Open Access Article
Open Access ArticleEffects of a low-strength magnetic field on the characteristics of activated sludge for membrane fouling mitigation
Ren Zhijun *abc,
Wang Pengfeia,
Tian Jiayua and
Zhang Zhiliub
*abc,
Wang Pengfeia,
Tian Jiayua and
Zhang Zhiliub
aSchool of Energy and Environmental Engineering, Hebei University of Technology, Tianjin, 300130, China. E-mail: renzhijun2003@126.com
bCollege of Aerospace and Civil Engineering, Harbin Engineering University, Harbin, 150001, China
cState Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, Harbin 150090, China
First published on 20th March 2019
Abstract
This study aims to investigate the performance of a low-strength magnetic field in membrane bioreactors (MBRs) for membrane fouling mitigation and its effects on sludge characteristics and microbial community. The continuous operation of MBR with magnetic powder (MP-MBR) is denoted as the control-MBR, and a magnetic field is added to the MP-MBR to form the magnetic-MBR (M-MBR). The comparison between MP-MBR and M-MBR was conducted to treat synthetic wastewater. The results showed that the application of a low-strength magnetic field not only decreased the zeta potential and increased the particle size, but also improved the dehydrogenase activity and stimulated microbes to produce fewer SMPs, which markedly contributed to the improved filtration performance of MBRs. The variations in the microbial communities from the two MBRs at genus levels confirmed that the addition of a low-strength magnetic field significantly affected the microbial community and composition, further altered the microbial metabolites, and consequently affected the membrane fouling evolution.
1 Introduction
Membrane fouling, which substantially reduces the flux permeability and thus deteriorates the filtration performance of membranes, is an inevitable phenomenon that occurs during the application of membrane bioreactors (MBRs).1,2 Recently, magnetic technology combined with membrane bioreactors (MBRs) has garnered attention from researchers. Liu et al.3 and Ren et al.4 reported that the size of the aerobic granular sludge with magnetic seeds increased as the particle size increased and the membrane fouling rate was significantly reduced by adding magnetic species. Magnetic bio-effect is considered to be the main factor in mitigating membrane fouling when magnetic powder is added in membrane bioreactors (MBRs) for membrane fouling mitigation.5 Wang et al.6 reported that magnetic powder improved the dehydrogenase activity, enhanced the biomass growth, and exhibited good performance in the mitigation of membrane fouling. Chen et al.7 found that there are a variety of microbial species in the reactor and that the membrane fouling rate can be decreased by adding magnetic particles to the MBR.Compared with other fouling mitigation techniques, the advantages of a magnetic-MBR include greater flexibility, less chemical usage, excellent effluent quality,8 and the ability to counter fouling by simultaneously integrating membrane filtration, magnetism, and biological treatment into a single system.9,10 In recent years, the physical, chemical and biological effects of magnetic fields on the microbial degradation process have been widely studied in biologically activated sludge.11,12 Both the growth of microorganisms13,14 and the biodegradation ability of microorganisms could be affected by magnetic field.15 However, the knowledge of the sludge properties, microbial community as well as their connection to membrane fouling by a magnetic field, especially low-intensity magnetic field, in MBRs is still limited. Also, the anti-fouling mechanisms of low-intensity magnetic field, fulfilled by magnetic bioeffects, have not been well examined and still need to be verified.
In this study, a permanent magnet was applied to a membrane bio-reactor (MBR) with magnetic activated sludge (MP-MBR) to form a magnetic-MBR (M-MBR). A comparison was conducted to evaluate the performance of M-MBR and MP-MBR based on sludge properties and membrane fouling. In order to link the microbial community and membrane fouling, high-throughput sequencing technology served to compare the microbial communities of bulk sludge and biofilm sludge in both MBRs at the genus level and possible anti-fouling mechanisms of a low-strength magnetic field were also examined and discussed.
2 Materials and methods
2.1 Operation of MBRs
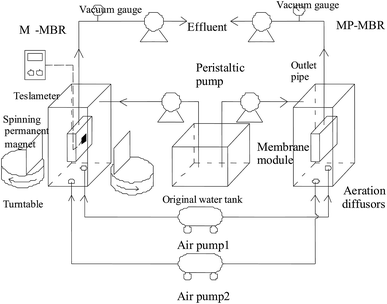
In order to better understand the mechanism of a magnetic field on membrane fouling control, parallel experiments of M-MBR and MP-MBR were investigated. The experimental apparatus is shown in Fig. 1.The CODcr of the wastewater was approximately 500 mg L−1. The operation of both the MBRs was as follows: the wastewater was fed into the reactor from the original water tank using a peristaltic pump, and an electronic timer was used to maintain a 10 minute water cycle that included a run time of 8 minutes and a stop time of 2 minutes, in which the content in the effluent was constant, the membrane flux was 25 L (m−2 h−1), the hydraulic retention time (HRT) was 4.8 h, the aeration was continuous, the dissolved oxygen was approximately 3 mg L−1, the temperature inside the reactor was kept between 15 °C and 25 °C, and the pH value was maintained between 6.5 and 7.5. A vacuum gauge was installed in the outlet pipe to monitor the TMP, and the membrane was cleaned when the TMP reached a critical value of 0.05.
In the M-MBR process, two permanent magnets on a turntable were symmetrically placed on both sides of the center of the flat film, which has dual purposes: directly influencing microbial activities and controlling the magnetic powder movement in MBR process. The length, width and height of the permanent magnets were 100 mm, 100 mm and 10 mm, respectively. The intensity of the magnetic field could be adjusted from 0 mT to 45 mT by regulating the distance from the turntable. The rotation rate of the turntable was controlled by the electronic timer, and the rotary run time![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) down time were 1 min
down time were 1 min![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 min, 2 min
2 min, 2 min![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 min, and 3 min
2 min, and 3 min![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 min. Based on the organic removal efficiency and the change in the MLSS and TMP under different magnetic field intensities and rotary run time
2 min. Based on the organic removal efficiency and the change in the MLSS and TMP under different magnetic field intensities and rotary run time![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) down time values, a magnetic field strength of 35 mT and a rotary run time
down time values, a magnetic field strength of 35 mT and a rotary run time![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) down time of 2 min
down time of 2 min![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 min were determined as the optimal experimental conditions. The results are not shown in this paper.16
2 min were determined as the optimal experimental conditions. The results are not shown in this paper.16
Activated sludge was collected from the secondary clarifier of the Daqing Oil Sewage Treatment Plant in Heilongjiang, China. The MLSS concentration was kept constant at approximately 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1.
000 mg L−1.
A frame plate membrane module from Shanghai Snapp Membrane Separation Technology Co., Ltd., was used. The membrane was composed of polyvinylidene fluoride (PVDF) and had a total area of 0.1 m2 and a pore size of 0.1 μm.
Fe3O4 powder, which is a common magnetic material with weak magnetic properties and strong adsorption ability due to its surface area, was used in this study. The powder size ranged from 1 μm to 12 μm, with 50% within the size range of 2–4 μm. The magnetic powder was pretreated as follows: 20 g of magnetic powder (with the Fe3O4 content greater than 98%) was added to a 1 L beaker and mixed with deionized water. The magnetic powder was allowed to settle for 30 minutes under the action of a round magnet placed in the bottom of the beaker, and then the supernatant was removed. The settling step was repeated twice, and then the beaker was placed in an oven at 105 °C for 24 h. Before it was added to the MBR process, the magnetic powder was transferred to a 1 L beaker, which was placed on the permanent magnet for 30 minutes to magnetize the magnetic powder.17
A quantification approach, indicated by mean SD, was used. Data in the figures are mean ± SD values of 3 replicates and mean was used for data analysis and comparison.
2.2 Analysis methods
All effluent samples were filtered through 0.45 μm membrane filters (mixed cellulose ester, Advantec) before chemical analysis. The MLSS concentration was measured using the standard method.18 Dehydrogenase was measured by following the Tracey method.19 The zeta potential of the supernatant was measured using a Zeta meter (ZetaSizer3000, England). The membrane samples were analyzed using attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy (Spectrum One, Perkin Elmer) to characterize the chemical functional groups of the dried sludge samples. The pretreatment process for sample preparation began with the slow removal of the loose sludge from the membrane surface using water, cleaning the surface gel layer of the diaphragm using deionized water, and drying the mixed liquid in a drying oven at 105 °C. The surface contaminants from the above procedure was mixed with KBr powder (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100), which was dried to a constant weight in a vacuum dryer and pressed into pellets using a tablet press. The spectra were recorded over the wavelength range of 4000 cm−1 to 400 cm−1.
100), which was dried to a constant weight in a vacuum dryer and pressed into pellets using a tablet press. The spectra were recorded over the wavelength range of 4000 cm−1 to 400 cm−1.
Proteins and polysaccharides are the largest components of SMPs; therefore, they can be used to infer the SMP content. The amounts of polysaccharides were measured using the anthrone–sulfuric acid method and those of the proteins were measured via UV-vis spectrophotometry.
The activated sludge samples were collected from the seed sludge and the biofilm samples were taken from the fouled membranes using a sterile steel knife. After DNA extraction and PCR amplification, the microbial communities were analyzed by Illumina MiSeq sequencing.
3 Results and discussion
After 3 months of operation, both the MBR processes exhibited a stable COD removal performance. Magnetic powder (650 mg L−1) was added to one of the MBR reactors to form an MP-MBR; magnetic powder with the same dosage and two permanent magnets was applied to another MBR to form an M-MBR. Both reactors were operated under the same conditions (details are given in Section 2.1).3.1 The performance of two MBRs
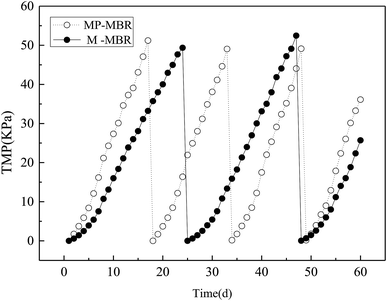
Fig. 2 shows the discrepancies in the TMP values of the M-MBR and the MP-MBR (control-MBR) during 60 days of operation. The TMP in the M-MBR reached above 50 kPa until day 17 with an average fouling rate of TMP of 3.01 kPa d−1, while the TMP in the M-MBR reached above 50 kPa until day 24 with an average fouling rate of TMP of 2.06 kPa d−1. A similar result was reported by Liu et al.5 The significantly low fouling rate in the M-MBR indicates that integrating the magnetic field unit into an MBR system is a promising method to alleviate the negative effects of membrane fouling, thereby prolonging the operation periods for MBRs.3.2 Zeta potential and particle sizes of mixed liquor colloid
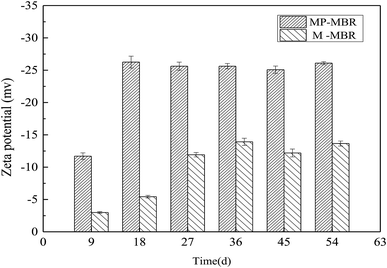
Zeta potential is an important indicator of membrane fouling. During the operation period, the value of the zeta potential of the two reactors decreased in the early stage and remained at a lower level in the later stage, with a small fluctuation. The zeta potential of the MP-MBR changed from −11.7 mV to −26.255 mV, with a mean value of −23.395 mV, while the zeta potential of the M-MBR changed from −2.989 mV to −13.917 mV, with a mean value of −10.020 mV. The value of the zeta potential of the M-MBR was 57.17% smaller than that of the MP-MBR, which indicated that the magnetic field played a very significant role in decreasing the zeta potential in the mixed liquor colloid. Zhou et al.,20 in their study, documented similar results and found that the maximum sludge zeta potential reduction (46.5% to 51.4%) was achieved when the center of the magnetic field was approximately 0.4 T. Li et al.21 also found that the zeta potential of the sludge was greatly influenced by a parallel magnetic field and that the largest reduction was to 46.5% (Fig. 3).One cause of the difference in membrane fouling rates was the variation in the distribution of particle sizes in the mixed liquor. The average particle sizes of the sludge in the two reactors were detected. In the control-MBR, the particle sizes of the sludge changed from 117.32 μm to 162.09 μm, which was not a significant increase. The size of the particles in the sludge in the M-MBR was always larger than that in the control-MBR, which increased evidently from 146.22 μm to 280.17 μm. There was a marked increase in the particle size from 28.89 μm to 118.08 μm, showing that the application of a magnetic field could significantly increase the sludge particle size.
3.3 Effect of a low-strength magnetic field on SMP
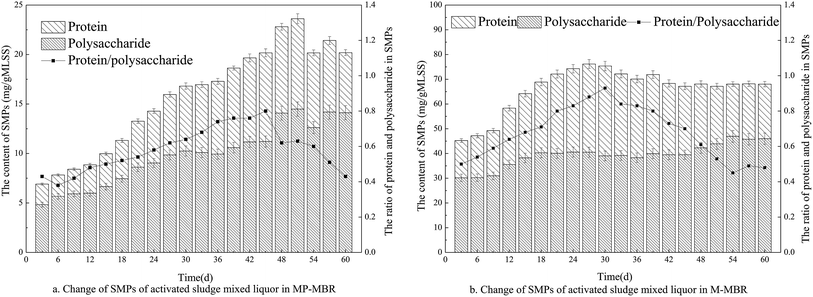
According to previous reports, SMPs have been found to play an important role in membrane fouling.22,23 Polysaccharides and proteins as the major components of SMP were analyzed periodically from the mixed liquors of each MBR.According to Fig. 4, the SMP content in the two reactors increased with time, and as expected, the SMP content and the proportion of proteins and polysaccharides in the M-MBR remained lower than those in the MP-MBR. It is evident that polysaccharides are the dominant component in the SMPs in both reactors. The lower protein to polysaccharide ratio in the M-MBR implies better settleability due to the hydrophobic nature of proteins. SMPs have been found to play an important role in membrane fouling.24 It has been reported that the variations in EPS and SMP simply reflect changes in the microbial community.25 Consequently, it is necessary to compare microbial communities of both the MBRs.
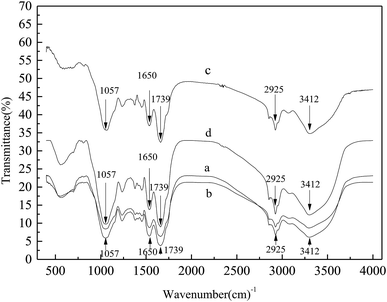
To corroborate the degradation of SMPs in bound water in sludge due to adsorption and bio-oxidation, FTIR analysis was performed to determine the variations in the functional groups of the sludge samples and the cake layer. As shown in Fig. 5, peaks of the functional groups of proteins and polysaccharides were clearly distinguished in the FTIR spectra.
From the spectra in Fig. 5, the trends of the cake layer on the membrane surface and for a mixed liquid were always the same. There were five additional significant absorption peaks in the mixed liquid spectra, whose wavenumbers were 1057 cm−1, 1650 cm−1, 1739 cm−1, 2925 cm−1 and 3412 cm−1.
The 3412 cm−1 absorption peak was generated by the oscillatory contraction of the O–H bond, possibly indicating the presence of organic acids. The 2925 cm−1 absorption peak was generated by the oscillatory contraction of the aliphatic C–H bond, and the 1650 cm−1 absorption peak was generated by the oscillatory contraction of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond and its amide-associated state, illustrating the existence of amide compounds; the structure of the amide compounds was the typical structure of proteins.26 The 1739 cm−1 absorption peak was generated by the carbonyl C
N bond and its amide-associated state, illustrating the existence of amide compounds; the structure of the amide compounds was the typical structure of proteins.26 The 1739 cm−1 absorption peak was generated by the carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, which may be related to polysaccharides, and the 1057 cm−1 absorption peak was generated by methyl C–H symmetric bending vibrations, which is very useful for the identification of methyl groups and may also be related to carbohydrates.27
O bond, which may be related to polysaccharides, and the 1057 cm−1 absorption peak was generated by methyl C–H symmetric bending vibrations, which is very useful for the identification of methyl groups and may also be related to carbohydrates.27
The amount of substances in the cake layer and the mixture of the absorption peaks in the M-MBR were significantly less than those in the MP-MBR, showing that the amounts of organic acids, polysaccharides and proteins in the M-MBR were significantly less than those in the MP-MBR. This conclusion is consistent with the conclusions presented in Section 3.1. As a type of protein, dehydrogenase (DHA) in living organisms can realize the hydrogen atom activation of organic matter in the oxidation process, the transfer of energy and the promotion of material circulation. The activity of dehydrogenase in a mixed liquid in the M-MBR was always higher than that in the MP-MBR. The dehydrogenase activity in the MP-MBR was between 41.27 mg TF g−1 MLSS h−1 and 47.22 mg TF g−1 MLSS h−1 and showed negligible changes, while the dehydrogenase activity in the M-MBR was between 60.10 mg TF g−1 MLSS h−1 and 63.32 mg TF g−1 MLSS h−1, which was increased by 27.07% in the case without a magnetic field. Jung et al.28 showed that magnetic fields could enhance the rate of phenol biodegradation in the activated sludge mixed culture by 30%. Ren et al.29 found that lower magnetic fields (15–25 mT) enhanced the permeability of the cell membrane and improved the activity of the oil-degrading bacteria. Thus, the appropriate magnetic field intensity plays a role in promoting the metabolism of microorganisms. The magnetic field stimulated the synthesis of microbial cells and the secretion of more dehydrogenase; thus, the total amount of dehydrogenase in the system increased, and more substrate was consumed.
3.4 Effect of a low-strength magnetic field on microbial community and composition
After necessary denoising and filtering, the effective reads from each sample were used for further analyses. The diversity estimators of Chao Index, Ace Shannon, Simpson and Good's coverage for each sample are illustrated in Table 1. In this study, the Good's coverage of all samples was above 0.93, indicating that the sequencing depth could reflect the real microbial community for both MBRs.
| Samples | Optimized sequences | OTUs | Shannon index | Ace index | Chao index | Good's coverage | Simpson |
|---|---|---|---|---|---|---|---|
| HHY0 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 139 139 |
1323 | 3.333 | 8408.230 | 4259.374 | 0.965 | 0.137 |
| HHY1 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 892 892 |
1774 | 4.015 | 9264.255 | 5246.579 | 0.954 | 0.078 |
| HHY2 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 573 573 |
1823 | 3.869 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 669.457 669.457 |
6867.809 | 0.955 | 0.070 |
| MO1 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
1937 | 4.839 | 9752.440 | 5240.780 | 0.932 | 0.068 |
| MO2 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 249 249 |
2572 | 4.381 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 802.746 802.746 |
7129.809 | 0.932 | 0.038 |
It was apparent that both the microbial richness (estimated by Chao 1 and Ace) and diversity (estimated by Shannon and Simpson) of bulk sludge and biofilm layer in M-MBR were higher than those in MP-MBR. Thus it could be inferred that the microbial community in M-MBR had greater richness and more diversity than that in MP-MBR, and further comparison of the microbial composition was conducted to reveal more information on the microbial community differences between those in the M-MBR and MP-MBR.
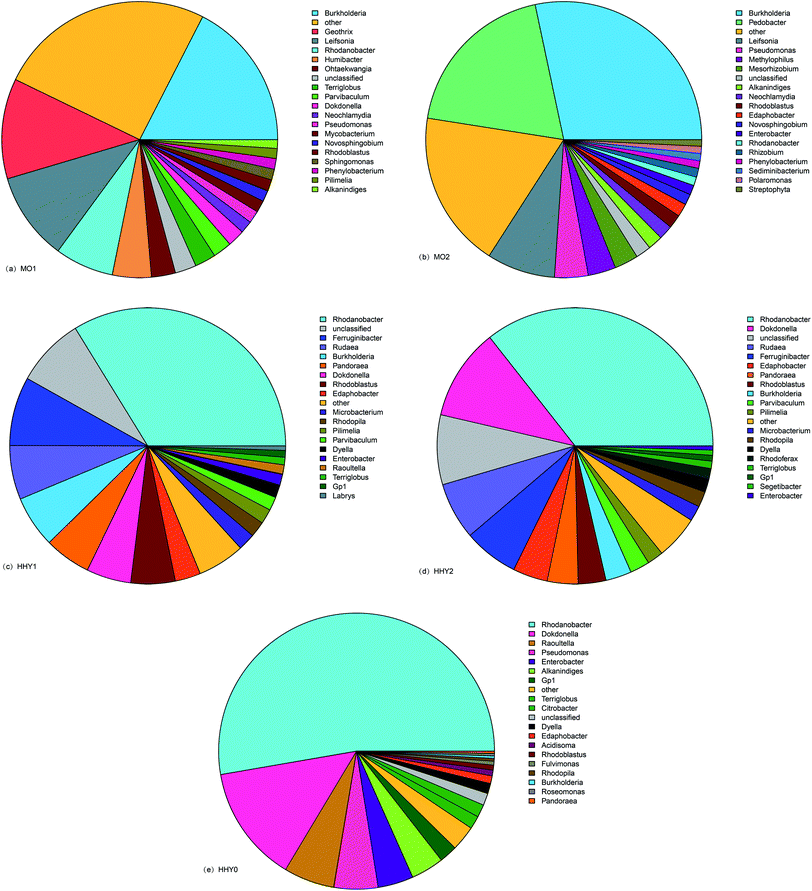
Fig. 6 shows that some abundance differences existed among sample species at the genus level; the abundances of MO1, MO2, HHY1, HHY2, and HHY0 were 20, 23, 17, 18 and 15, respectively. From the viewpoint of the overall structure, MO1 and MO2, HHY1 and HHY2 were similar, but HHY0 was different from the others. The deep blue slices of the MO1 and MO2 pie charts represent the Burkholderia genus, which accounted for 17% of MO1 and 28% of MO2, 5% of HHY1 and 4% of HHY2, respectively; HHY0 did not contain this genus. The content of this genus on the membrane surface was much higher than that in the mixture. The members of the genus Burkholderia were formerly classified as Pseudomonas, whose surface has Gram-negative bacteria, high movability and aerobic rod-shaped bacteria. This genus plays a large role in the degradation of organic matter; thus, it is likely that this genus can degrade contaminants on the membrane surface. The red slice of the MO1 pie chart represents the genus of bacteria, whose proportion was 12%, but there were no such bacteria in MO2 and the other samples; thus, there are currently only a few studies of this species, and the degradation of organic matter is not obvious. The green slice of the MO2 pie chart represents Bacillus which accounted for 23% and only existed in MO2. And Bacillus is widely distributed in the reduction environment. These species are particularly helpful in organic matter degradation, which also explains the lesser membrane pollution in the M-MBR. The dark gray slices of the MO1 and MO2 pie charts represent the Lai Salmonella genus which accounted for 12% and 11%, respectively. Although there were fewer studies on these bacteria, they played an irreplaceable role in the degradation of pollutants on the membrane surface in this study.
The above results show that a change in the microbial community occurs under a magnetic field.30,31 Shi32 found that a succession of alternate dominant species of different metabolic types was formed with an increase in the magnetic induction intensity. Shan33 found that the magnetic effect increases the activity of some bacterial species, which adapt to the magnetic environment. The other species that are less adaptive are eliminated. The superior species will dominate as the variety decreases. These types of species are more likely to form the steady structure of the community. Ni34 also showed that denitrifying bacteria biomass under a constant magnetic field are higher in activated sludge samples.
4 Conclusion
This study provides a promising technique for significantly alleviating membrane fouling by introducing a low-strength magnetic field in MBRs from the two main findings: (1) the application of a magnetic field decreases the zeta potential and increases the particle size, which markedly contributes to the better filtration performance of MBRs; (2) the addition of a low-strength magnetic field significantly affected the microbial community and composition, which has greater richness and more diversity in M-MBRs, and consequently affected the membrane fouling evolution.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 51209053) and the Open Project of the State Key Laboratory of Urban Water Resource and Environment of the Harbin Institute of Technology (No. QA201619).References
- B. Lesjean, S. Rosenberger, C. Laabs, M. Jekel, R. Gnirss and G. Amy, Correlation between membrane fouling and soluble/colloidal organic sub-stances in membrane bioreactors for municipal wastewater treatment, Water Sci. Technol., 2005, 51(6–7), 11–18 Search PubMed.
- L. Deng, W. Guo, H. H. Ngo, H. Zhang, J. Wang, J. Li, S. Xia and Y. Wu, Biofouling and control approaches in membrane bioreactors, Bioresour. Technol., 2016, 221, 656–665 CrossRef CAS PubMed.
- A. P. Liu, Z. Y. Chen, K. M. Li and S. Guo, Cultivation of aerobic granular sludge with magnetic seed and its effect on membrane fouling in MBR, China Water Wastewater, 2010, 26, 1–4 CAS.
- Z. J. Ren and J. C. Shao, Mechanism of Membrane Fouling Control in the Magnetic Activated Sludge (MAS) Process, Desalin. Water Treat., 2017, 66, 111–116 CrossRef CAS.
- Y. Liu, Q. Liu, J. Li, H. H. Ngo, W. Guo, J. Hu, M. T. Gao, Q. Wang and Y. Hou, Effect of magnetic powder on membrane fouling mitigation and microbial community/composition in membrane bioreactors (MBRs) for municipal wastewater treatment, Bioresour. Technol., 2017, 249(10), 377–385 Search PubMed.
- H. Wang, Z. Chen, J. Miao and Y. Li, A novel approach for mitigation of membrane fouling: concomitant use of flocculant and magnetic powder, Bioresour. Technol., 2016, 209, 318–326 CrossRef CAS PubMed.
- Q. Chen, W. Nong and W. Cui, Mechanism and Application of Magnetic Technology in Wastewater Treatment, J. Environ. Sci. Manage., 2012, 8, 110–114 Search PubMed.
- G. U. Semblante, S. D. R. Tampubolon, L. Zhang, S. J. You, Y. F. Lin, T. C. Chang and F. C. Yen, Fouling reduction in membrane reactor through magnetic particles, J. Membr. Sci., 2013, 435(10), 62–70 CrossRef CAS.
- E. Illes and E. Tombacz, The role of variable surface charge and surface complexation in the adsorption of humic acid on magnetite, Colloids Surf., A, 2003, 230(1–3), 99–109 CrossRef CAS.
- L. Ai, C. Zhang and Z. Chen, Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite, J. Hazard. Mater., 2011, 192(3), 1515–1524 CrossRef CAS PubMed.
- Q. X. Han and F. Q. Shao, The Increasing effect of magnetic field on wastewater treatment by activated sludge process, J. Fushun Pet. Inst., 2002, 8–10 CAS.
- F. Ma, Q. Wang and X. S. Zhu, Application Status and Development Trend of Magnetic Technology in Wastewater Treatment, China Water Wastewater, 2010, 26(14), 34–37 CAS.
- Z. Zhou, B. X. Bian and S. Li, Magnetic treatment for lowering zeta potential of biological sludge, Environ. Pollut., 2007, 29, 174–177 CAS.
- M. Lebkowska, A. Rutkowska-Narozniak, E. Pajor, A. Tabernacka and M. Zaleska-Radziwill, Impact of a static magnetic field on biodegradation of wastewater compounds and bacteria recombination, Environ. Sci. Pollut. Res., 2018, 25(23), 22571–22583 CrossRef CAS PubMed.
- H. Yavuz and S. S. Celebi, Effects of magnetic field on activity of activated sludge in wastewater treatment, Enzyme Microb. Technol., 2000, 26(1), 22–27 CrossRef CAS.
- Z. L. Zhang, Analysis on Efficiency of Magnetic Field Improved MP-MBR for Vessel Oily Wastewater Treatment and Membrane Fouling Control, Harbin Engineering University, 2016 Search PubMed.
- W. F. Yuan, B. S. Liu and Z. N. Wang, Purifying Oil-Bearing Sewage Water By Using Magnetic Powder, Environ. Chem., 1991, 4, 12–18 Search PubMed.
- A. D. Eaton, L. S. Clesceri and A. E. Greenberg, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington, DC, 2005, pp. 20001–23710 Search PubMed.
- T. Burdock, M. Brooks, A. Ghaly and D. Dave, Effect of assay conditions on the measurement of dehydrogenase activity of using triphenyl tetrazolium chloride, Adv. Biosci. Biotechnol., 2011, 2(4), 12 Search PubMed.
- S. Li, B. X. Bian and Z. Zhou, Study on Effect of Magnetic Treatment on Sludge Dewatering Ability, Res. Environ. Sci., 2007, 20(3), 119–123 Search PubMed.
- J. Li, Y. L. Yi, Y. Jiao, D. G. Zhang, Ch. L. Yuan, X. L. Cheng and J. D. Sun, Effect of magnetic field on soil microbes and soil enzyme activities in brown earth, Chin. J. Soil Sci., 2007, 38, 957–961 CAS.
- S. Rosenberger and M. Kraume, Filterability of activated sludge in membrane bioreactors, Desalination, 2002, 146(1–3), 373–379 CrossRef CAS.
- J. Li, F. Yang, Y. Li, F. S. Wong and H. C. Chua, Impact of biological constituents and properties of activated sludge on membrane fouling in a novel submerged membrane bioreactor, Desalination, 2008, 225(1–3), 356–365 CrossRef CAS.
- S. Rosenberger, H. Evenblij, S. Poele, T. Wintgens and C. Laabs, The importance of liquid phase analyses to understand fouling in membrane assisted activated sludge processes-six case studies of different European research groups, J. Membr. Sci., 2005, 263(1–2), 113–126 CrossRef CAS.
- Y. Ding, Y. Tian, J. Liu, N. Li, J. Zhang, W. Zuo and Z. Li, Investigation of microbial structure and composition involved in membrane fouling in the forward osmosis membrane bioreactor treating anaerobic bioreactor effluent, Chem. Eng. J., 2016, 286, 198–207 CrossRef CAS.
- A. Omoike and J. Chorover, Adsorption to goethite of extracellular polymeric substances from Bacillus subtilis, Geochim. Cosmochim. Acta, 2006, 70(4), 827–838 CrossRef CAS.
- J. R. Pan, Y. C. Su, C. Huang and H. C. Lee, Effect of sludge characteristics on membrane fouling in membrane bioreactors, J. Membr. Sci., 2010, 349(1), 287–294 CrossRef CAS.
- J. Jung, B. Sanji, S. Godbole and S. Sofer, Biodegradation of Phenol: A comparative study with and without applying magnetic fields, J. Chem. Technol. Biotechnol., 2010, 56(1), 73–76 CrossRef.
- Z. J. Ren, X. D. Leng, Z. X. Zhang and H. N. Feng, Effect of low-strength magnetic fields on the oil removal performance of oil-degrading microorganisms, Desalin. Water Treat., 2018, 120, 133–140 CrossRef.
- H. Chen, Research on PHAs production from activated sludge under the co-effect of “feast”/” “famine” condition and magnetic field, Zhejiang University, 2008 Search PubMed.
- R. Radhakrishnan and B. D. Kumari, Influence of pulsed magnetic field on soybean (Glycine max L.) seed germination, seedling growth and soil microbial population, Indian J. Biochem. Biophys., 2013, 50(4), 312–317 CAS.
- B. Shi, Study on the effects of low-intensity steady magnetic field on wastewater treatment with biofilm reactor, Jinan University, 2010 Search PubMed.
- J. Shan, Effect of magnetic system in SBR-denitrification and the microbial community dynamic analysis, Harbin Institute of Technology, 2010 Search PubMed.
- J. Y. Ni, The effect of stationary magnetic field on the anaerobic activated sludge performance, Shandong University, 2012 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |