Open Access Article
Open Access ArticleAn efficient Nozaki–Hiyama allenylation promoted by the acid derived MIL-101 MOF†
Yi Luan *a,
Zonghui Caia,
Xiujuan Lia,
Daniele Ramellab,
Zongcheng Miaoc and
Wenyu Wang*d
*a,
Zonghui Caia,
Xiujuan Lia,
Daniele Ramellab,
Zongcheng Miaoc and
Wenyu Wang*d
aSchool of Materials Science and Engineering, University of Science and Technology Beijing, 30 Xueyuan Road, Haidian District, Beijing 100083, P. R. China. E-mail: yiluan@ustb.edu.cn
bDepartment of Chemistry, Temple University-Beury Hall, 1901, N. 13th Street, Philadelphia, PA 19122, USA
cKey Laboratory of Organic Polymer Photoelectric Materials, School of Science, Xijing University, Xi'an, 710123, China
dBroad Institute, 415 Main Street, Cambridge, Massachusetts 02142, USA
First published on 7th March 2019
Abstract
A concise synthesis of the sulfonic acid-containing MIL-101 MOF catalyst was reported using commercially available materials. A series of characterization of as-synthesized MIL-101-SO3H including SEM, XRD, FTIR, BET and TGA was also demonstrated. Using MIL-101-SO3H as a catalyst, an efficient Nozaki–Hiyama allenylation reaction was achieved to generate various polyfunctionalized α-allenic alcohols in high yield and good selectivity. Taking advantage of the high acidity of the MIL-101-SO3H MOF structure, such transformations were also achieved under mild reaction conditions and short reaction times. Based on our observed evidence during this study, a mechanism was proposed involving a substrate activation/γ-nucleophilic addition reaction sequence. In addition, the MIL-101-SO3H catalyst can be recycled ten times during the Nozaki–Hiyama allenylation reaction without compromising the yield and selectivity.
Introduction
Allenes have proven to be versatile and useful intermediates or synthons1 for synthesis of drug-like molecules or natural products due to the existence of two orthogonal π-bonds.2 Recently, α-allenic alcohols have drawn much attention because they can be easily transformed to dihydrofurans3 and cyclopropanes,4 and iodination of the allene could provide subsequent access to vinyl epoxides,5 syn-1,2-diols6 and syn-1,2-amino alcohols7 in high diastereoselectivities. Synthesis of α-allenic alcohols has been achieved through allenylation of carbonyls with allenyl metal or propargyl metal species.2l However, controlling the formation of allenols over homopropargyl alcohols is challenging due to the facile isomerization between the allenyl metal and the corresponding propargyl metal species, especially given the fact that propargyl alcohols are generally thermodynamically favored over the allenols.Recently, metal–organic frameworks (MOFs) have emerged as a new class of solid catalysts,8 by virtue of their highly tailorable nature, porous structure and large surface area.9 Incorporation of Brønsted acidity into MOFs materials have also been developed and due to their well-defined porous acidic micro structure, they have been used as the efficient solid catalysts for several catalytic transformations.10 Among all the reported examples, a stable and recyclable MOF structure bearing sulfonic acid functionality can serve as a low pKa catalytic material,11 and we eventually choose to use a chemically stable and previously described MIL-101-SO3H MOF in our catalytic reaction development study.12 Due to its chemical and physical stabilities, MIL-101-SO3H has been utilized as an efficient catalyst for several organic reactions.13
Herein, we reported a highly efficient Nozaki–Hiyama allenylation reaction catalyzed by MIL-101-SO3H MOF using propargyl boronates, which provided rapid access to the α-allenic alcohol products. This newly synthesized MIL-101-SO3H catalyst bearing aromatic sulfonic acid groups was obtained using commercially available monosodium 2-sulfoterephthalate. A broad scope of propargyl boronates and aldehydes were tested to be tolerable under the optimal reaction conditions with high catalytic reactivities. Furthermore, the as-synthesized MIL-101-SO3H catalyst can be readily filtered and separated from the allenylation reaction mixture. Recycling the MIL-101-SO3H up to ten times doesn't compromise yield or selectivity of the benzaldehyde allenylation.
Results and discussion
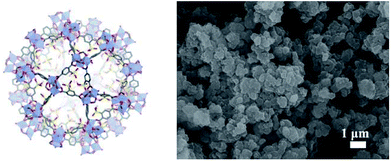
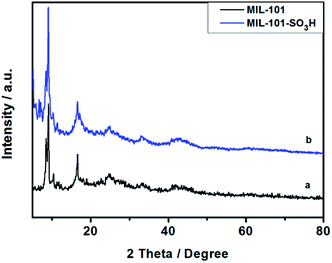
MIL-101-SO3H can be readily synthesized and purified according to the reported hydrothermal methods as a crystalline powder.14 It is isostructural to MIL-101 (Fig. 1), and thus has the same pore structures. The SEM images of MIL-101-SO3H showed (Fig. 1) the rectangular crystals of MIL-101-SO3H are evenly distributed with a size of 200 nm.Subsequently, powder X-ray diffraction (PXRD) measurements were conducted (Fig. 2) and showed good agreement with literature values.15 In addition, the obtained XRD data of MIL-101-SO3H demonstrated high similarity to the XRD patterns of the MIL-101, which also indicated our successful preparation of the MIL-101-SO3H MOF.
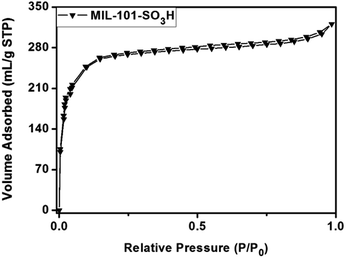
The specific surface areas of the products were then analyzed by N2 adsorption–desorption measurements at 77 K. As shown in Fig. 3, the trace of the MIL-101-SO3H showed a type-I isotherm. N2 gas sorption isotherms reveal a Brunauer–Emmett–Teller (BET) surface area of 1243 m2 g−1.
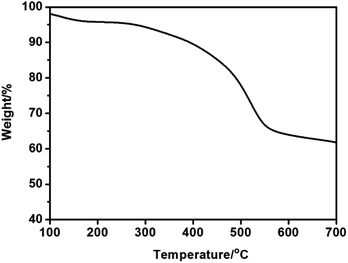
Additionally, the thermal structural stabilities of MIL-101-SO3H were examined by thermal gravimetric analysis (TGA). A weight loss at about 300 °C was observed during the TGA measurement,16 which indicated high thermal stability of the acid derived MIL-101-SO3H sample, which should provide reliable stability during the catalytic reaction temperature range (Fig. 4).
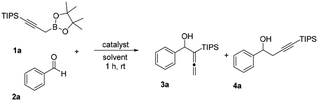
With the MOF catalyst in hand, we started our catalytic Nozaki–Hiyama allenylation reaction studies. Several reaction factors such as nature of the catalyst and solvent, were tested in order to reveal the optimal reaction condition (Table 1). First, a control experiment was performed to study the background rate of the allenylation reaction of propargyl boronate 1a. As expected, no conversion was observed for propargyl boronate 1a in the absence of catalyst or using biphenol as the catalyst (Table 1, entries 1–2) suggesting that a low pKa acid is indeed necessary for the reaction to occur. Subsequently, HCl showed modest yield at 1 mol% catalytic loading and the reaction was slow due to the low catalyst loading and undesired α-addition byproduct 4a (Table 1, entry 3) was also observed. p-TsOH provided satisfying yield and selectivity at 1 mol% catalyst loading, despite the fact it is not recyclable and being corrosive to several equipments (Table 1, entry 4). As expected, MIL-101 bearing no acidic functional group provided only traces of product at 23 °C in CH2Cl2 solvent (Table 1, entry 5). We expect metal–organic framework material bearing –SO3H can provide the high reaction efficiency and recyclable nature at the same time. Our previously reported UiO-66-RSO3H indeed provided an increase for the yield.17 However, alkyl sulfonic acid functional group inside the UiO-66-RSO3H catalyst was not acidic enough to provide the quantitative conversion (Table 1, entry 6). In contrast, 1 mol% MIL-101-SO3H showed almost quantitative conversion and over 99% selectivity for the allenylation 1a.17 The greatly enhanced acidity could efficiently allow the rearrangement of boronate for γ-nucleophilic addition to aldehyde to provide the desired α-allenic alcohol 2a in excellent yield (Table 1, entry 7). Toluene gave almost comparable yield for the allenylation reaction (Table 1, entry 8). Polar nonprotic/protic solvents, such as THF and ethanol, gave decent yield of the desired allenylation product with diminished yields (Table 1, entries 9–10). Mildly basic solvent, DMF was not suitable for the acidic heterogeneous MIL-101-SO3H promoted allenylation reaction (Table 1, entry 11). Further solvent evaluation indicated that dichloromethane is the optimal reaction solvent for further allenylation reaction studies (Table 1, entries 7–11). Optimization of catalyst structure demonstrated that MIL-101-SO3H can efficiently catalyze the allenylation reaction using propargyl boronates and aldehyde as the starting material.
| Entry | Catalyst | Solvent | Yield of 3a | Selectivity |
|---|---|---|---|---|
| a Reaction condition: 1 mol% catalyst, TIPS propargyl boronate 1a and benzaldehyde 2a were stirred in the solvent (0.2 M) for 1 h at room temperature. | ||||
| 1 | — | CH2Cl2 | <5% | N.A. |
| 2 | Biphenol | CH2Cl2 | <5% | N.A. |
| 3 | HCl | CH2Cl2 | 53% | 71% |
| 4 | p-TsOH | CH2Cl2 | 91% | 99% |
| 5 | MIL-101 | CH2Cl2 | <5% | N.A. |
| 6 | UiO-66-RSO3H | CH2Cl2 | 77% | 96% |
| 7 | MIL-101-SO3H | CH2Cl2 | 99% | 99% |
| 8 | MIL-101-SO3H | PhCH3 | 98% | 99% |
| 9 | MIL-101-SO3H | THF | 68% | 99% |
| 10 | MIL-101-SO3H | EtOH | 44% | 99% |
| 11 | MIL-101-SO3H | DMF | 23% | 99% |
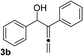
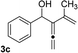
With an optimal catalyst and reaction condition identified, the scope of propargyl boronate reaction partners were next investigated. Simple TIPS-protected boronate 1a and benzaldehyde 2a resulted in quantitative formation of 3a without any undesired propargyl α-addition byproduct (Table 2, entry 1). Phenyl substituted propargyl boronate 1b also tolerated this chemistry, which gave allenic alcohol 3b in 95% yield. The 1,1-disubstituted vinyl moiety on 1c is known to undergo protonation to generate a stabilized tertiary carbocation which potentially provide us a diminished yield of the desired allenylation product. Gratifyingly, the complex vinylpropargyl borate 1c also gave desired 3c in excellent yield (Table 2, entry 3) under identical reaction condition, which illustrated the versatility of MIL-101-SO3H as a catalyst in this reaction. Consistently, acid labile substrate 1d also provided desired product 3d in great yield without losing TBS group (Table 2, entry 4).
| Entry | Boronate | Product | Yield |
|---|---|---|---|
| a Reaction condition: 1 mol% MIL-101-SO3H, propargyl boronate 1 and benzaldehyde 2a were stirred in CH2Cl2 for 1 h at room temperature. | |||
| 1 | 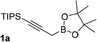 |
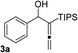 |
99% |
| 2 | 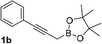 |
 |
98% |
| 3 | 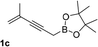 |
 |
95% |
| 4 | 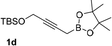 |
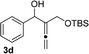 |
97% |
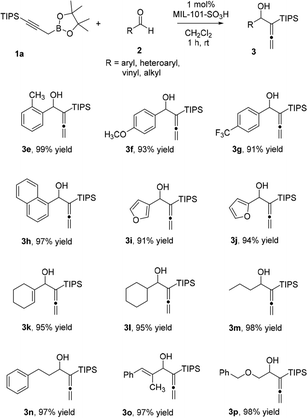
We next examined MIL-101-SO3H catalyst in allenylation of a wide variety of aldehydes (Table 3). Electronic rich and deficient benzaldehydes as well as polyaromatic benzaldehyde (2e–2h) were all shown to be tolerant for such transformation, as well as a heterocyclic aldehyde such as furfurals (2i and 2j). The α,β-unsaturated aldehyde 2k was also tested and only the desired addition product was observed in 95% yield, although boronates are known to undergo 1,4-addition into enones. More challenging aliphatic aldehydes (2l–2n, 2p) also provide excellent yields and selectivity. Furthermore, alkene aldehydes (2k, 2o) were shown to be well tolerant in our catalytic system to provide corresponding allenic alcohols in more than 95% yields.
| a Reaction condition: 1 mol% MIL-101-SO3H, propargyl boronate 1 and benzaldehyde 2a were stirred in CH2Cl2 for 1 h time at room temperature. |
|---|
 |
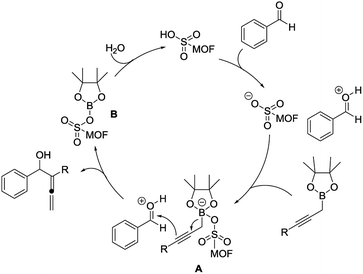
A detailed reaction mechanism has been proposed. First, the Brønsted acidic site on MIL-101-SO3H catalyst would activate the aldehyde substrate by protonation, and simultaneously, the generated sulfonate anion on the backbone of the MOF catalyst could further activate the boronate species to form intermediate A (Scheme 1). Therefore, by using our heterogeneous catalyst MIL-101-SO3H formation of the key zwitterionic intermediate A could introduce a better reactant approximation for the ideal reactivity to occur whereas using traditional acid, such as HCl, would bring less control of the desired reactivity which lead to formation of the undesired propargyl alcohols (cf. Table 1). The well-defined porous structure of the catalyst will facilitate this double activation of both reactant and trigger the desired γ-nucleophilic addition from boronate to the aldehyde.
However, if such double activation did not work properly, the undesried α-nucleophilic addition could also happen to give the propargyl byproduct. Subsequently, the catalyst could be regenerated by hydrolysis of borate intermediate B.
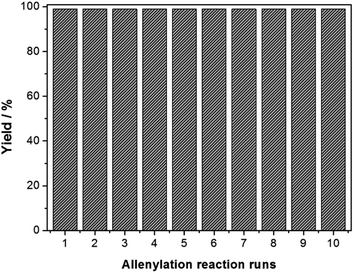
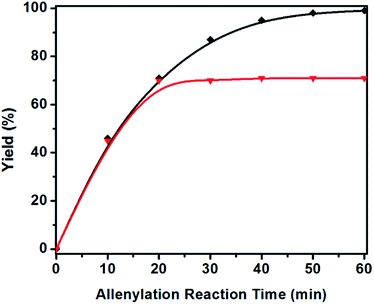
Additionally, we tested recyclability of the acidic MIL-101-SO3H catalyst and showed that it can be recycled up to ten times without compromising the yield and selectivity of the allenylation reaction of aldehyde 1a. The recyclability of the MIL-101-SO3H catalyst was evaluated using TIPS-propargyl boronate 1a in 2 mL of CH2Cl2 at room temperature. As for MIL-101-SO3H, the yield of desired 3a remained 98% after usage of the same recycled catalyst with 10 batches of fresh reagent (Fig. 5).
A hot filtration test was also performed for the MIL-101-SO3H catalyzed allenylation reaction. After 20 minutes of reaction, the solid MIL-101-SO3H catalyst was filtered off and at this point, the allenylation of aldehyde 1a did not further proceed, which further suggested no acid leaching into the reaction solution (Fig. 6). Meanwhile SEM images and X-ray powder diffraction spectrum of the MIL-101-SO3H catalyst were also recorded after ten allenylation reaction cycles. These data were found to be identical to those of the freshly prepared catalyst (Fig. S1 and S2†), which also suggested the high chemical stability of the MIL-101-SO3H catalyst.
Experimental
Synthetic procedures
Conclusions
In conclusion, a heterogeneous MIL-101-SO3H catalyst bearing an aromatic sulfonic acid group was synthesized and applied to allenylation reaction of aldehydes for the efficient synthesis α-allenic alcohols. Simple reaction setup using mild reaction conditions and low catalyst loadings are hallmarks of this MOF promoted allenylation methodology. The MIL-101-SO3H promoted reaction was found to be general for aldehydes and the ability to vary the alkyne substitution on the boronate, which makes this methodology a powerful tool for the generation of a wide range of α-allenic alcohol products. The structural morphology and properties of MIL-101-SO3H were fully characterized by SEM, XRD, TGA, FTIR and BET. The newly developed MIL-101-SO3H showed higher reaction efficiency and selectivity in the allenylation reaction at 1 mol% catalyst loadings in comparison with several other catalyst systems. The high chemical stability of the MIL-101-SO3H catalyst was due to its strong covalent bond, and did not suffer from leaching. Further studies involving new synthetic applications of the MIL-101-SO3H catalyst are currently in progress and will be reported in the due course.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by Beijing Natural Science Foundation (No. 2172037) and National Natural Science Foundation of China (No. 51673157). We also thank the Natural Science Basic Research Plan in Shaanxi Province of China (No. 2018JQ5028, No. 2017JM5134 and No. 2018JM5047) and the Science Research Foundation of Xijing University (Grant No. XJ16T02) for financial support.Notes and references
- J. A. Marshall, B. W. Gung and M. L. Grachan, in Modern Allene Chemistry, ed. N. Krause and A. S. K. Hashmi, Wiley-VCH, Weinheim, 2004 Search PubMed.
- (a) S. Ma and S. Zhao, J. Am. Chem. Soc., 2001, 123, 5578 CrossRef CAS PubMed; (b) N. Krause, A. Hoffmann-Roeder and J. Canisius, Synthesis, 2002, 1759 CrossRef CAS; (c) B. Alcaide, P. Almendros and C. Aragoncillo, Chem.–Eur. J., 2002, 8, 1719 CrossRef CAS PubMed; (d) D. Xu, Z. Li and S. Ma, Chem.–Eur. J., 2002, 8, 5012 CrossRef CAS PubMed; (e) E. Yoneda, S. Zhang, D. Zhou, K. Onitsuka and S. Takahashi, J. Org. Chem., 2003, 68, 8571 CrossRef CAS PubMed; (f) W. Wang, A. Clay, R. Krishnan, N. J. Lajkiewicz, L. E. Brown, J. Sivaguru and J. A. Porco Jr, Angew. Chem., Int. Ed., 2017, 56, 14479 CrossRef CAS PubMed; (g) C. Qi, W. Wang, K. D. Reichl, J. McNeely and J. A. Porco Jr, Angew. Chem., Int. Ed., 2018, 57, 2101 CrossRef CAS PubMed; (h) S. Liu, W. Wang, L. E. Brown, C. Qiu, N. J. Lajkiewicz, T. Zhao, J. Zhou, J. A. Porco Jr and T. Wang, EBioMedicine, 2015, 11, 1600 CrossRef PubMed; (i) W. Wang, R. Cencic, L. Whitesell, J. Pelletier and J. A. Porco Jr, Chem.–Eur. J., 2016, 22, 12006 CrossRef CAS PubMed; (j) C. Mukai, I. Nomura and S. Kitagaki, J. Org. Chem., 2003, 68, 1376 CrossRef CAS PubMed; (k) A. S. K. Hashima, M. C. Blanco, D. Fischer and J. W. Bats, Eur. J. Org. Chem., 2006, 2006, 1387 CrossRef; (l) H. C. Brown, U. R. Khire and G. Narla, J. Org. Chem., 1995, 60, 8130 CrossRef CAS.
- (a) A. Hoffmann-Röder and N. Krause, Org. Lett., 2001, 3, 2537 CrossRef; (b) F. Zamani, S. G. Pyne and C. J. T. Hyland, J. Org. Chem., 2017, 82, 6819 CrossRef CAS PubMed; (c) R. R. Tata and M. Harmata, Org. Lett., 2016, 18, 5684 CrossRef CAS PubMed; (d) B. Yang, C. Zhu, Y. Qiu and J. Bäckvall, Angew. Chem., Int. Ed., 2016, 55, 5568 CrossRef CAS PubMed.
- (a) M. Tomoya, N. Takayuki, B. Tsuneaki and M. Masahiro, Chem. Lett., 2015, 44, 700 CrossRef; (b) H. K. Grover, M. R. Emmett and M. A. Kerr, Org. Biomol. Chem., 2015, 13, 655 RSC; (c) H. Hori, S. Arai and A. Nishida, Adv. Synth. Catal., 2017, 359, 1170 CrossRef CAS; (d) M. Yasui, R. Ota, C. Tsukano and Y. Takemoto, Nat. Commun., 2017, 8, 674 CrossRef PubMed.
- R. W. Friesen and M. Blouin, J. Org. Chem., 1993, 58, 1653 CrossRef CAS.
- (a) R. W. Friesen and A. Giroux, Tetrahedron Lett., 1993, 34, 1867 CrossRef CAS; (b) C. S. Adams, R. D. Grigg and J. M. Schomaker, Chem. Sci., 2014, 5, 3046 RSC.
- R. W. Friesen and A. E. Kolaczewska, J. Org. Chem., 1991, 56, 4888 CrossRef CAS.
- Y. Huang, J. Liang, X. Wang and R. Cao, Chem. Soc. Rev., 2017, 46, 126 RSC.
- (a) X. Cao, C. Tan, M. Sindoro and H. Zhang, Chem. Soc. Rev., 2017, 46, 2660 RSC; (b) Q. Yang, Q. Xu and H. Jiang, Chem. Soc. Rev., 2017, 46, 4774 RSC.
- J. Jiang and O. M. Yaghi, Chem. Rev., 2015, 115, 6966 CrossRef CAS PubMed.
- (a) J. Juan-Alcaniz, R. Gielisse, A. B. Lago, E. V. Ramos-Fernandez, P. Serra-Crespo, T. Devic, N. Guillou, C. Serre, F. Kapteijn and J. Gascon, Catal. Sci. Technol., 2013, 3, 2311 RSC; (b) A. Klinkebiel, N. Reimer, M. Lammert, N. Stock and U. Luning, Chem. Commun., 2014, 50, 9306 RSC; (c) H. Li, K. Wang, D. Feng, Y. Chen, W. Verdegaal and H. Zhou, ChemSusChem, 2016, 9, 2832 CrossRef CAS PubMed.
- H. Li, K. C. Wang, D. W. Feng, Y. P. Chen, W. Verdegaal and H. C. Zhou, ChemSusChem, 2016, 9, 2832 CrossRef CAS PubMed.
- (a) M. B. Boroujeni, A. Hashemzadeh, M. T. Faroughi, A. Shaabani and M. M. Amini, RSC Adv., 2016, 6, 100195 RSC; (b) M. Saikia and L. Saikia, RSC Adv., 2016, 6, 15846 RSC.
- Y. Zhou, Y. Chen, Y. Hu, G. Huang, S. Yu and H. Jiang, Chem.–Eur. J., 2014, 20, 14976 CrossRef CAS PubMed.
- G. Chang, M. Huang, Y. Su, H. Xing, B. Su, Z. Zhang, Q. Yang, Y. Yang, Q. Ren, Z. Bao and B. Chen, Chem. Commun., 2015, 51, 2859 RSC.
- (a) Y. Luan, Y. Qi, H. Gao, R. S. Andriamitantsoa, N. Zheng and G. Wang, J. Mater. Chem. A, 2015, 3, 17320 RSC; (b) X. Du, X. Li, H. Tang, W. Wang, D. Ramella and Y. Luan, New J. Chem., 2018, 42, 12722 RSC.
- (a) Y. Luan, N. Zheng, Y. Qi, J. Yu and G. Wang, Eur. J. Inorg. Chem., 2014, 2014, 4268 CrossRef CAS; (b) J. Zhao, W. Wang, H. Tang, D. Ramella and Y. Luan, Mol. Catal., 2018, 456, 57 CrossRef CAS; (c) Y. Qi, Y. Luan, J. Xu, X. Peng and G. Wang, Chem.–Eur. J., 2014, 20, 1–10 CrossRef PubMed.
- (a) G. Akiyama, R. Matsuda, H. Sato, M. Takata and S. Kitagawa, Adv. Mater., 2011, 23, 3294 CrossRef CAS PubMed; (b) G. Ferey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surble and I. Margiolaki, Science, 2005, 309, 2040 CrossRef CAS PubMed.
- H. C. Brown, C. D. Roy and R. Soundararajan, Tetrahedron Lett., 1997, 38, 765 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra09600g |
| This journal is © The Royal Society of Chemistry 2019 |