the adsorption mechanism.
The pseudo-first-order kinetics of linear and non-linear equations are represented as follows:27
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − K1t (linear) qe − K1t (linear)
| (4) |
| qt = qe(1 − e−K1t) (non-linear) | (5) |
The pseudo-second-order kinetics of linear and non-linear equations are represented as follows:27
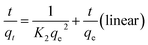 | (6) |
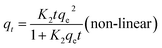 | (7) |
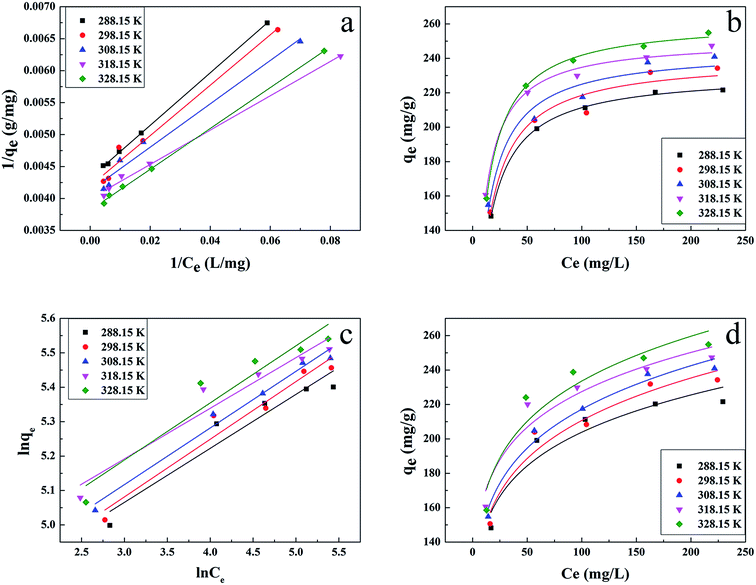
The results are shown in Fig. 4 and Table 1. Compared with other kinetic models, the value of R2 (0.997) for the linear pseudo-second-order fitted best. In addition, the calculated equilibrium adsorption capacity (187.6 mg g−1) from the linear pseudo-second-order was close to the actual experiment adsorption capacity (170.1 mg g−1) shown in Fig. 3. These results indicated that the adsorption of uranium obeys the linear pseudo-second-order model, which suggested that the rate-controlling step of the adsorption process may be the chemical adsorption.28
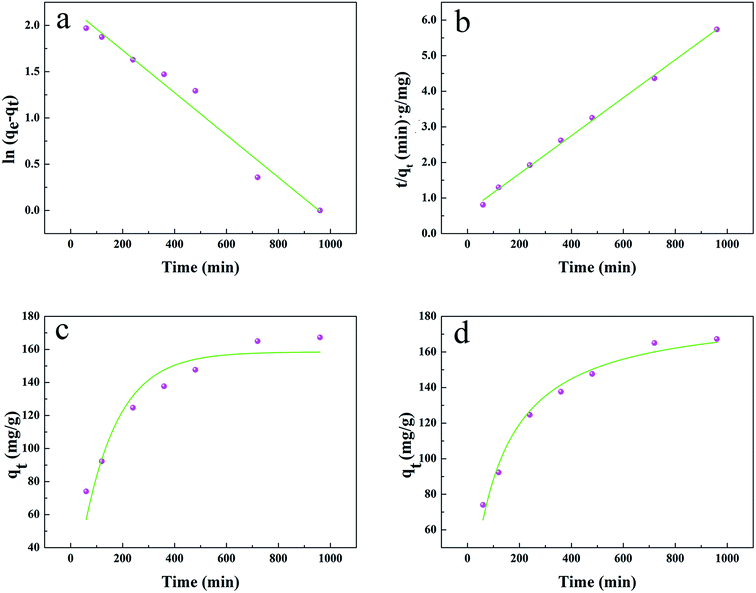 | ||
| Fig. 4 (a) and (b) Linear plots of the pseudo-first-order kinetics and pseudo-second-order kinetics, (c) and (d) non-linear plots of the pseudo-first-order kinetics and pseudo-second-order kinetics. | ||
| Type | Pseudo-first-order kinetic | Pseudo-second-order kinetic | ||||
|---|---|---|---|---|---|---|
| qe (mg g−1) | K1 (min−1) | R2 | qe (mg g−1) | K2 (g (mg min)−1) | R2 | |
| Linear model | 8.935 | 0.002 | 0.967 | 187.617 | 2.841 × 10−5 | 0.997 |
| Non-linear model | 158.487 | 0.007 | 0.903 | 184.131 | 5.009 × 10−5 | 0.977 |
In order to get a better understanding of the uranium adsorption mechanism and to quantify the adsorption data, Langmuir and Freundlich models were used to fit the experimental data.
The Langmuir isotherm is based on the assumption that monolayer adsorption occurs on a uniform surface and the adsorbed layer will be one molecule thick. Furthermore, it is assumed that all the adsorption sites have equal affinities for molecules of the adsorbate.30
Linear and non-linear Langmuir equations are expressed by the following:
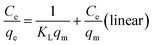 | (8) |
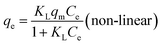 | (9) |
The Freundlich model considers multilayer adsorption with a heterogeneous energetic distribution of active sites.31
Linear and non-linear Freundlich equations are expressed by the following:
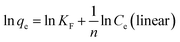 | (10) |
| qe = KFCe1/n (non-linear) | (11) |
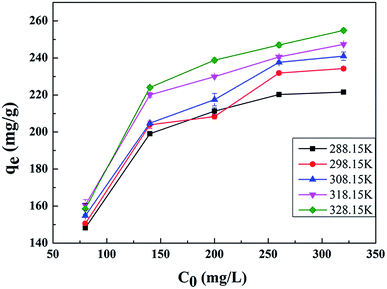
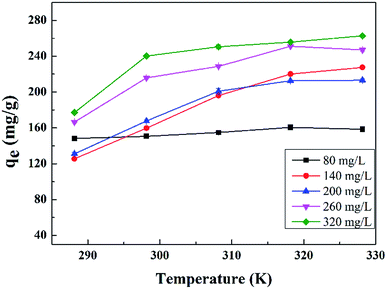
The results are presented in Fig. 6 and Table 2, with the Langmuir and Freundlich isotherm parameters calculated from the intercept and slope of the plots. Clearly, it could be seen that the linear Langmuir model fitted the experimental data well according to the high correlation coefficients (R2), which confirmed that the Langmuir model could properly describe the sorption data. Furthermore, these results suggested a monolayer adsorption happened on the homogeneous surface of the GPNB-BT adsorbent.
| Type | T (K) | Langmuir constant | Freundlich constant | ||||
|---|---|---|---|---|---|---|---|
| KL (L mg−1) | qm (mg g−1) | R2 | n | KF(mg g−1), (L mg−1)1/n | R2 | ||
| Linear model | 288.15 | 0.105 | 231.482 | 0.999 | 6.375 | 98.998 | 0.901 |
| 298.15 | 0.106 | 238.663 | 0.972 | 5.970 | 97.417 | 0.937 | |
| 308.15 | 0.121 | 242.719 | 0.971 | 6.077 | 101.849 | 0.975 | |
| 318.15 | 0.149 | 250.001 | 0.995 | 6.798 | 115.639 | 0.920 | |
| 328.15 | 0.120 | 261.780 | 0.999 | 6.025 | 108.923 | 0.899 | |
| Non-linear model | 288.15 | 0.105 | 231.461 | 0.999 | 6.864 | 104.233 | 0.889 |
| 298.15 | 0.102 | 240.019 | 0.949 | 6.252 | 100.862 | 0.931 | |
| 308.15 | 0.113 | 244.908 | 0.950 | 6.292 | 104.492 | 0.971 | |
| 318.15 | 0.146 | 250.853 | 0.990 | 7.260 | 120.614 | 0.913 | |
| 328.15 | 0.119 | 262.108 | 0.997 | 6.558 | 115.738 | 0.888 | |
The essential characteristics of the Langmuir isotherm can be explained in terms of a dimensionless equilibrium parameter (RL), which can be expressed by the following equation:
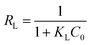 | (12) |
The values of RL indicate the shape of the isotherm: unfavorable adsorption (RL > 1), linear adsorption (RL = 1), favorable adsorption (0 < RL < 1), or irreversible adsorption (RL = 0). The obtained RL values as shown in Table 3 are 0–1, which indicates that the adsorption of uranium from aqueous solutions by GPNB-BT is favorable.
| Temperature (K) | Uranium concentration (mg L−1) | ||||
|---|---|---|---|---|---|
| 80 | 140 | 200 | 260 | 320 | |
| 288.15 | 0.106 | 0.064 | 0.045 | 0.035 | 0.029 |
| 298.15 | 0.105 | 0.063 | 0.045 | 0.035 | 0.029 |
| 308.15 | 0.093 | 0.056 | 0.040 | 0.031 | 0.025 |
| 318.15 | 0.077 | 0.046 | 0.032 | 0.025 | 0.021 |
| 328.15 | 0.095 | 0.056 | 0.040 | 0.031 | 0.025 |
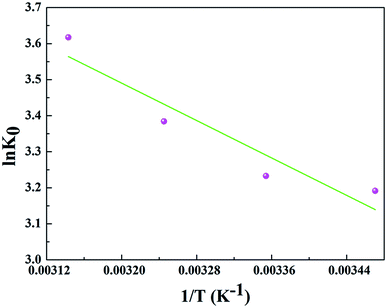
In order to further evaluate the effect of temperature on the adsorption, the thermodynamic parameters for uranium adsorption were calculated by the following equations.32
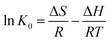 | (13) |
| ΔG = ΔH − TΔS | (14) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 versus 1/T and the results are represented in Table 4 and Fig. 8.
K0 versus 1/T and the results are represented in Table 4 and Fig. 8.
| T (K) | ΔH (kJ mol−1) | ΔS [J (mol K)−1] | ΔG (kJ mol−1) |
|---|---|---|---|
| 288.15 | 10.790 | 63.546 | −7.646 |
| 298.15 | 10.790 | 63.546 | −8.013 |
| 308.15 | 10.790 | 63.546 | −8.670 |
| 318.15 | 10.790 | 63.546 | −9.569 |
The positive value of ΔH (10.790 kJ mol−1) and negative values of ΔG suggested that the adsorption process of uranium on GPNB-BT was endothermic and spontaneous. Furthermore, the positive value of ΔS [63.546 J (mol K)−1] implied the increased randomness at the solid/solution interface during the adsorption of uranium by GPNB-BT. The endothermic adsorption nature might due to the desolvation of the uranyl ions and activation of the surface of GPNB-BT.33
3.2. Characteristics of GPNB-BT
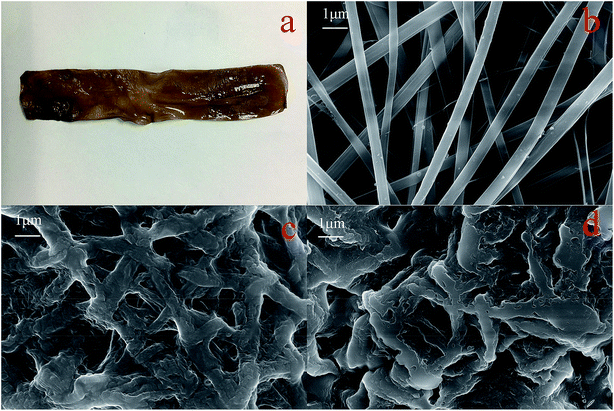 | ||
| Fig. 12 Digital photo of GPNB-BT (a), SEM images of GPNB (b), GPNB-BT before and after the adsorption of uranium (c) and (d). | ||
Contact angle tests were carried out to investigate the hydrophilicity of GPNB-BT. The contact angle of GPNB-BT was 19 ± 1.71° compared to pure PVA (62 ± 1.19°), which revealed the existence of plenty of hydrophilic functional groups in GPNB-BT.41 Therefore, the GPNB-BT exhibits excellent hydrophilic property and has good advantages in the adsorption process.
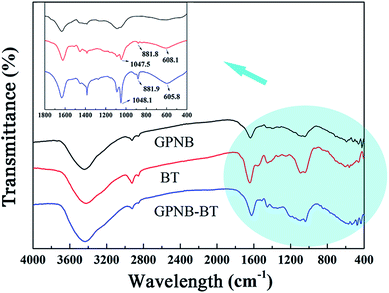
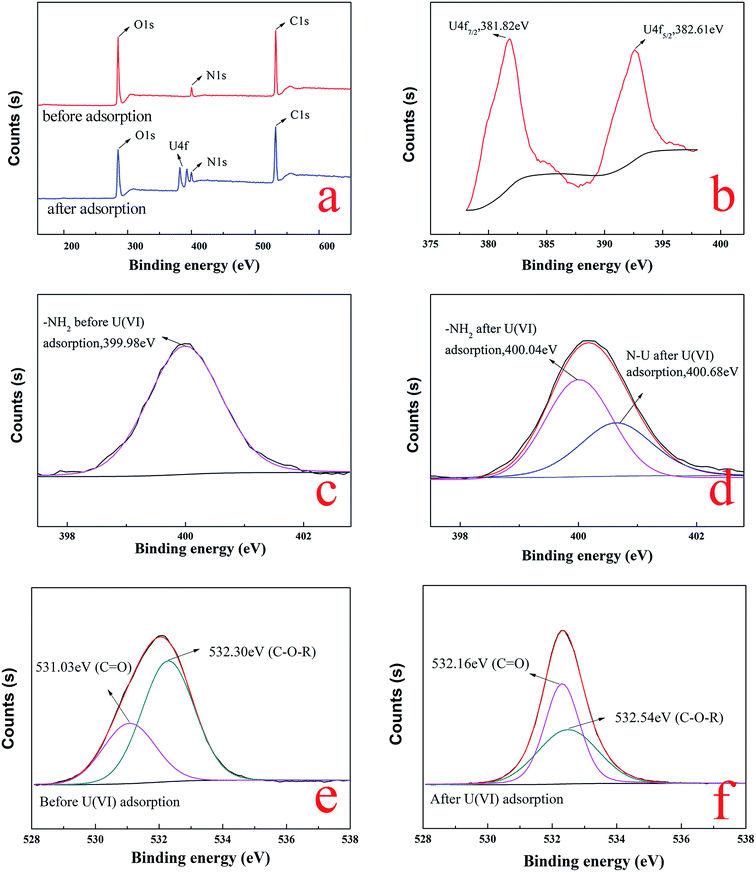
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of carboxyl and C–O–R of methoxyl groups on BT,48,49 respectively. After the adsorption of uranium, the two peaks of C
O of carboxyl and C–O–R of methoxyl groups on BT,48,49 respectively. After the adsorption of uranium, the two peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O–R were significantly shifted to 532.16 eV and 532.54 eV, as shown in Fig. 14f, which illustrated that the carboxyl of gelatin and methoxyl groups of BT were also involved in the adsorption process. The results of the XPS analysis confirmed that not only the BT but also gelatin was involved in uranium adsorption.
O and C–O–R were significantly shifted to 532.16 eV and 532.54 eV, as shown in Fig. 14f, which illustrated that the carboxyl of gelatin and methoxyl groups of BT were also involved in the adsorption process. The results of the XPS analysis confirmed that not only the BT but also gelatin was involved in uranium adsorption.
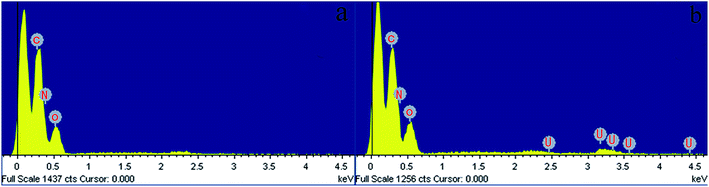 | ||
| Fig. 15 EDX images of (a) GPNB-BT before the adsorption of uranium, (b) GPNB-BT after the adsorption of uranium. | ||
| Element | Weight% | Atomic% |
|---|---|---|
| C | 34.34 | 39.82 |
| N | 37.94 | 37.74 |
| O | 25.63 | 22.32 |
| U | 2.08 | 0.12 |
3.3. Mechanism analysis
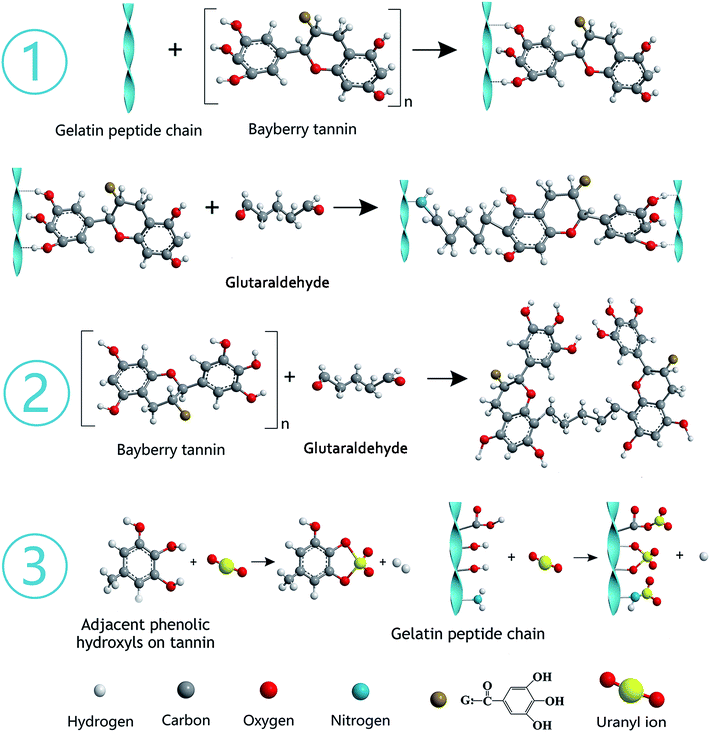
The gelatin was hydrolyzed from collagen, which contains abundant functional groups, such as –OH, –COOH, and –NH2. BT represents polyphenols derived from the barks of bayberry plants and belong to condensed tannins with highly nucleophilic sites (C-6 and C-8 of A ring) in molecules. The synthesis mechanism diagram of GPNB-BT is shown in Fig. 16 ① and ②. The phenolic hydroxyl groups of BT are combined with the peptide chain of gelatin by hydrogen bonding. The phenolic hydroxyl groups of BT and –NH2 on the peptide chain are combined by a covalent bond through a Mannich reaction, when the bifunctional crosslinking agent of glutaraldehyde is added (Fig. 16 ①).50 Furthermore, the tannin molecules can also be crosslinked and agglomerated themselves by glutaraldehyde to become larger, and implanted in the pores of GPNB such that they can hardly be removed from the GPNB fibers (Fig. 16 ②). Meanwhile, it was also proved by experiment that GPNB-BT could really withstand water extraction and no leaked BT was detected in all the adsorption experiments.The reaction mechanism diagram of uranium removal by GPNB-BT is shown in Fig. 16 ③. The uranium can react with –OH, –COOH, and –NH2 of gelatin fiber and the phenolic hydroxyl groups of GPNB-BT. However, compared with functional groups of GPNB, such as –OH, –COOH, and –NH2, the phenolic hydroxyl groups of BT loaded on GPNB will have a higher complexing ability with uranium, due to the fact that a stable five-membered ring can be formed between adjacent phenolic hydroxyl and uranium. Furthermore the porosity and high specific surface area of GPNB-BT, which show good mass-transfer performance, can greatly improve the adsorption ability of the GPNB-BT for uranium, compared with other tannin-immobilized fiber adsorbents.
3.4. Comparison of various adsorbents
There are several advantages and properties of the GPNB-BT, including: (1) GPNB-BT possesses excellent hydrophilic and floating performance to promote the adsorption of uranium; (2) GPNB-BT has superfine nano-fibers and abundant pores, more tannins immobilized, and more active sites to bind uranium than other materials; (3) GPNB-BT is like a kelp in shape and shows an excellent regeneration–reuse performance, which is good for recycling in seawater; (4) the nanometer fiber band avoids the problem that the nanofibers materials tend to agglomerate in aqueous solution. A comparison of the adsorption capacities of different adsorbents for uranium is shown in Table 6. All of these factors suggest that GPNB-BT will have good potential application in the removal of uranium from seawater.4. Conclusions
In this study, GPNB-BT was synthesized by electrostatic spinning and crosslinking and employed to remove uranium from simulated seawater. The performance of GPNB-BT in the removal of uranium(VI) was evaluated. Batch experiments of the GPNB-BT adsorbent revealed that the pH of the solution has a great impact on the adsorption of uranium(VI). The maximum adsorption capacity (254.8 mg g−1) was obtained at a solution pH value of 5.5, adsorbent dosage of 0.02 g, contact time of 12 h, and temperature of 298.15 K. In addition, the adsorption kinetics and isotherms were well fitted by the pseudo-second-order kinetics model and Langmuir isotherm model, respectively. The adsorption process was spontaneous, endothermic, and increased randomness. Furthermore, the GPNB-BT performed with good reusability, stability, and ultrahigh removal efficiency (above 90%) of trace uranium in the simulated seawater. The maximum adsorption capacity of uranium was 7.2 μg g−1 even at an extremely low initial concentration of 3 μg L−1 in the simulated seawater for 24 h.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors want to thank the technology support of Engineering Research Center of Biomass Materials, Ministry of Education, Southwest University of Science and Technology, and State Key Laboratory of NBC Protection for Civilian. This work was sponsored by Longshan academic talent research support plan of Southwest University of Science and Technology (18LZX315).References
- M. J. Monreal and P. L. Diaconescu, Nat. Chem., 2010, 2, 424 CrossRef CAS PubMed.
- S. Das, S. Brown, R. T. Mayes and C. J. Janke, Chem. Eng. J., 2016, 298, 125–135 CrossRef CAS.
- J. Q. Li, L. L. Gong and X. F. Feng, Chem. Eng. J., 2017, 316, 154–159 CrossRef CAS.
- M. Koh, J. Yoo, P. Yong, D. Bae and K. Park, Ind. Eng. Chem. Res., 2006, 45, 5308–5313 CrossRef CAS.
- E. A. Santos and A. C. Ladeira, Environ. Sci. Technol., 2011, 45, 3591–3597 CrossRef CAS PubMed.
- K. L. Ang, D. Li and A. N. Nikoloski, Miner. Eng., 2018, 123, 8–15 CrossRef CAS.
- S. B. Xie, C. Zhang, X. H. Zhou, J. Yang and X. J. Zhang, J. Environ. Radioact., 2009, 100, 162–166 CrossRef CAS PubMed.
- J. Zhou, W. Zhu, J. Yu, H. Zhang and Y. Zhang, Appl. Surf. Sci., 2018, 435, 920–927 CrossRef CAS.
- S. E. Bailey, T. J. Olin, R. M. Bricka and D. D. Adrian, Water Res., 1999, 33, 2469–2479 CrossRef CAS.
- A. Gajowiak, M. Majdan and K. Drozdzal, Przem. Chem., 2009, 88, 190–196 CAS.
- I. Zhuravlev, O. Zakutevsky, T. Psareva, V. Kanibolotsky and V. Strelko, J. Radioanal. Nucl. Chem., 2002, 254, 85–89 CrossRef CAS.
- K. Oshita, M. Oshima, Y. Gao, K. H. Lee and S. Motomizu, Anal. Chim. Acta, 2003, 480, 239–249 CrossRef CAS.
- J. Choi and J. W. Park, Geosci. J., 2005, 9, 53–61 CrossRef CAS.
- T. S. Anirudhan and P. S. Suchithra, Appl. Clay Sci., 2008, 42, 214–223 CrossRef CAS.
- I. Chibata, T. Tosa, T. Mori, T. Watanabe and N. Sakata, Enzyme Microb. Technol., 1986, 8, 130–136 CrossRef CAS.
- T. Sakaguchi and A. Nakajima, Sep. Sci., 1987, 22, 1609–1623 CrossRef CAS.
- A. Nakajima and T. Sakaguchi, J. Chem. Technol. Biotechnol., 1987, 40, 223–232 CrossRef CAS.
- J. Zhou, Indian J. Chem. Technol., 2018, 25, 361–367 Search PubMed.
- D. Yang, Y. Li and J. Nie, Carbohydr. Polym., 2007, 69, 538–543 CrossRef CAS.
- M. Okhawilai, R. Rangkupan, S. Kanokpanont and S. Damrongsakkul, Int. J. Biol. Macromol., 2010, 46, 544–550 CrossRef CAS PubMed.
- J. Ju, W. Kang, N. Deng, L. Li, Y. Zhao and X. Ma, Microporous Mesoporous Mater., 2017, 239, 416–425 CrossRef CAS.
- J. Yu, M. Guo, F. Muhammad, A. Wang and F. Zhang, Carbon, 2014, 69, 502–514 CrossRef CAS.
- W. Li, Q. Liu and J. Liu, Appl. Surf. Sci., 2017, 403, 378–388 CrossRef CAS.
- L. Tan, Q. Liu and X. Jing, Chem. Eng. J., 2015, 273, 307–315 CrossRef CAS.
- J. Zhu, Q. Liu, Z. Li, J. Liu and H. Zhang, J. Hazard. Mater., 2018, 353, 9–17 CrossRef CAS PubMed.
- W. S. Ngah and S. Fatinathan, J. Environ. Manage., 2010, 91, 958–969 CrossRef CAS PubMed.
- M. T. Sikder, S. Tanaka and T. Saito, J. Environ. Chem. Eng., 2014, 2, 370–376 CrossRef.
- L. Zhou, J. Jin, Z. Liu, X. Liang and C. Shang, J. Hazard. Mater., 2011, 185, 1045–1052 CrossRef CAS PubMed.
- V. Tomar, S. Prasad and D. Kumar, Adsorptive removal of fluoride from water samples using Zr–Mn composite material, Microchem. J., 2013, 111, 116–124 CrossRef CAS.
- M. S. Sivakami, T. Gomathi, J. Venkatesan, H. S. Jeong, S. K. Kim and P. N. Sudha, Int. J. Biol. Macromol., 2013, 57, 204–212 CrossRef CAS PubMed.
- C. Bai, M. Zhang and B. Li, J. Hazard. Mater., 2015, 300, 368–377 CrossRef CAS PubMed.
- M. A. Zulfikar, S. Afrita, D. Wahyuningrum and M. Ledyastuti, Environmental Nanotechnology, Monitoring & Management, 2016, 6, 64–75 Search PubMed.
- Q. Song, L. Ma and J. Liu, J. Colloid Interface Sci., 2012, 386, 291–299 CrossRef CAS PubMed.
- R. Li, C. Rong and L. Qi, J. Hazard. Mater., 2017, 338, 167–176 CrossRef CAS PubMed.
- G. Huang, W. Li, Q. Liu and J. Y. Liu, New J. Chem., 2017, 42, 168–176 RSC.
- S. M. Yakout and M. A. Rizk, Desalin. Water Treat., 2013, 53, 1917–1922 CrossRef.
- P. Yang, Q. Liu and J. Liu, J. Mater. Chem. A, 2018, 5, 17933–17942 RSC.
- C. Gunathilake, M. Jaroniec, J. Gorka and S. Dai, J. Mater. Chem. A, 2015, 3, 11650–11659 RSC.
- X. Xie, Y. Tian, Z. Qin, Q. Yu, H. Wei, D. Wang, X. Li and X. Wang, Sci. Rep., 2017, 7, 1 CrossRef PubMed.
- Y. Zhang, X. Lin, S. Hu, X. Zhang and X. Luo, RSC Adv., 2016, 6, 73959–73973 RSC.
- M. P. Mani and S. K. Jaganathan, Bioinspired, Biomimetic Nanobiomater., 2018, 7, 213–218 CrossRef.
- W. Zhao, X. Lin, Y. Qin, H. Cai, Y. Chen and X. Luo, J. Radioanal. Nucl. Chem., 2017, 314, 1853–1864 CrossRef CAS.
- B. Teng, X. Jian, Y. Gao and W. Chen, J. Cleaner Prod., 2016, 112, 972–979 CrossRef CAS.
- F. Liu, Z. Wang and G. Li, Desalin. Water Treat., 2014, 52, 7172–7179 CrossRef CAS.
- L. Falcao and M. E. M. Araujo, J. Cult. Herit., 2013, 14, 499–508 CrossRef.
- L. Wu, X. Lin, X. Zhou and X. Luo, Appl. Surf. Sci., 2016, 384, 466–479 CrossRef CAS.
- D. Yang, X. Wang and G. Song, Sci. Bull., 2017, 62, 1609–1618 CrossRef CAS.
- J. Yu, X. Luo and B. Liu, J. Mater. Chem. A, 2018, 6, 15359–15370 RSC.
- B. Li, L. Ma and Y. Tian, J. Hazard. Mater., 2014, 271, 41–49 CrossRef CAS PubMed.
- C. Pena, K. D. L. Caba, A. Eceiza, R. Ruseckaite and I. Mondragon, Bioresour. Technol., 2010, 101, 6836–6842 CrossRef CAS PubMed.
- Y. M. Cheng, X. Sun, X. P. Liao and B. Shi, Chin. J. Chem. Eng., 2011, 19, 592–597 CrossRef CAS.
- X. P. Liao, H. W. Ma, R. Wang and B. Shi, J. Membr. Sci., 2004, 243, 235–241 CrossRef CAS.
- X. Sun, X. Huang and X. P. Liao, J. Hazard. Mater., 2010, 179, 295–302 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra09297d |
| This journal is © The Royal Society of Chemistry 2019 |