Open Access Article
Open Access ArticleACE-inhibitory and antioxidant peptides from coconut cake albumin hydrolysates: purification, identification and synthesis†
Yajun Zheng‡
 *ab,
Yan Li‡ab and
Guifeng Lia
*ab,
Yan Li‡ab and
Guifeng Lia
aCollege of Food Science, Shanxi Normal University, Linfen, 041004, China. E-mail: zyj_coconut@163.com; Fax: +86-0357-2051482; Tel: +86-13976563642
bCollege of Life Sciences and Food Engineering of Hebei Engineering University, Handan 056038, China
First published on 18th February 2019
Abstract
Coconut cake albumin was hydrolysed by sequential digestion with alcalase, flavourzyme, pepsin and trypsin to purify bioactive peptides with ACE-inhibitory and antioxidant activities. Following fractionation with sequential ultrafiltration, Sephadex gel chromatography and RP-HPLC, three novel peptides KAQYPYV, KIIIYN and KILIYG were identified. KAQYPYV, KIIIYN and KILIYG provided an IC50 value of 37.06 μM, 58.72 μM and 53.31 μM on ACE-inhibitory activity, respectively. For hydroxyl radical scavenging activity, KAQYPYV, KIIIYN and KILIYG demonstrated an IC50 value of 70.84 μM, 77.62 μM and 95.23 μM, respectively. All the three peptides exhibited a mixed modality of noncompetitive and uncompetitive inhibition on ACE and KAQYPYV showed good stability against gastrointestinal enzymes digestion. Moreover, these peptides could effectively lower intracellular endothelin-1 content without significant cytotoxicity, and protected vascular endothelial cells from reactive oxygen species mediated damage. Furthermore, KAQYPYV, KIIIYN and KILIYG also demonstrated high ion chelating ability (62.13% ± 4.21%, 64.66% ± 5.51% and 69.82% ± 7.24% at 0.1 mg mL−1, respectively) and considerable superoxide radical scavenging activity (39.30% ± 2.72%, 46.79% ± 1.70% and 51.16% ± 3.23% at 1.0 mg mL−1, respectively). These results indicate that coconut cake albumin is a potential source of bioactive peptides possessing ACE-inhibitory and antioxidant activities.
1. Introduction
It has been demonstrated that hypertension is a significant risk factor for cardiovascular diseases and threatens the health of one fourth of the adults in the world.1,2 Angiotensin-I converting enzyme (ACE) makes a positive contribution to hypertension since it can catalyze the conversion of inactive angiotensin-I into angiotensin-II (a potent vasoconstrictor) and deactivate bradykinin (a potent vasodilator) in the renin-angiotensin system, leading to increase in blood pressure.3 Thus, hypertension is commonly treated by drugs such as captopril and enalapril, which can lower the blood pressure by inhibiting ACE activity. Except for the renin–angiotensin system, the regulation of blood pressure is regulated by other complex systems, involving the sympathetic nervous system, the endothelin system, kidney and fluid balance mechanisms and the nitric oxide system.4 In the endothelin system, the endothelin converting enzyme cleaves the biologically inactive intermediate termed big endothelin-1 to form endothelin-1 (ET-1) which has powerful vasoconstrictor and pressor properties, cause the rise of blood pressure.5 Moreover, increasing epidemiological evidence demonstrated that oxidative stress and associated oxidative damage were mediators in hypertension and other chronic diseases like arthritis, diabetes and neurodegenerative diseases.6 Oxidative stress also can disturb the balance between oxidants and antioxidants in cells which is very important to human's health.7More studies demonstrated that the control and improvement of diet could handle both hypertension and oxidative stress, which is helpful to prevent the occurrence of hypertension and other cardiovascular diseases. Therefore development of nutraceuticals or functional foods would be a low-cost alternative to conventional synthetic therapeutics which always has possible harmful side effects.4 In the recent years, ACE-inhibitory and/or antioxidant peptides obtained from food natural sources receiving an increasing interest in the world. Many ACE-inhibitory and/or antioxidant peptides has been purification and identified from food-derived proteins, including animal proteins, plant proteins and marine proteins.7–17 These bioactive peptides are usually short protein sequences (2–20 amino acids long), and some of them can reveal other functions like antimicrobial, beyond antioxidative and ACE-inhibitory activity.9
Albumin is commonly known as water-soluble protein and makes a significant contribution to the human diet. Some albumins such as lactoalbumin, ovalbumin and wheat albumin can exert physiological hormone-like effects on humans beyond their nutritional value.3,18 Coconut cake is a byproduct of the coconut oil industry, contains about 10% to 16% of protein, in which albumin and globulin are the predominant fractions, accounting for about 31% and 60%, respectively.19 Previous study found that coconut albumin could effectively protect DNA against oxidative damage and exhibited considerable superoxide radical scavenging activity (14.99%), reducing power (0.122 ± 0.010) and ACE inhibition activity (12.67 ± 3.08%),20 indicating that bioactivity peptides with antioxidant and potential antihypertensive effect may be isolated from it. Although three antimicrobial peptides (Cn-AMP1, Cn-AMP2 and Cn-AMP3) were identified from green coconut water,21 and several ACE-inhibitory and antioxidant peptides (Pro-Gln-Phe-Tyr-Trp, Val-Val-Leu-Tyr-Arg and Arg-Pro-Glu-Ile-Val) were identified from coconut cake globulin hydrolysates.22,23 There is no study on antioxidant and ACE-inhibitory peptides derived from coconut cake albumin. The annual production of coconut cake is about 63 million tons in the world,24 meaning that a large amount of albumin could be produced. If peptides with high antihypertensive or antioxidant activity could be obtained from it, the usage of coconut cake will be expanded and remarkable economic benefits may be achieved. Therefore, the current study aimed at the isolation, identification, synthesis and characterization of ACE-inhibitory and/or antioxidative peptides from coconut cake albumin.
2. Materials and methods
2.1. Materials and reagents
Coconut cake was obtained from South Coconut Food Processing Co., China. Flavourzyme (5 × 104 U g−1), alcalase (1 × 105 U g−1), pepsin (1 × 105 U g−1) and trypsin (1 × 105 U g−1) were purchased from Shanghai Biotech. Co., Ltd. (China). ACE (from rabbit lung) and N-Hippuryl-His-Leu hydrate (HHL) were purchased from Sigma Co., USA. The human endothelial cell line (EA.hy926) was from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Sijiqing Biological Co., China. Human endothelin-1 (ET-1) Elisa kit was purchased from Ruiqi Biological Co. Ltd., China. GSH (glutathione), ferrozine and 2-Deoxy-D-ribose were purchased from Yeyuan Biotech. Co., Ltd., Shanghai, China. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and other chemicals and reagents were of analytical grade. Three isolated peptides (KAQYPYV, KIIIYN and KILIYG) were synthesized using the method of the solid phase procedure described by Zhang et al.252.2. Extraction of coconut cake albumin (CCA)
CCA was prepared according to the method described by Kwon et al.26 Coconut cake was defatted three times with n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, g mL−1) and dried, ground and passed through a sieve of 0.2 mm mesh. The obtained defatted coconut cake were mixed with distilled water (1
10, g mL−1) and dried, ground and passed through a sieve of 0.2 mm mesh. The obtained defatted coconut cake were mixed with distilled water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, g mL−1), stirred at 4 °C for 1 h and then the mixture was filtrated. The filtrate was collected and the residue was resuspended in distilled water and stirred at 4 °C for 1 h. This step was repeated in three times. The filtrate was pooled and centrifuged at 10000g for 30 min, the supernatant was collected, dialyzed against distilled water for 24 h at 4 °C. Then the dialyzation was centrifuged at 10000g for 30 min, and the supernatant was collected and lyophilized. Then CCA was obtained and stored at −20 °C.
15, g mL−1), stirred at 4 °C for 1 h and then the mixture was filtrated. The filtrate was collected and the residue was resuspended in distilled water and stirred at 4 °C for 1 h. This step was repeated in three times. The filtrate was pooled and centrifuged at 10000g for 30 min, the supernatant was collected, dialyzed against distilled water for 24 h at 4 °C. Then the dialyzation was centrifuged at 10000g for 30 min, and the supernatant was collected and lyophilized. Then CCA was obtained and stored at −20 °C.
2.3. Hydrolysis of coconut cake albumin
The enzymatic hydrolysis of CCA was performed following the method reported by Zheng et al.27 The CCA solution (5 g/100 mL) was transferred into polyethylene bags, sealed under vacuum and subjected to high pressure treatment using a HPP-L3 high-pressure apparatus (Tianjin HuaTaiSenMiao Biotechnology Co. Ltd., China) at 400 MPa, 35 °C for 15 min. Then the CCA solution was separately hydrolyzed by alcalase, flavourzyme, pepsin, trypsin and a mixture of these four enzymes. The reaction conditions of each enzyme and hydrolysis degree (DH), ACE-inhibitory and antioxidant activity of coconut cake albumin hydrolysates (CCAH) were shown in Table 1. Results showed that when CCA was sequentially hydrolyzed by alcalase, flavourzyme, pepsin and trypsin, CCAH demonstrated the highest ACE-inhibitory and antioxidant activity. Thus, these four enzymes were chose to hydrolyze CCA in the following sequence: alcalase, flavourzyme, pepsin and trypsin. Moreover, the use of pepsin and trypsin was to improve the stability of peptides from coconut cake albumin against the gastrointestinal enzymes digestion. At the end of the hydrolysis, the reaction solutions were incubated in 100 °C for 5 min to inactivate the enzyme, and then centrifuged at 10000g, 4 °C for 20 min. The supernatants were collected and lyophilized to obtain CCAH. The degree of hydrolysis (DH), ACE-inhibitory ability and hydroxyl radical (·OH) scavenging activity of coconut cake albumin hydrolysis (CCAH) were determined using the methods of Adler-Nissen, Jimsheena & Gowda and the 2-deoxyribose oxidation method,8,28,29 respectively.| Proteolysisb | DH (%) | ACE-inhibitory activity (%) | ·OH scavenging activity (%) |
|---|---|---|---|
| a Before the proteolysis, coconut cake albumin was subjected to the pretreatments of high pressure (400 MPa, 15 min).b Proteolysis separately by each enzyme at respective conditions as follows: alcalase at 45 °C, pH 8.5, usage dose: 2 g/100 g protein, hydrolyzed time: 2 h; flavourzyme at 50 °C, pH 7.0, usage dose: 2 g/100 g protein, 2 h; pepsin at 37 °C, pH 2.0, usage dose: 2 g/100 g protein, 2 h; and trypsin at 37 °C, pH 7.0, usage dose: 2 g/100 g protein, 2 h.c Proteolysis using enzymes in sequence of alcalase (45 °C, pH 8.5, usage dose: 0.5 g/100 g protein, hydrolyzed time: 2 h), flavourzyme (50 °C, pH 7.0, usage dose: 0.5 g/100 g protein, 2 h), pepsin (37 °C, pH 2.0, usage dose: 0.3 g/100 g protein, 1 h), trypsin (37 °C, pH 7.0, usage dose: 0.3 g/100 g protein, 1 h). Before the addition of each enzyme, the temperature and pH of reaction solution were adjusted to the optimum of the enzyme. Small letters on (d–g) the columns match results that are significantly different (P < 0.05). | |||
| Alcalase | 20.94 ± 2.26e | 35.05 ± 3.67e | 42.56 ± 2.46e |
| Flavourzyme | 16.29 ± 3.11ef | 29.10 ± 3.33f | 36.33 ± 2.79f |
| Pepsin | 3.15 ± 0.61g | 9.94 ± 1.63h | 17.37 ± 1.09h |
| Trypsin | 10.67 ± 1.34f | 20.80 ± 3.08g | 24.02 ± 1.93g |
| Alcalase, flavourzyme, pepsin and trypsinc | 27.62 ± 3.94d | 48.34 ± 1.41d | 55.02 ± 6.28d |
2.4. Degree of hydrolysis (DH)
The DH of CCAH was analyzed using the trinitrobenzenesulfonic acid (TNBS) method.28 Briefly, 250 μL of coconut cake albumin hydrolysate solution was mixed with 2.0 mL of phosphate buffer (0.2 M, pH 8.2) and 2.0 mL of TNBS solution (1 mg mL−1). The mixture was incubated at 50 °C for 60 min in dark, and then 4 mL of 0.1 M HCl was added to terminate the reaction. After cooled at room temperature for 30 min, the absorption was measured at 340 nm to quantitate free amino nitrogen. L-Leucine (0–2.0 mM, dissolved in 1% SDS) was used to construct a standard curve. Total amino nitrogen in protein was obtained by acid hydrolysis with 6.0 M HCl at 110 °C for 24 h, filtered and neutralized. The DH was calculated as follows:| DH (%) = (AN2 − AN1)/Npb × 100 | (1) |
2.5. ACE-inhibitory activity and inhibition pattern
According to the method of Jimsheena & Gowda,29 50 μL of sample solution, 50 μL of ACE (25 mill units permL) and 150 μL of 8.3 mM HHL were mixed and incubated at 37 °C for 60 min, followed by termination of the reaction by adding 250 μL of 1 M HCl. Then 1.4 mL of ethyl acetate was added and the mixture was vortexed for 5 s and centrifuged at 14100g for 5 min. One mL of the upper organic phase was transferred into a test tube and placed in a vacuum oven at 80 °C for 1 h. Subsequently, 2 mL of distilled water was added and the absorbance at 228 nm was read. The IC50 value was defined as the concentration of inhibitor required to inhibit 50% of the ACE activity. The inhibition (%) was calculated as below:| ACE inhibition (%) = (1 − AS/AC) × 100 | (2) |
2.6. Scavenging activity of hydroxyl radical
Hydroxyl radical (·OH) scavenging activity was assayed using the 2-deoxyribose oxidation method.8 100 μL of CCAH solution was mixed with 1.4 mL of sodium phosphate buffer (0.1 M, pH 7.4), 100 μL of 10 mM 2-deoxyribose, 100 μL of 10 mM FeSO4, 100 μL of 10 mM ethylenediaminetetra acetic acid (EDTA) and 100 μL of H2O2 (10 mM). After incubation at 37 °C for 1 h, 1.0 mL of trichloroacetic acid (28 mg mL−1) and 1.0 mL of 2-thiobarbituric acid (10 mg mL−1) were added. Then the mixture was kept in boiling water bath for 20 min. After cooled to room temperature, the absorbance of the mixture at 532 nm was read. The activity was determined using the equation:| ·OH scavenging activity (%) = [1 − (AS − AB)/AC] × 100 | (3) |
2.7. Purification of the bioactivity peptides
CCAH was separated by four steps in the following sequence: ultrafiltration, Sephadex G-25 gel chromatography, Sephadex G-15 gel chromatography and reversed-phase high performance liquid chromatography (RP-HPLC). Firstly, the CCAH was filtered sequentially using an ultrafiltration unit (Pellicon XL, Millipore, USA) through two ultrafiltration membranes with molecular weight (MW) cut-off of 5 and 3 kDa, respectively. Three fractions were obtained: CCAH-I with MW > 5 kDa, CCAH-II with MW 3–5 kDa and CCAH-III with MW < 3 kDa. The fraction with the highest ACE-inhibitory activity was further separated by a Sephadex G-25 gel filtration column (Φ2.6 cm × 60 cm) equilibrated with distilled water at 1.0 mL min−1. The column was eluted with distilled water and monitored at 220 nm. Fractions were collected, lyophilized and their ACE-inhibitory activity and ·OH scavenging activity were measured. The fraction showing the highest ACE-inhibitory activity and/or ·OH scavenging activity was separated by Sephadex G-15 chromatography (Φ1.2 cm × 100 cm) equilibrated with distilled water at 0.8 mL min−1. The column was eluted with distilled water and monitored at 220 nm. The fraction showing the highest ACE-inhibitory activity and/or ·OH scavenging activity was separated by RP-HPLC on a Zorbax semi-preparative C18 column (Φ9.4 mm × 250 mm, Agilent Technologies, USA), using a linear gradient of acetonitrile containing 0.1% TFA (5–30%, in 30 min) at a flow rate of 2.5 mL min−1. The fraction showing the highest ACE-inhibitory activity and/or ·OH scavenging activity was further isolated on a Zorbax analysis C18 column (Φ4.6 mm × 250 mm, Agilent Technologies, USA) with a linear gradient of acetonitrile containing 0.1% TFA (5–25%, in 20 min) at a flow rate of 1.0 mL min−1. The fractions with high ACE-inhibitory activity and/or antioxidant activity were rechromatographed to confirm their purity on the same analytical C18 column at a flow rate of 1.0 mL min−1 with a linear gradient of acetonitrile containing 0.1% TFA (15–30%, in 15 min). The fractions with high ACE-inhibitory activity and/or ·OH scavenging activity were subjected to LC-MS/MS analysis.2.8. Molecular mass and amino acid sequence of the purified peptides
To identify molecular mass and amino acid sequence, the purified fractions from RP-HPLC with highly ACE-inhibitory and antioxidant activity, namely A4e-3 was analyzed by LC-MS/MS with a coupled Eksigent Nano LC (Eksigent Technologies, Dublin, CA, USA) and Thermo LTQ linear ion trap mass spectrometer (Thermo Fisher, San Jose, CA, USA). Briefly, acetonitrile (2%, v/v) containing 0.1% formic acid was used as mobile phase A and acetonitrile (80%, v/v) with 0.1% formic acid as mobile phase B for Nano-LC separation. Gradient elution was carried out according to the following process: phase B was linearly increased from 5% to 50% within 85 min; afterwards, phase B was increased to 95% within the following 10 min, and then maintained at 95% for 30 min. Sequence identification of the eluted peptides were analyzed by MS/MS, and the parameters were: spray voltage: 2.2 kV, normalized collision energy: 35%, capillary temperature: 200 °C, and scan range: m/z 300–2000. The acquired MS/MS data were interpreted using the bioinformatics search engine Mascot version 2.1.0 (Matrix Sciences, London, UK). The peptide sequences were matched to the published sequences of coconut proteins from the National Center for Biotechnology Information (NCBI, Bethesda, MD, USA) database.2.9. Stability of the synthetic peptides
Following the method of Tavares et al.,31 peptide solutions were adjusted to pH 2.0, mixed with pepsin (E/S = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35) and incubated at 37 °C for 1 h. Afterwards, the reaction mixture was adjusted to pH 7.0, pancreatin was added (1 g/25 g peptide) and incubated at 37 °C for 2 h, and then the digestion was terminated by boiling for 10 min. The ACE-inhibitory activity and ·OH scavenging activity of the treated synthetic peptides and the untreated synthetic peptides were determined.
35) and incubated at 37 °C for 1 h. Afterwards, the reaction mixture was adjusted to pH 7.0, pancreatin was added (1 g/25 g peptide) and incubated at 37 °C for 2 h, and then the digestion was terminated by boiling for 10 min. The ACE-inhibitory activity and ·OH scavenging activity of the treated synthetic peptides and the untreated synthetic peptides were determined.
2.10. Cell culture and cell viability determination
The EA.hy926 cells were seeded in a 96-well plate at a density of 1.0 × 105 cells per mL, cultured in DMEM (containing 1% non-essential amino acid solution, 10% FBS, 100 IU mL−1 penicillin and 100 μg mL−1 streptomycin) in a humidified atmosphere of 5% CO2 at 37 °C for 24 h. Then the wells were washed with phosphate buffer solution (PBS) and cultured in fresh DMEM but without FBS at 37 °C for 12 h. The DMEM without FBS was removed and the cells were cultured in DMEM at 37 °C for another 24 h. Then the cells were treated with different sample concentrations (1.0, 1.5 and 2.0 mg mL−1) for 24 h. Captopril (1 mg mL−1) was used as the positive control, whereas the negative control was treated with only DMEM. Each treatment was repeated in six wells. After treatment, viability of the cells was measured using MTT method as described by Mosmann.32 Briefly, the cells were washed with PBS and 20 μL of MTT (5 mg mL−1) were added into each well and kept at 37 °C for 4 h. Then MTT solution was removed and 100 μL of dimethyl sulfoxide were added. The absorbance was measured at 490 nm by a Varioskan Flash (Thermo Scientific, USA).2.11. Effect on intracellular ET-1 content
The EA.hy926 cells were seeded in a 96-well plate at a density of 1.0 × 105 cells per mL. After being cultured in the DMEM at 37 °C for 24 h, the cells were treated with different concentrations of test samples (1.0, 1.5 and 2.0 mg mL−1) for 48 h. Captopril (1 mg mL−1) was used as positive control and the negative control was treated with only DMEM. After treatment, the growth medium of each well was collected to quantify ET-1 content using the ET-1 Elisa kit following the manufacturer's instructions.2.12. Antioxidant activity of the peptides
| Protective ability% = (AS − AC)/(AB − AC) × 100 | (4) |
| Chelating activity (%) = [1 − (AS − A B)/AC] × 100 | (5) |
2.13. Statistical analysis
All the experiments were repeated at least three times and mean values were used. Data were subjected to analysis of variance and Duncan value with a confidence interval of 95% was calculated to compare means.3. Results and discussion
3.1. ACE-inhibitory and antioxidant activity of CCAH
Subjection to the high pressure treatment and the hydrolysis by the four enzymes, the DH of CCAH was 27.62% ± 3.94%, the ACE inhibition activity and ·OH scavenging ability were 48.34% ± 1.41% and 55.02% ± 6.28%, respectively. High pressure treatment was used to improve the DH and bioactivity of CCAH, for it can increase the susceptibility of proteins to enzymatic action by exposing new cleavage sites that allow enzymes to reach otherwise buried hydrolysis sites, thereby increasing the DH of AACH and release more peptides with antioxidant and/or ACE-inhibitory activity.353.2. Separation by ultrafiltration and Sephadex G-25 gel chromatography
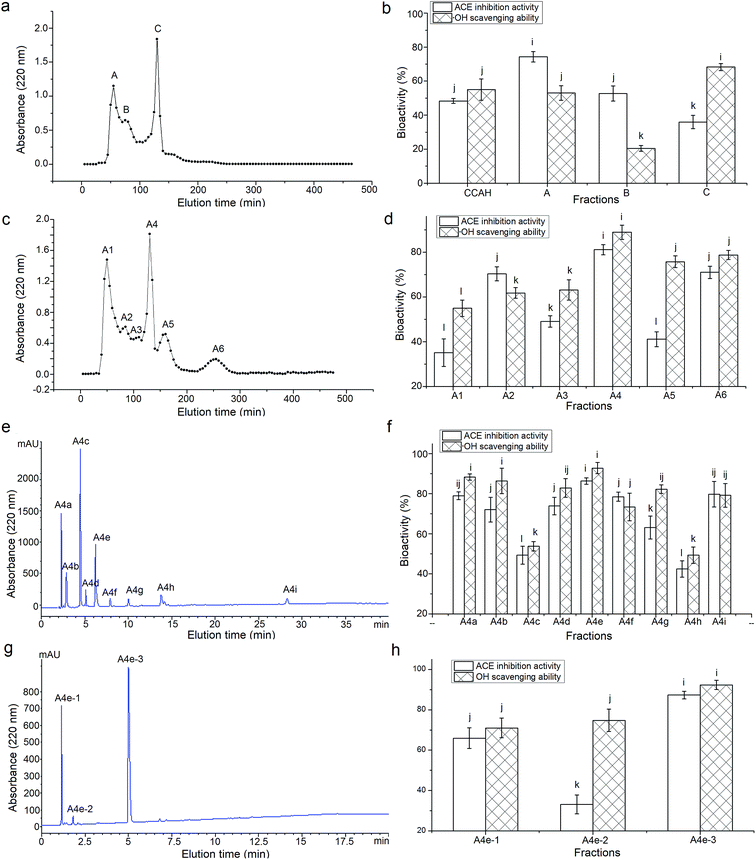
Among the three fractions from ultrafiltration, CCAH-III (MW < 3 kDa) exhibited a significantly higher ACE-inhibitory ability (63.58 ± 5.14% at 1 mg mL−1) than those of CCAH-I (41.64 ± 3.94%) and CCAH-II (49.88 ± 5.02%), which confirm the report that molecular weight (MW) makes a negative contribution to ACE-inhibitory activity of polypeptides.34 Through the Sephadex G-25 gel chromatography, CCAH-III was separated into three major fractions (Fig. 1a), of which the fraction A showed the highest ACE-inhibitory activity (74.29 ± 3.07%, Fig. 1b) among the fractions, while it's ·OH scavenging activity (53.01 ± 4.29%) is lower than that of fraction C (68.27 ± 2.05%). This meant that MW plays important role in the bioactivity of peptides, but it's not the risk factor. Peptides with high ACE-inhibitory don't always have high antioxidant activity at the same time. Both ACE-inhibitory activity and antioxidant ability were the evaluation indicators to separate and choose CCAH, while ACE-inhibitory activity is the first factor. Hence the fraction A was chosen to further fractionation.3.3. Purification of the ACE-inhibitory and antioxidant peptides
As shown in Fig. 1c, the fraction A was separated into six major fractions through the Sephadex G-15 gel chromatography, of which fraction A4 showed the highest ACE-inhibitory activity (81.15% ± 2.27%) and antioxidant capacity (88.92% ± 3.16%, Fig. 1d). The fraction A4 was further isolated by RP-HPLC on a semi-preparative C18 column and nine major peaks named as A4a to A4i were obtained and collected (Fig. 1e). The fraction A4e with the highest ACE-inhibitory activity (86.33 ± 1.65%) and ·OH scavenging capacity (92.79 ± 2.79%, Fig. 1f) was further purified by RP-HPLC in an analytical C18 column (Φ4.6 mm × 250 mm). As shown in Fig. 1g, A4e was separated into three fractions named A4e-1, A4e-2 and A4e-3. However, the ACE-inhibitory and ·OH scavenging activity of A4e and A4e-3 have no significant differences (P > 0.05), and the bioactivities of A4e-1 and A4e-2 were lower than those of A4e (Fig. 1h). This indicated that there may be synergistic effect among the fractions A4e-1, A4e-2 and A4e-3.25 The yield of fraction A4e and A4e-3 were 12.33 g/100 g fraction A4 and 40.05 g/100 g fraction A4e, respectively. The fraction A4e-3 showed the highest ACE-inhibitory activity (87.31% ± 1.89%) and ·OH scavenging activity (92.32% ± 2.25%), then it was subjected to ESI-MS/MS analysis.3.4. Characterization of the purified peptides
As shown in Table 2, three peptides Lys-Ala-Gln-Tyr-Pro-Tyr-Val (867.45 Da), Lys-Ile-Ile-Ile-Tyr-Asn (762.47 Da) and Lys-Ile-Leu-Ile-Tyr-Gly (705.44 Da) were identified in A4e-3. Their mass spectra by Nano-LC-ESI-Q-TOF MS/MS are shown in Fig. 2a–c. It was obvious that the identified peptides are all oligopeptides with 6 or 7 amino acid residues, corresponding to the report that the majority of ACE-inhibitory and/or antioxidant peptides are short sequences with 2–12 amino acids.3 Moreover, these peptides are all rich in hydrophobic and branched amino acids (Ile and Leu). Especially to KIIIYN and KILIYG, the content of branched amino acids (Ile and Leu) is reach to 50%. More important, all these peptides have aromatic amino acid (Tyr) and branched-chain amino acids (Ile and Val) in the C-terminal tripeptide, and have Lys in the N-terminus. This may be attributed to the trypsin used during CCAH preparation which prefers to hydrolyze peptide bonds with Lys or Arg on the C-terminal end.18 Respect to KAQYPYV and KIIIYN, they have Val and Asn in the C-terminus, respectively.| Peptide | MW (Da) | Matched sin Elaeis guineensis b | Hydrophobic residues content | IC50 (μM) on ACE inhibition activity | IC50 (μM) on ·OH scavenging activity |
|---|---|---|---|---|---|
| a Different small letters (c–e) in the same row means significant difference (P < 0.05).b From National Center for Biotechnology Information (NCBI). | |||||
| KAQYPYV Lys-Ala-Gln-Tyr-Pro-Tyr-Val | 867.45 | Q.KAQYPYV.K | 42.86% | 37.06d | 70.84e |
| KIIIYN Lys-Ile-Ile-Ile-Tyr-Asn | 762.47 | N. KIIIYN.K | 50.00% | 58.72c | 77.62d |
| KILIYG Lys-Ile-Leu-Ile-Tyr-Gly | 705.44 | N.KILIYG.K | 50.00% | 53.31c | 95.23c |
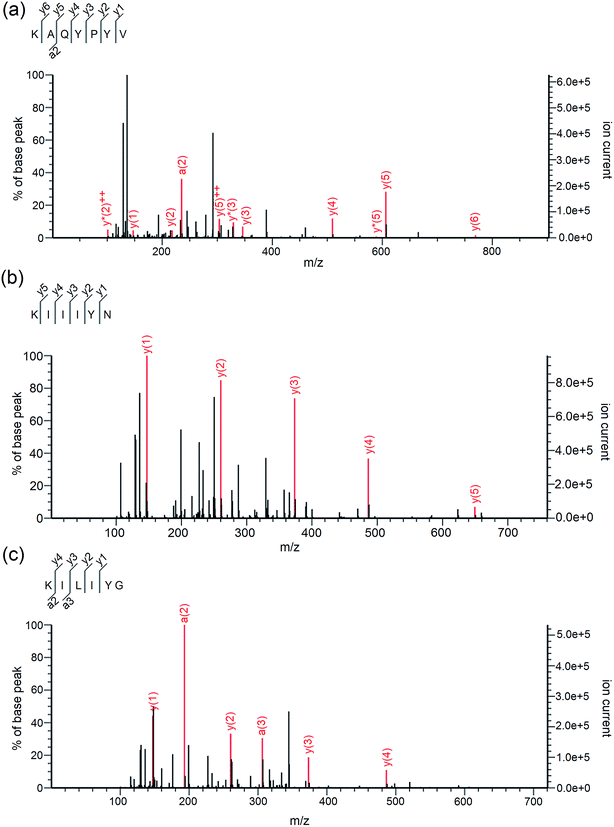 | ||
| Fig. 2 Mass spectra of peptides KAQYPYV (a), KIIIYN (b) and KILIYG (c) identified by LC-MS/MS in coconut cake albumin hydrolysates (CCAH). | ||
In recent years, the correlation between structure and activity of various ACE-inhibitory peptides indicate that the C-terminal tripeptide residues play a predominant role in competitive binding to the active site of ACE, and peptides with a Pro and/or an aromatic residue (Trp, Tyr or Phe) and/or a branched aliphatic amino acid residue (Val, Ile, or Leu) in the C-terminal tripeptide always showed strong ACE-inhibitory effects.3,7 Activity data also suggests that the positive charge either on the guanidine group of C-terminal Arg or on the ε-amino group of N-terminal Lys side chains contributes substantially to the inhibitory activity against ACE.4,27,36 Moreover, Rao et al. found that the Asn residue at the C-terminal of peptides (Ser-Leu-Gly-Asn and Ile-Thr-Ala-Ser-Val-Asn) and Lys residue at the N-terminal of peptides (Lys-Ile-Val-Ser-Asp and Lys-Val-Phe) also contributed to the ACE-inhibitory potency.37
Similarly, the structure–activity relationship of antioxidative peptides also demonstrated that the presence of aromatic, positively charged, and hydrophobic amino acid residues in the peptide are important in contributing to the radical-scavenging capacity of antioxidant peptides through donating hydrogen to reactive oxygen species.18 Moreover, the peptides with a Pro at C-terminal (like His-His-Pro and Pro-Asn-Arg-Pro-Gln-Phe) and the peptides with Val residues at either C-terminal or N-terminal (e.g. Val-Lys-Leu and Val-Lys-Val) could show strong ·OH scavenging ability.10,31 All the identified peptides in this paper are well fit for these structural features of ACE-inhibitory and antioxidant peptides. In addition, Zareia et al. have identified a peptide (Lys-Ile-Ile-Ile-Tyr) from palm kernel cake proteins, which had a similar amino acid sequence with the peptide Lys-Ile-Ile-Ile-Tyr-Asn obtained the current study.38 However, KIIIY demonstrated higher ACE-inhibitory ability (IC50: 47.00 ± 1.60 μM) than that of KIIIYN (IC50: 58.72 μM) obtained in the current study. This proves once again that an aromatic amino acid residue in the C-terminal is very important to the ACE-inhibitory activity of peptides.
Though with a lager MW, KAQYPYV demonstrated the highest ACE-inhibitory activity (37.06 μM) and antioxidant activity (IC50: 70.84 μM) among the three peptides, probably attributed to the presence of Tyr, Pro and Val residues at the C-terminal simultaneously. It has been demonstrated that ACE has four functional amino acid residues of Tyr, Arg, Glu and Lys at the active site and three hydrophobic binding subsites, thus peptides with both aromatic residues (Tyr) and hydrophobic residues (Pro and Val) in the C-terminus have high affinity with these active sites.36
Furthermore, the IC50 values on ACE-inhibitory activity of the three peptides (37.06 to 58.72 μM) were lower than that of peptides identified from corn (Ala-Tyr, IC50: 146.8 μM), egg white lysozyme (Ala-Met-Lys, IC50: 94.2 μM) and sweet sorghum grain (Thr-Leu-Ser, IC50: 102.1 μM).37,39,40 And the ·OH scavenging activity of the three peptides (IC50: 70.84 to 95.23 μM) were also higher than that of peptides identified from tilapia skin gelatin (Leu-Ala-Arg-Leu, IC50: 310.0 μM), salmon (Phe-Leu-Asn-Glu-Phe-Leu-His-Val, IC50: 152 μM) and bovine casein (FYYEQNL, IC50: 119.11 μM).7,18,41 This indicated that the peptides identified in the current study had a relatively high ACE-inhibitory and antioxidant activity.
3.5. Stability and inhibition pattern of synthetic peptides
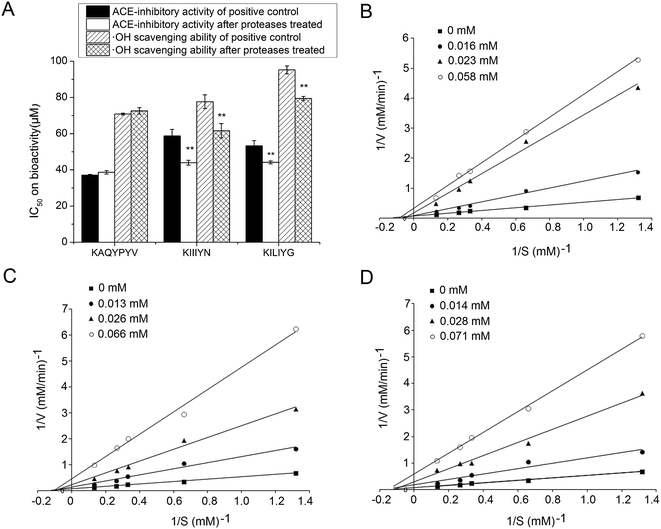
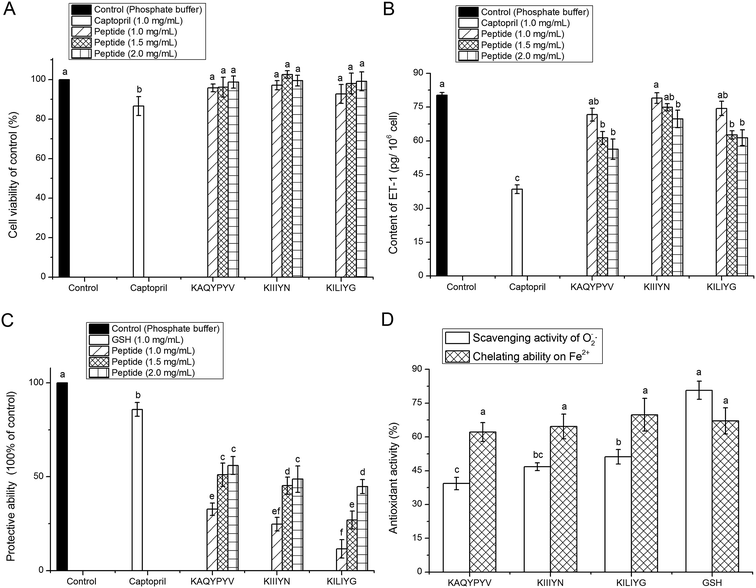
The ACE-inhibitory and antioxidant peptides identified in CCAH were synthesized to confirm their activity and evaluate the stability against the gastrointestinal enzymes digestion. As shown in Fig. 3A, after digestion with pepsin followed by pancreatin, the IC50 values of KIIIYN and KILIYG decreased remarkably (P < 0.05). It was reported that pepsin preferentially cleaves the C-terminal to Phe, Leu and Glu, while pancreatin hydrolyses peptide bonds with aromatic side chains (Tyr, Trp and Phe) at N-terminal or C-terminal.18,42 The peptide KIIIYN can be split into KIIIY and N by pancreatin, while KILIYG can be split into KILIY and G. The bioactivity of split peptide KIIIY and KILIY with Tyr at C-terminal was expected to increase after digestion. In contrast, there was no significant decrease (P > 0.05) in the ACE-inhibitory and antioxidant activity of the peptides KAQYPYV, suggesting that it could effectively retain the activity in the gastrointestinal digestion system. Although also with Tyr residue in C-terminals, KAQYPYV has a Pro in the C-terminus, which has a significant role in the stability of ACE-inhibitory peptides against some proteases.36ACE-inhibitory peptides were found to inhibit ACE via different inhibition modalities such as competitive, noncompetitive, uncompetitive and mixed inhibition manners. The kinetic constants of ACE in the presence of peptides KAQYPYV, KIIIYN and KILIYG revealed that, the Vmax decreased but Km changed differently (Fig. 3B–D), indicating a mixed modality of noncompetitive and uncompetitive inhibition. Similar peptides like EVSQGRP, SAAVGSP and VVLYK also exhibited mixed-mode inhibition patterns.11,27
3.6. Effect on EA.hy926 cells proliferation and intracellular ET-1 content
Result in Fig. 4A revealed that all the peptides (KAQYPYV, KIIIYN and KILIYG) showed no significant (P > 0.05) cytotoxicity towards the EA.hy926 cells at the tested concentrations, meaning that these peptides are all safe material in terms of cellular toxicity and thereby could be used as a safe inhibitor of ACE and as effective antioxidant with no cytotoxicity to vascular endothelial cells.4Other than the renin angiotensin system (RAS), the endothelin system has an increasingly recognized role in blood pressure regulation, in which ET-1 has powerful vasoconstrictor and pressor properties. Thus, peptides that can inhibit the intracellular ET-1 also have potential antihypertensive properties.5 Results in Fig. 4B showed that all the three peptides could effectively inhibit the intracellular ET-1 content with a concentration-dependent increase, indicating their potential antihypertensive effect, though which was lower than that of captopril (P < 0.05). Previous studies also demonstrated that ACE-inhibitory peptides like Val-Phe-Tyr, Val-Val-Leu-Tyr-Lys and Val-Ile-Glu-Pro-Arg could play antihypertensive effects through inhibiting the expression of ET-1 in vascular endothelial cells.27,43
3.7. Antioxidant activity of the ACE-inhibitory peptides
Both O2−˙ and ·OH are the most reactive among the oxygen radicals that can attract adjacent biomolecules, initiate oxidative chain reaction and induce the production of various radicals; and the presence of some metal ions like Fe2+ can extremely accelerate the oxidation reaction.42 In this point of view, the results in this paper demonstrated that the three peptides could exhibit high antioxidant activity via inhibiting the generation of radical or chelating Fe2+ or scavenging the radical produced during the oxidation reaction, and thereby protect vascular endothelial cells from oxidative damage.44
Furthermore, increasing studies demonstrated that ACE-inhibitory peptides could play antihypertensive effect by one or more of the following ways: (1) inhibiting the ACE activity or improving activity of angiotensin-II converting enzyme (ACE2), (2) restraining the expression of ET-1 in vascular cells, (3) protecting vascular cells against oxidative damage, and (4) other ways.11,27,43 The results in the current study showed that the identified peptides in CCAH exhibited high ACE inhibition activity and antioxidant ability, and could effectively decrease the intracellular ET-1 and protect vascular cells against oxidative damage, indicating their potential antihypertensive effect.
4. Conclusions
Three novel ACE-inhibitory and antioxidant peptides comprised of 6–7 amino acids were identified in CCAH. The obtained peptides showed high ACE-inhibitory activity (IC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37.06 to 58.72 μM), and high ·OH scavenging capacity (IC50: 70.84 to 95.23 μM), high Fe2+ chelating activity and considerable O2−˙ scavenging ability. Moreover, the three peptides could effectively reduce the intracellular ET-1 content and protect vascular endothelial cells from reactive oxygen species mediated damage, indicating their potential antihypertensive effect. Therefore, these peptides isolated from CCAH could be used as a functional food ingredient to control ACE activity and antioxidation.
37.06 to 58.72 μM), and high ·OH scavenging capacity (IC50: 70.84 to 95.23 μM), high Fe2+ chelating activity and considerable O2−˙ scavenging ability. Moreover, the three peptides could effectively reduce the intracellular ET-1 content and protect vascular endothelial cells from reactive oxygen species mediated damage, indicating their potential antihypertensive effect. Therefore, these peptides isolated from CCAH could be used as a functional food ingredient to control ACE activity and antioxidation.
Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
This work was supported by the Key Research Project of Hainan Province (No. ZDYF2017061).References
- World Health Organization, Causes of death 2008: data sources and methods, World Health Organization, Geneva, 2010, available at http://www.who.int/healthinfo/global_burden_disease/cod_2008_sources_methods.pdf Search PubMed.
- L. Lin, S. Lv and B. F. Li, Angiotensin-I-converting enzyme (ACE)-inhibitory and antihypertensive properties of squid skin gelatin hydrolysates, Food Chem., 2012, 131, 225–230 CrossRef CAS.
- B. Hernández-Ledesma, M. M Contreras and I. Recio, Antihypertensive peptides: Production, bioavailability and incorporation into foods, Adv. Colloid Interface Sci., 2011, 165, 23–25 CrossRef PubMed.
- S. W. A. Himaya, D. H. Ngo, B. Ryu and S. K. Kim, An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress, Food Chem., 2012, 132, 1872–1882 CrossRef CAS.
- S. M. Wallace, L. Yasmin, C. M. McEniery, M. Makj-Petaja, A. D. Booth, J. R. Cockcroft and L. B. Wilkinson, et al., Isolated systolic hypertension is characterized by increased aortic stiffness and endothelial dysfunction, Hypertension, 2007, 50, 228–233 CrossRef CAS PubMed.
- B. J. Bhuyan and G. Mugesh, Synthesis, characterization and antioxidant activity of angiotensin I converting enzyme inhibitors, Org. Biomol. Chem., 2011, 9, 1356–1365 RSC.
- A. B. Shazly, Z. He, M. A. EI-Aziz, M. Zeng, S. Zhang, F. Qin and J. Chen, Fractionation and identification of novel antioxidant peptides from buffalo and bovine casein hydrolysates, Food Chem., 2017, 232, 753–762 CrossRef CAS PubMed.
- J. Ren, M. Zhao, J. Shi, J. Wang, Y. Jiang, C. Cui, Y. Kakuda and S. J. Xue, Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry, Food Chem., 2008, 108, 727–736 CrossRef CAS PubMed.
- P. Behera, R. Kumar, I. V. R. Sandeep, R. Kapila, A. K. Dang and S. Kapila, Casein hydrolysates enhance osteoblast proliferation and differentiation in mouse bone marrow culture, Food Biosci., 2013, 2, 24–30 CrossRef CAS.
- L. Wattanasiritham, C. Theerakulkait, S. Wickramasekara, C. S. Maier and J. F. Stevens, Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein, Food Chem., 2016, 192, 156–162 CrossRef CAS PubMed.
- B. Forghani, M. Zarei, A. Ebrahimpour, R. Philip, J. Bakar, A. Abdul-Hamid and N. Saari, Purification and characterization of angiotensin converting enzyme-inhibitory peptides derived from Stichopus horrens: Stability study against the ACE and inhibition kinetics, J. Funct. Foods, 2016, 20, 276–290 CrossRef CAS.
- S. Y. Lee and S. J. Hur, Antihypertensive peptides from animal products, marine organisms, and plants, Food Chem., 2017, 228, 506–517 CrossRef CAS PubMed.
- R. Z. Chiozzi, A. L. Capriotti, C. Cavaliere, G. L. Barbera, S. Piovesana and A. Laganà, Identification of three novel angiotensin-converting enzyme inhibitory peptides derived from cauliflower by-products by multidimensional liquid chromatography and bioinformatics, J. Funct. Foods, 2016, 27, 262–273 CrossRef.
- R. Z. Chiozzi, A. L. Capriotti, C. Cavaliere, G. L. Barbera, S. Piovesana, R. Samperi and A. Laganà, Purification and identification of endogenous antioxidant and ACE-inhibitory peptides from donkey milk by multidimensional liquid chromatography and nanoHPLC-high resolution mass spectrometry, Anal. Bioanal. Chem., 2016, 408, 5657–5666 CrossRef PubMed.
- S. Piovesana, A. L. Capriotti, C. Cavaliere, G. L. Barbera, C. M. Montone, R. Z. Chiozzi and A. Laganà, Recent trends and analytical challenges in plant bioactive peptide separation, identification and validation, Anal. Bioanal. Chem., 2018, 410, 3425–3444 CrossRef CAS PubMed.
- C. M. Montone, A. L. Capriotti, C. Cavaliere, G. L. Barbera, S. Piovesana, R. Z. Chiozzi and A. Laganà, Peptidomic strategy for purification and identification of potential ACE-inhibitory and antioxidant peptides in Tetradesmus obliquus microalgae, Anal. Bioanal. Chem., 2018, 410, 3573–3586 CrossRef CAS PubMed.
- C. M. Montone, A. L. Capriotti, C. Cavaliere, G. L. Barbera, S. Piovesana, R. Z. Chiozzi and A. Laganà, Characterization of antioxidant and angiotensin-converting enzyme inhibitory peptides derived from cauliflower by-products by multidimensional liquid chromatography and bioinformatics, J. Funct. Foods, 2018, 44, 40–47 CrossRef CAS.
- A. Sila and A. Bougatef, Antioxidant peptides from marine by-products: Isolation, identification and application in food systems: A review, J. Funct. Foods, 2016, 21, 10–26 CrossRef CAS.
- U. Patil and S. Benjakul, Characteristics of albumin and globulin from coconut meat and their role in emulsion stability without and with proteolysis, Food Hydrocolloids, 2017, 69, 220–228 CrossRef CAS.
- Y. Li, Y. Zheng, Y. Zhang, J. Xu and G. Gao, Antioxidant Activity of Coconut (Cocos nucifera L.) Protein Fractions, Molecules, 2018, 23(3), 707 CrossRef PubMed.
- M. J. Santana, A. L. D. Olivera, L. H. K. Q. Júnior, S. M. Mandal, C. O. Matos, R. D. Dias, O. L. Franco and L. M. Liao, Structural insights into Cn-AMP1, a short disulfide-free multifunctional peptide from green coconut water, FEBS Lett., 2015, 589, 639–644 CrossRef CAS PubMed.
- Y. Li, Y. Zheng, Y. L. Zhang, L. Y. Liu and S. L. Zhao, Purification, characterization, synthesis, in vivo and in vitro antihypertensive activity of bioactive peptides derived from coconut (Cocos nucifera L.) cake globulin hydrolysates, RSC Adv., 2016, 6(95), 92688–92698 RSC.
- Y. J. Zheng, Y. Li, Y. L. Zhang and S. L. Zhao, Purification, characterization and synthesis of antioxidant peptides from enzymatic hydrolysates of coconut (Cocos nucifera L.) cake protein isolates, RSC Adv., 2016, 6, 54346–54356 RSC.
- FAO, production, http://faostat3.fao.org/browse/Q/*/E/, 2013, accessed 18 May, 2015.
- M. Zhang, T. H. Mu and M. J. Sun, Purification and identification of antioxidant peptides from sweet potato protein hydrolysates by alcalase, Food Chem., 2014, 7, 191–200 Search PubMed.
- K. Kwon, K. H. Park and K. C. Rhee, Fractionation and characterization of proteins from coconut (Cocos nucifera L.), J. Agric. Food Chem., 1996, 44(7), 1741–1745 CrossRef CAS.
- Y. J. Zheng, Y. Li, Y. L. Zhang, X. H. Ruan, Y. F. Zhang and S. L. Zhao, Purification, characterization, synthesis, in vivo ACE inhibition and in vitro antihypertensive activity of bioactive peptides derived from palm kernel expeller glutelin-2 hydrolysates, J. Funct. Foods, 2017, 28(2), 48–58 CrossRef CAS.
- J. Adler-Nissen, Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid, J. Agric. Food Chem., 1979, 27, 1256–1262 CrossRef CAS PubMed.
- V. K. Jimsheena and L. R. Gowda, Colorimetric, high-throughput assay for screening angiotensin I-converting enzyme inhibitors, Anal. Chem., 2009, 81, 9388–9394 CrossRef CAS PubMed.
- K. Bush, P. R. Henry and D. S. Slusarchyk, Muraceins-muramyl peptides produced by Norcardia orientalis as angiotensin converting enzyme inhibitors, J. Antibiot., 1984, 37, 330–335 CrossRef CAS PubMed.
- T. Tavares, M. D. Contreras, M. Amorim, M. Pintado, I. Recio and F. X. Malcata, Novel whey-derived peptides with inhibitory effect against angiotensin-converting enzyme: in vitro effect and stability to gastrointestinal enzymes, Peptides, 2011, 32, 1013–1019 CrossRef CAS PubMed.
- T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS.
- S. Marklund and G. Marklund, Involvement of the superoxide anion radical in the autoxidation of pyrogallal and a convenient assay for superoxide dismutase, Eur. J. Biochem., 1974, 47, 469–474 CrossRef CAS.
- J. B. Jeong, B. O. De Lumen and H. J. Jeong, Lunasin peptide purified from Solanum nigrum L. protects DNA from oxidative damage by suppressing the generation of hydroxyl radical via blocking fenton reaction, Cancer Lett., 2010, 293, 58–64 CrossRef CAS PubMed.
- M. J. Eisenmenger and J. I. Reyes-De-Corcuera, High pressure enhancement of enzymes: a review, Enzyme Microb. Technol., 2009, 45, 331–347 CrossRef CAS.
- A. Alemán, B. Giménez, E. Pérez-Santin, M. C. Gómez-Guillén and P. Montero, Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate, Food Chem., 2011, 125, 334–341 CrossRef.
- S. Rao, J. Sun, Y. Liu, H. Zeng, Y. Su and Y. Yang, ACE inhibitory peptides and antioxidant peptides derived from in vitro digestion hydrolysate of hen egg white lysozyme, Food Chem., 2012, 135, 1245–1252 CrossRef CAS PubMed.
- M. Zareia, B. Forghania, A. Ebrahimpoura, A. Abdul-Hamida, F. Anwarc and N. Saaria, In vitro and in vivo antihypertensive activity of palm kernel cake protein hydrolysates: sequencing and characterization of potent bioactive peptides, Ind. Crops Prod., 2015, 76, 112–120 CrossRef.
- F. Lin, L. Chen, R. Liang, Z. Zhang, J. Wang, M. Cai and Y. Li, Pilot-scale production of low molecular weight peptides from corn wet milling byproducts and the antihypertensive effects in vivo and in vitro, Food Chem., 2011, 124, 801–807 CrossRef CAS.
- Q. Wu, J. Du, J. Jia and C. Kuang, Production of ACE inhibitory peptides from sweet sorghum grain protein using alcalase: Hydrolysis kinetic, purification and molecular docking study, Food Chem., 2016, 199, 140–149 CrossRef CAS.
- Y. L. Zhuang and L. P. Sun, Preparation of reactive oxygen scavenging peptides from tilapia (Oreochromis niloticus) skin gelatin: optimization using response surface methodology, J. Food Sci., 2011, 76, C483–C489 CrossRef CAS.
- B. H. Sarmadi and A. Ismail, Antioxidative peptides from food proteins: a review, Peptides, 2010, 31, 1949–1956 CrossRef CAS PubMed.
- D. Liu, L. Zhang, S. Li, F. Liu and S. Z. Liang, Influence of angiotensin-converting enzyme inhibitory peptide on endothelial cell proliferation and endothelin expression in human umbilical vein cells, Chin. J. Clin. Rehabil., 2006, 10(25), 160–163 CAS.
- E. Babini, D. Tagliazucchi, S. Martini, L. D. Più and A. Gianotti, LC-ESI-QTOF-MS identification of novel antioxidant peptides obtained by enzymatic and microbial hydrolysis of vegetable proteins, Food Chem., 2017, 228, 186–196 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra10269d |
| ‡ The two authors contribute equally to this work and they are co-first author. |
| This journal is © The Royal Society of Chemistry 2019 |