Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Decontamination of organic pollutants from aqueous media using cotton fiber–graphene oxide composite, utilizing batch and filter adsorption techniques: a comparative study
A. I. Abd-Elhamid*a,
A. A. Nayl bc,
Ahmed A. El. Shanshorya,
Hesham M. A. Solimana and
H. F. Alyc
bc,
Ahmed A. El. Shanshorya,
Hesham M. A. Solimana and
H. F. Alyc
aAdvanced Technology and New Materials Research Institute, City for Scientific Research and Technology Applications, SRTA, P. O. Box, 21934, Egypt
bChemistry Department, College of Science, Jouf University, Sakakah, Saudi Arabia. E-mail: aanayl@yahoo.com
cHot Laboratories Center, Atomic Energy Authority, Nasr 13759, Egypt
First published on 18th February 2019
Abstract
Cotton fiber–graphene oxide (C–GO) composite with high adsorptive properties towards the cationic dye, crystal violet (CV), was successfully fabricated by simple mixing of cotton fiber and GO in aqueous solution using a homogenizer. The as-prepared composite was characterized using TEM, SEM, LOM, XRD, FTIR, Raman and TGA. The characterization indicated that the formation of a homogeneous composite occurred via adequate mixing of the cotton fiber and GO. The fine structure of the obtained composite was successfully used in two adsorption techniques, namely batch adsorption and filter adsorption. Various parameters affecting batch adsorption, such as contact time, dye concentration, composite dose, NaCl dose, temperature and pH were investigated. In the filter adsorption mode, dye concentration, composite dose, NaCl dose, temperature, flow rate and pH were studied. A comparison study between the two techniques, i.e., batch adsorption and filter adsorption, are reported. The filter adsorption technique shows higher adsorption efficiency than the batch one, which was evident from the maximum adsorption capacity (Q°) values, obtained from the Langmuir isotherm. Further, the filter technique was developed and evaluated. This was achieved by regeneration, scaling-up and, finally, using another model of cationic dye (methylene blue).
1. Introduction
Discharge of dye containing effluents to the environment is harmful to various kinds of living organisms. Cationic dyes (methylene blue and crystal violet), anionic dyes (methyl orange and eriochrome black T) and/or azo dyes (acid red 1 and acid red 40) may be present in the dye effluents. Annually, several hundred thousand tons of dye stuff are produced.1–4 Many essential industries, such as textile, paper, and plastic, use dyes to color their products. Moreover, these industries require a huge amount of water. Hence, a significant quantity of colored wastewater effluent should be treated before it is released into the environment for both toxicological and esthetical reasons.5Several approaches, including physical, chemical and biological, have been studied by researchers to remove dyes from waste. These methods suffer from some drawbacks, such as the formation of by-products, release of aromatic amines, methane and hydrogen sulfide production as a result of anaerobic breakdown, high cost, and the requirement for a lot of dissolved O2. Adsorption is one of the methods, applied to treat wastewater, which is considered to be an efficient and superior tool in many cases for wastewater treatment technology.6 This method may be desirable owing to its low initial cost, simplicity of design, ease of operation and lack of harmful exhaust.
Because of its unique structure, GO is estimated to be one of the more powerful adsorbents. GO monolayer is one C-atom thick, and it is decorated with a large number of oxygen-rich active groups, such as OH, on the basal plane and COOH edges.7
Cotton is a suitable substrate material due to its high surface area, mechanical support and low weight. Numerous techniques have been investigated for creating hybridization between GO and the cotton fiber, such as dipping and drying,8,9 layer by layer coating,10 dipping,11 and brush-coating and drying.12 However, these methods are time consuming, and they require a multi-step preparation. Also, they produce GO-layers which coat the fiber surface, and GO-monolayers cannot penetrate the fiber.
The present study deals with preparation of a low cost adsorbent with a high tendency to adsorb a cationic dye form an aqueous solution. The composite was prepared via a one-pot reaction using a homogenizer by simply mixing the cotton fiber and GO in an aqueous solution.
In detail, the homogenizer allows the cotton fiber, pumped at high pressure, to pass through a small gap inside it. These gaps are created by a valve and an impact ring. When working, the valve is rapidly opened and closed. This subjects the cotton fibers to high shear and impact forces, which provoke cellulose fiber nanofibrillation. By introducing GO into the homogenization media, monolayers get in between the cotton nanofibers. Hence, the GO monolayers will be distributed overall the cotton fiber used. Moreover, this method provides a link between the cotton fiber and GO, preventing disintegration of the composite during the treatment process.
Additionally, the main problem with using GO is the placement of its monolayers, which are compacted upon drying. In this state, most of the active groups on the both sides of the layer will be hindered from the adsorbed species, and it will take a time for them to be available for the adsorption process. In this study we assume that the GO-monolayers will be separated by the cotton nanofiber. As a result, GO will keep its efficiency of adsorbing the pollutant species. On the other hand, the removal of GO from the treated media appears to be a difficult process due to its high dispensability, but strong interaction between GO and the cotton fiber makes the isolation process easier. The soft structure of the obtained composite was found suitable for use in various adsorption techniques. Therefore, two techniques for removal of the cationic dye from water were studied: batch adsorption and filter system. A comparison between the two techniques was performed and discussed.
2. Experimental
2.1. Materials and laboratory equipment
All chemicals were of analytical grade and used as received. These were Crystal Violet (CV) and Methylene Blue (MB) (99%, Sigma-Aldrich), sulfuric acid (95–97%, Riedel de Haen), potassium permanganate (99%, Long live), hydrogen peroxide (36%, Pharaohs Trading and Import), graphite (200 mesh, 99.99%, Alpha Aesar), potassium persulfate (≥99.0%, Sigma-Aldrich), hydrochloric acid (30%, El Salam for Chemical Industries), and cotton (from the local market).The following equipment was used: homogenizer (T18 Basic, Ika, Germany), water distillatory (2108, GLF, Germany) for double distilled water, pH meter (3510, Genway), hot plate stirrer (SB 162, Stuart, UK), centrifuge, (Mikro 220R, Hettich, UK), UV/Vis Spectrophotometer-Double beam (T80+, PG instruments Ltd., UK), and analytical balance (CP 2245, Sartorius, USA).
2.2. Preparation of GO and cotton–GO composite
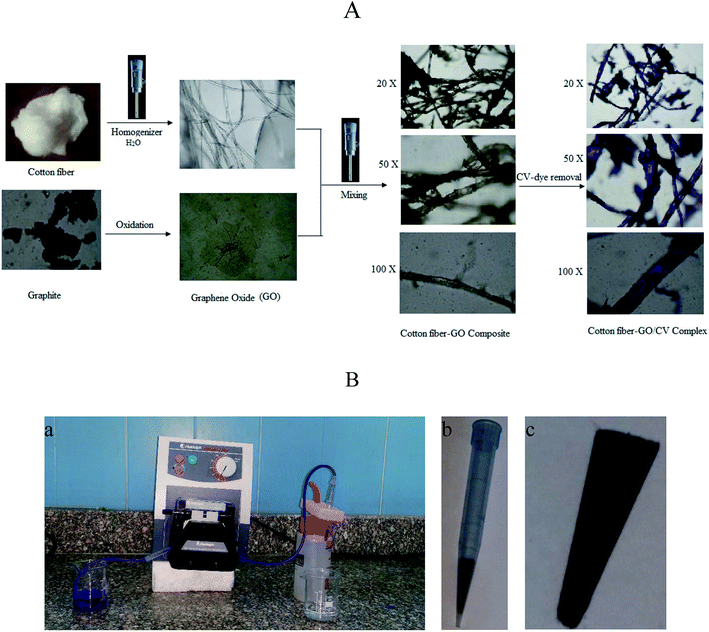
GO was prepared as described by Ji Chen et al.13 with some modifications. Briefly, to 70.0 mL concentrated sulfuric acid solution in 150 mL glass beaker 3.0 g graphite powder was added under stirring for 10 minutes. This suspension was kept at 20 °C in a water bath and 9.0 g KMnO4 was gradually added, followed by gradual addition of 3.0 g potassium persulfate. The temperature of this mixture was raised to 40 °C under vigorous stirring for about 30 min. The slurry was added to 150 mL double distilled water and the solution was stirred for 15 min at 95 °C. The volume of the final slurry was increased to 500 mL using double distilled water, followed by a slow addition of 15 mL H2O2 (30%). The color of the suspension turned from dark brown to yellow. The mixture was filtered and the filtrate of the prepared GO was washed with 250 mL of 10% HCl aqueous solution. Finally, the GO product was washed with 250 mL double distilled water and dried at 35 °C for 99 h. The resultant powder was used for further experiments.The cotton–GO composite was prepared by dispersing 10.0 g of cotton fiber in 500 mL double distilled water using homogenizer. To this suspension 0.5 g of the prepared GO powder was added under homogenization until complete mixing between the cotton fiber and GO was achieved. Finally, the solution of cotton–GO composite was removed by filtration and dried at 70 °C for 24 h. Schematic diagram of optical images explaining the different stages of preparation of C–GO composite and the composite after the adsorption process are presented in Fig. 1A.
2.3. Characterization
2.4. Batch adsorption of CV
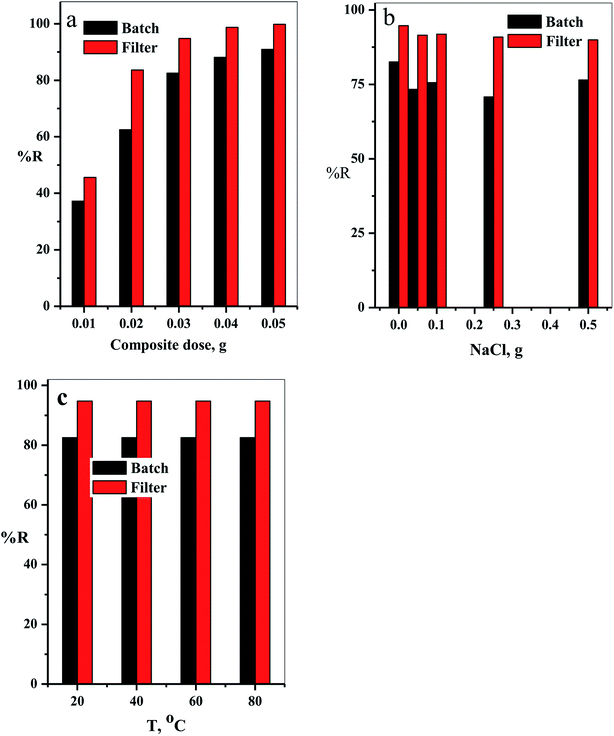
One g L−1 of CV-dye solution was prepared as a stock solution and the desired concentrations, needed for the subsequent studies, were prepared by further dilution using double distilled water. The effect of adsorption time on CV-dye removal was studied by adding 0.03 g of the prepared composite to a 50.0 mL solution containing 10 mg L−1 of the dye at pH 7.0 and 25 °C at known time intervals until equilibrium was reached. Known concentrations of the dye solution in the range (10–50 mg L−1) were used to study the effect of the initial dye concentration. Certain known weights of the composite (0.01–0.05 g) were added to 50.0 mL of CV-dye solutions with concentration of 30 mg L−1. The initial solution pH range was adjusted to 2.5–9.5 using 0.5 mol L−1 HCl or NaOH. Salinity test was performed by adding different amounts (0.0–0.5 g) of NaCl to 30 mg L−1 of the dye solution. The effect of the temperature on the adsorption process was investigated in the range of 20–80 °C. The obtained liquid samples were isolated by centrifugation and CV concentration was determined using UV-spectrophotometer at the wavelength of 590 nm and in case of MB at 662 nm. The dye removal efficiency (% R) is defined as
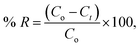 | (1) |
2.5. Continuous removal of CV
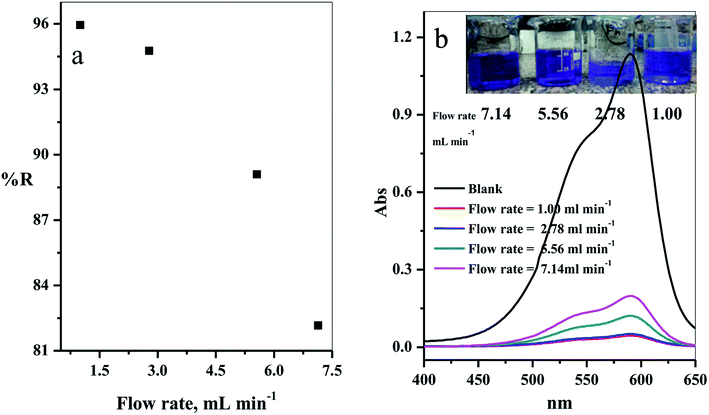
The equipment used for the continuous removal process contained a plastic pipe. One end of this pipe was used for withdrawing the dye solution (input). The other end was connected to a plastic tip (1.0 mL) of a micropipette. This plastic tip was loaded with different known amounts of the prepared composite (0.01–0.05 g) and served as the outlet for the treated water, as illustrated in Fig. 1B.The removal of CV-dye from the aqueous solution using the filter system was carried out by passing the solution of the dye through the packed composite. The treatment experiments were performed by immersing of 50 mL of the CV solution of various concentrations (10–50 mg L−1) in a 100 mL glass beaker. Composite dose in the range of 0.01–0.05 g was used and the concentration of the dye supplied was 30 mg L−1. The effect of salinity was tested by adding NaCl in the range of 0.0–0.50 g to the 30 mg L−1 dye solution at different temperatures and variant flow rate in the pH range of 2.5–9.50. Finally, 0.5 mL of the output solution was diluted to 5 mL by double distilled water, and CV concentration was determined as previously described. The dye removal efficiency (% R) was determined using eqn (1).
2.6. Regeneration experiments
To regenerate the composite for further use, a 10 mL of 4% HCl solution was passed through the packed filter, followed by 5 mL of distilled water for washing, 10 mL of 20% EDTA-aqueous solution and, finally, 5 mL of distilled water for washing with a flow rate of 0.625 mL min−1 by the peristaltic pump. The removal percentage of the dye in each cycle was calculated using eqn (1).3. Results and discussion
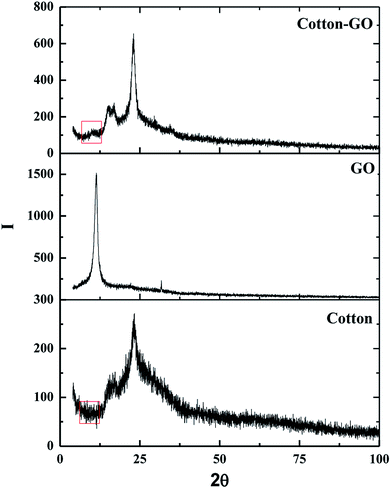
3.1. Composite characterization
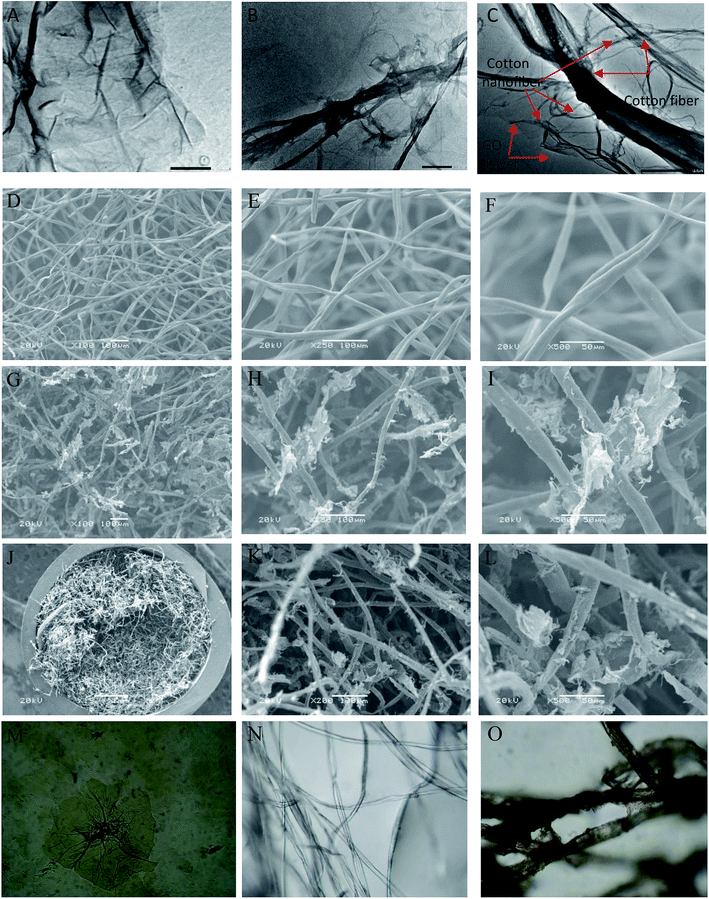
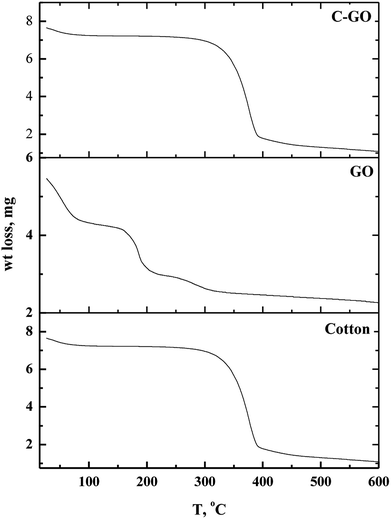
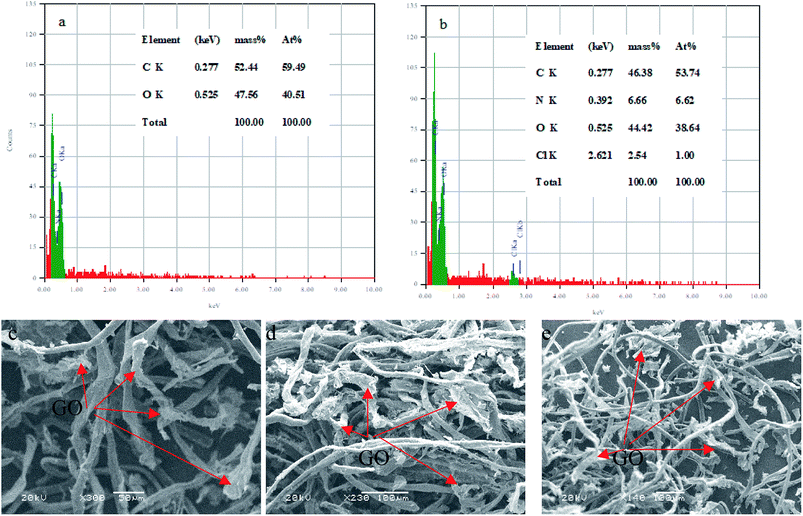
The SEM images, presented in Fig. 2D–F, show the cotton fiber photographs at different magnification. They indicates that the cotton fiber has a uniform size and a smooth surface. On the other hand, by mixing GO and the cotton fiber, we can see that the GO-sheets are well mixed with the cotton fiber (Fig. 2G–I). This observation is also supported by Fig. 2J–L, which contain the SEM images of C–GO composite packed in the filter system at different magnifications. In addition, in the LOM image, Fig. 2M, GO-nanosheets appear as a light brown layer.7,14 The cotton fiber preformed as a white-colored fiber, Fig. 2N. Finally, the optical image of the C–GO composite shows a brown fiber of the cotton–GO complex, Fig. 2O.
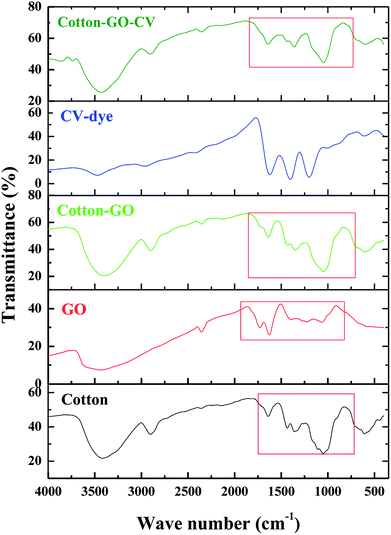
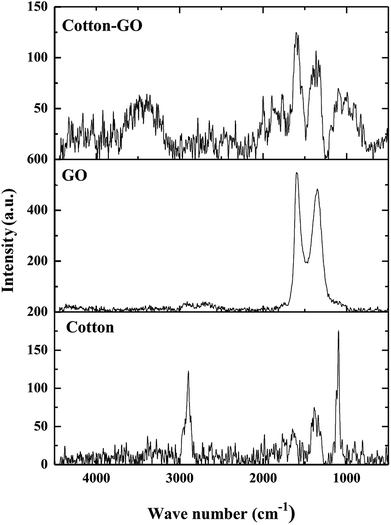
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, O–H deformation11 and alkoxy C–O stretching vibration, respectively. The characteristic GO bands represent O–H stretching vibration at 3433 cm−1, COOH group at 1730 cm−1,17 O–H bending vibration at 1629 cm−1, O–H deformation vibration at 1394 cm−1 and alkoxy C–O stretching vibration at 1064 cm−1. Cotton–GO composite, Fig. 4, exhibits absorption bands at 3406 cm−1 corresponding to O–H stretching of the adsorbed water, C–H stretching vibration at 2901 cm−1, COOH group shoulder at 1730 cm−1, O–H bending vibration at 1637 cm−1, and C–O stretching vibration at 1049 cm−1. Additionally, the bands characteristic of the CV-dye are seen in Fig. 4. When the CV-dye was adsorbed onto the C–GO composite, the bands at 3406 cm−1, 2901 cm−1, 1637 cm−1, 1348 cm−1, and 1049 cm−1 shifted to 3431 cm−1, 2904 cm−1, 1641 cm−1, 1435 cm−1, 1359 cm−1, and 1047 cm−1, respectively. These results confirmed the adsorption of the dye onto the composite.
O stretching vibration, O–H deformation11 and alkoxy C–O stretching vibration, respectively. The characteristic GO bands represent O–H stretching vibration at 3433 cm−1, COOH group at 1730 cm−1,17 O–H bending vibration at 1629 cm−1, O–H deformation vibration at 1394 cm−1 and alkoxy C–O stretching vibration at 1064 cm−1. Cotton–GO composite, Fig. 4, exhibits absorption bands at 3406 cm−1 corresponding to O–H stretching of the adsorbed water, C–H stretching vibration at 2901 cm−1, COOH group shoulder at 1730 cm−1, O–H bending vibration at 1637 cm−1, and C–O stretching vibration at 1049 cm−1. Additionally, the bands characteristic of the CV-dye are seen in Fig. 4. When the CV-dye was adsorbed onto the C–GO composite, the bands at 3406 cm−1, 2901 cm−1, 1637 cm−1, 1348 cm−1, and 1049 cm−1 shifted to 3431 cm−1, 2904 cm−1, 1641 cm−1, 1435 cm−1, 1359 cm−1, and 1047 cm−1, respectively. These results confirmed the adsorption of the dye onto the composite.
| Sample | D-band peak | G-band peak | ID/IG | ||
|---|---|---|---|---|---|
| Raman shift (cm−1) | FWHM (cm−1) | Raman shift (cm−1) | FWHM (cm−1) | ||
| GO | 1340 | 137 | 1614′ | 80 | 1.71 |
| C–GO | 1363 | 148 | 1604 | 116 | 1.28 |
3.2. Adsorption process
3.2.1.1. Adsorption kinetics. The adsorption kinetics of the CV-dye adsorption process can be explained using pseudo first order (eqn (2)),24 pseudo second order (eqn (5))25 and intraparticle diffusion models (eqn (6))26 and illustrated in Fig. 7c–e, respectively.
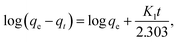 | (2) |
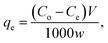 | (3) |
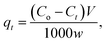 | (4) |
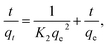 | (5) |
| qt = kit0.5 + C, | (6) |
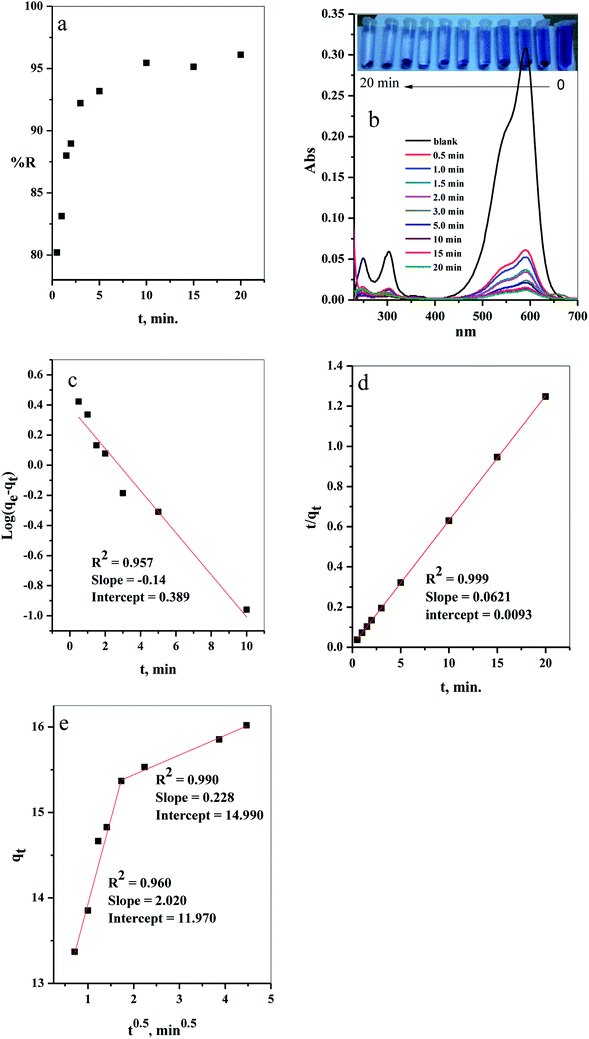
The kinetic parameters of the experiments, such as qe exp, K1, qe cal, R2 and K2, were calculated using Fig. 7c and d and listed in Table 2. The results demonstrate that the adsorption of CV-dye onto the composite follows the pseudo second order. This phenomenon is attributed to the fact that the amount of crystal violet, adsorbed at equilibrium and calculated using the pseudo second order (qe cal = 16.10 mg g−1), is closer to the adsorbed amount, calculated using experiment data (qe exp = 16.0 mg g−1). Moreover, the correlation coefficient (R2 = 0.999), which is related to pseudo second order model, is larger than the one related to pseudo first order model (R2 = 0.957).
| Dye | qe exp (mg g−1) | First-order kinetic parameter | Second-order kinetic parameter | ||||
|---|---|---|---|---|---|---|---|
| K1 (min−1) | qe cal (mg g−1) | R2 | K2 (g mg−1 min−1) | qe cal (mg g−1) | R2 | ||
| CV | 16.02 | −0.322 | 2.450 | 0.957 | 0.415 | 16.103 | 0.999 |
Furthermore, various stages of adsorption process were analyzed, applying the intraparticle diffusion model (Fig. 7e). The adsorption process contained two adsorption stages. The first one involved the diffusion of the dye-molecule on the external surface of the composite. The second stage was the gradual adsorption stage.
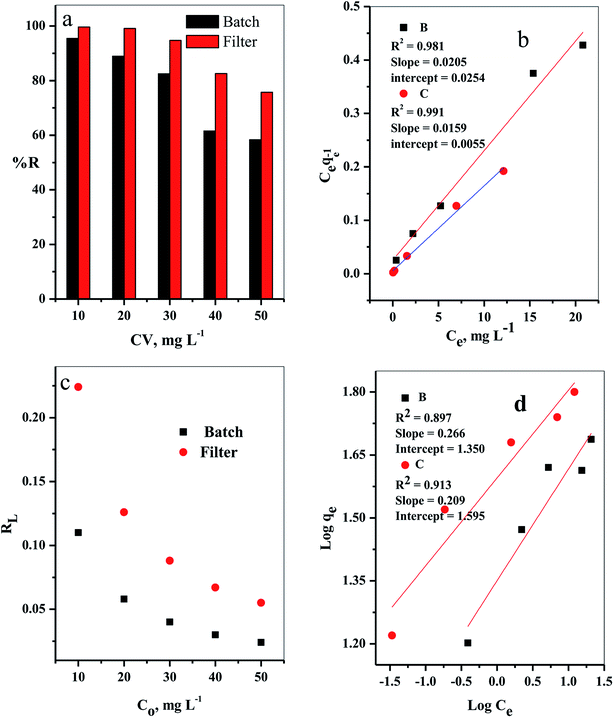
It is important to indicate that the filter shows higher ability to remove the CV-dye from the aqueous solution than batch adsorption, as clarified in Fig. 8a.
3.2.2.1. Adsorption isotherm. The adsorption isotherm models were studied by Langmuir27 and Freundlich28 isotherm, eqn (7) and (8), respectively.
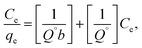 | (7) |
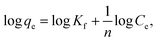 | (8) |
The results, obtained from the experimental data, were plotted in Fig. 8b–d, and the isotherm parameters were calculated and recorded in Table 3. When using Langmuir isotherm, it is assumed that the dye molecules adsorb homogeneously onto the active sites, and no further adsorption takes place at that site. The Langmuir model, related to this study (Table 3), has high correlation coefficients, R2 = 0.981 and 0.991 for batch and filter, respectively, which suggest homogenous adsorption of CV-dye onto the C–GO composite surface. The monolayer maximum adsorption capacity, Q°, and b for batch are 48.780 mg g−1 and 0.810 L mg−1, whereby for filter, they are 62.890 mg g−1 and 0.346 L mg−1. On the other hand, Freundlich model assumes that the active sites located on the adsorbent surface sites have inequivalent binding energies, and the powerful binding sites are occupied first. Furthermore, the Freundlich constant, 1/n, values for batch (0.266) and filter (0.210) imply favorable adsorption of CV-dye on the C–GO composite (Table 3). The high values of Kf, Table 3, indicate that the C–GO composite shows high adsorption capacity for CV-dye in aqueous solution.
| Technique | Langmuir isotherm model | Freundlich isotherm model | ||||
|---|---|---|---|---|---|---|
| Q° (mg g−1) | b (L mg−1) | R2 | 1/n | Kf (mg g−1) | R2 | |
| Batch | 48.780 | 0.810 | 0.981 | 0.266 | 22.420 | 0.900 |
| Filter | 62.890 | 0.346 | 0.991 | 0.210 | 39.252 | 0.916 |
In addition, the dimensionless constant RL,29 separation factor, values corresponding Langmuir isotherm is less than the unit, RL < 1, as explained in Fig. 8c, and show favorable adsorption.30 Finally, the correlation coefficients, corresponding to the Langmuir model are higher than those corresponding to the Freundlich model (Table 3). This observation indicated that the adsorption of CV-dye onto the C–GO composites as a monolayer using batch adsorption or filter adsorption is favorable.
Moreover, the maximum adsorption capacity of various adsorbents, utilized for the removal of CV-dye,31–37 and the results of the current study are summarized in Table 4. The present comparison shows that Q° values obtained in this study are higher than those obtained for the reported adsorbents.
| Adsorbent | Q° (mg g−1) | Ref. |
|---|---|---|
| Jute fiber carbon | 27.99 | 32 |
| Coniferous pinus bark powder | 32.78 | 33 |
| TiO2-based nanosheet | 58.30 | 34 |
| Moroccan pyrophyllite | 9.58 | 35 |
| NaOH-modified rice husk | 44.876 | 36 |
| Punica granatum shell | 50.21 | 37 |
| Artocarpus heterophyllus (jackfruit) leaf powder (JLP) | 43.39 | 38 |
| PAN/β-CD/GO | 16.47 | 45 |
| CNF-based PVDF membrane | 2.948 | 46 |
| Meldrum's acid modified CNFbased PVDF membrane | 3.984 | 46 |
| WCNF | 48 | 47 |
| Batch adsorption | 48.780 | This study |
| Filter adsorption | 62.890 | This study |
The intensity of the maximum absorption peak increased as the flow rate increased, shown in Fig. 10b, and the intensity of violet color increased as seen in the photo-image inset of Fig. 10b.
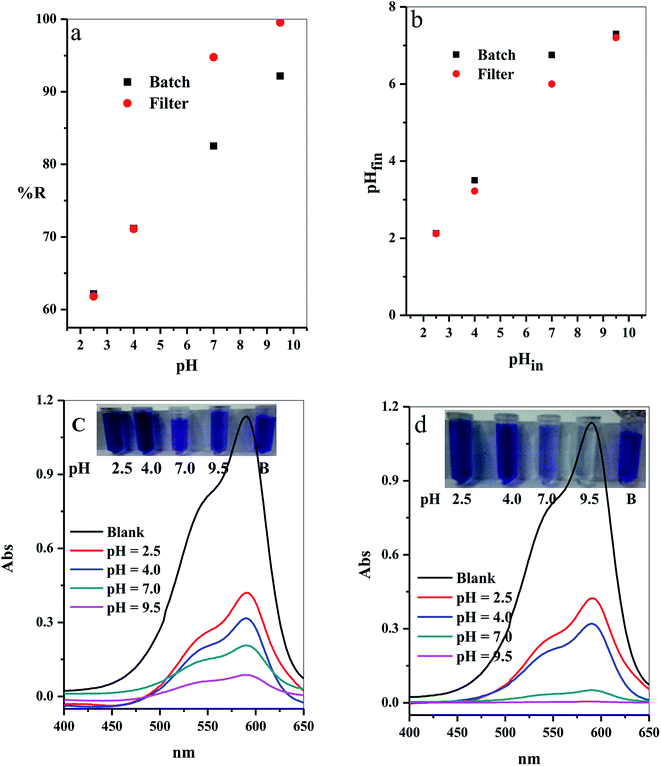
The relation between the initial pHin and the final pHfin of the dye solution is shown in Fig. 11b and the calculated values are listed in Table 5. The analysis of the data, obtained from Fig. 11b and Table 5, indicated that the initial pH decreased over the entire pH range. Moreover, we observed that the highest decrease in the pHin occured in the alkaline medium, where % R exhibits the highest value (Table 5). These results support the above suggested mechanism, which indicates that adsorption of the dye molecules causes liberation of H+ ions in the solution. This leads to a decrease in the pHin value and the ΔpH value increases as a result of the increasing % R.
| Technique | pHin | pHfin | ΔpH = pHin − pHfin | % R |
|---|---|---|---|---|
| Batch | 2.5 | 2.13 | 0.37 | 62.16 |
| 4 | 3.50 | 0.50 | 71.17 | |
| 7 | 6.75 | 0.25 | 82.523 | |
| 9.5 | 7.30 | 2.20 | 92.16 | |
| Filter | 2.5 | 2.13 | 0.37 | 61.8 |
| 4 | 3.22 | 0.80 | 71.1 | |
| 7 | 6.00 | 1.00 | 94.77 | |
| 9.5 | 7.20 | 2.30 | 99.55 |
The optical images and UV-Vis spectra of the CV-dye samples, separated at different pH values after the treatment process using batch technique and filter technique, are presented in Fig. 11c and d. They support the experimental results in terms of the variation in the color of the treated solutions.
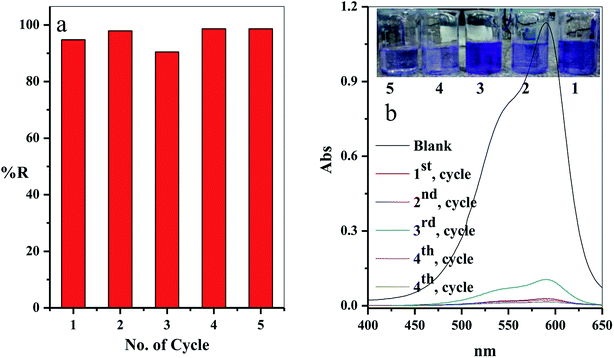
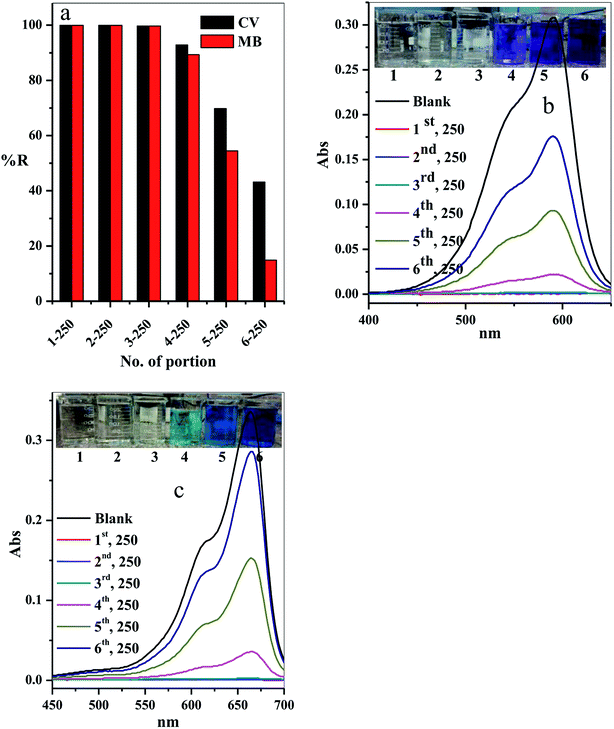
The residual concentration in both treated dye solutions (crystal violet and methylene blue) was plotted as a function of the number of the treated portions, as shown in Fig. 13a. It is clear that the dyes (crystal violet and methylene blue) were completely removed from the first three portions (% R = 99.90%). The % R gradually decreased in the last three portions to 92.86, 69.81 and 43.18, respectively, for crystal violet dye and to 89.29, 54.46 and 14.88, respectively, for methylene blue. These results can be observed by the liquids collected and the absorption spectra for crystal violet (Fig. 13b) and methylene blue (Fig. 13c) for various portion number during dye adsorption experiments.
4. Conclusion
C–GO composite was successfully prepared and used for the adsorption of cationic dye from the aqueous solution. Batch adsorption and filter adsorption were performed in this study. The filter adsorption process achieved better removal of CV-dye with Q° = 62.89 mg g−1, which is higher than Q° = 48.78 mg g−1 in batch adsorption. This phenomenon may be attributed to the fact that in the filter, all the dye species in the solution are forced to contact active sites, increasing the removal efficiency. The filter in our system is easy to regenerate and its production can be scaled up, enhancing its application on industrial scale.Conflicts of interest
There are no conflicts to declare.References
- G. McMullan, C. Meehan, A. Conneely, N. Kirby, T. Robinson, P. Nigam, I. M. Banat, R. Marchant and W. F. Smyth, Appl. Microbiol. Biotechnol., 2001, 56, 81–87 CrossRef CAS PubMed.
- C. I. Pearce, J. R. Lloyd and J. T. Guthrie, Dyes Pigm., 2003, 58, 179–196 CrossRef CAS.
- J. W. Lee, S. P. Choi, R. Thiruvenkatachari, W. G. Shim and H. Moon, Dyes Pigm., 2006, 69, 196–203 CrossRef CAS.
- M. Rafatullah, O. Sulaiman, R. Hashim and A. Ahmad, J. Hazard. Mater., 2010, 177, 70–80 CrossRef CAS PubMed.
- H. Metivier-Pignon, C. Faur-Brasquet and P. L. Cloirec, Sep. Purif. Technol., 2003, 31, 3–11 CrossRef CAS.
- R. Batmaz, N. Mohammed, M. Zaman, G. Minhas, R. M. Berry and K. C. Tam, Cell, 2014, 21(3), 1655–1665 CrossRef CAS.
- A. I. Abd-Elhamid, H. F. Aly, H. A. M. Soliman and A. A. El-Shanshory, J. Mol. Liq., 2018, 265, 226–237 CrossRef CAS.
- G. Cai, Z. Xu, M. Yang, B. Tang and X. Wang, Appl. Surf. Sci., 2017, 393, 441–448 CrossRef CAS.
- L.-L. Xu, M.-X. Guo, S. Liu and S.-W. Bian, RSC Adv., 2015, 5, 25244–25249 RSC.
- Z. Nooralian, M. P. Gashti and I. Ebrahimi, RSC Adv., 2016, 6, 23288–23299 RSC.
- F. Yaghoubidoust, D. H. B. Wicaksono, S. Chandren and H. Nur, J. Mol. Struct., 2014, 1075, 486–493 CrossRef CAS.
- W.-W. Liu, X.-B. Yan, J.-W. Lang, C. Peng and Q.-J. Xue, J. Mater. Chem., 2012, 22, 17245–17253 RSC.
- J. Chen, B. Yao, C. Li and G. Shi, Carbon, 2013, 64, 225–229 CrossRef CAS.
- M. A. Dimiev and J. M. Tour, ACS Nano, 2014, 8, 3060–3068 CrossRef PubMed.
- E. M. Teixeira, T. J. Bondancia, K. B. R. Teodoro, A. C. Corrêa, J. M. Marconcini and L. H. C. Mattoso, Ind. Crops Prod., 2011, 33, 63–66 CrossRef.
- A. Hebeish, S. Farag, S. Sharaf, A. M. Rabie and Th. I. Shaheen, Egypt. J. Chem., 2013, 56(4), 271–289 CrossRef.
- Y. Jiang, R. Raliya, P. Liao, P. Biswasa and J. D. Fortner, Nanotechnol. Environ. Sci., 2017, 4, 1484–1493 CAS.
- P. Eronen, M. Osterberg and A. S. Jaaskelainen, Cell, 2009, 16, 167–178 CrossRef CAS.
- Y. L. Liu, S. Kokot and T. J. Sambi, Analyst, 1998, 123, 633–636 RSC.
- L. Cabrales, N. Abidi and F. Manciu, Fibers, 2014, 2, 285–294, DOI:10.3390/fib2040285.
- L. Shahriary and A. A. Athawale, Int. J. Renew. Energy Environ. Eng., 2014, 2(1), 58–63 Search PubMed.
- X. Feng, K. Zheng, C. Wang, F. Chu and Y. Chen, Fibers Polym., 2016, 17(13), 371–379 CrossRef CAS.
- Y. Wu, Y. Yang, H. Liu, X. Yao, F. Leng, Y. Chen and W. Tian, RSC Adv., 2017, 7, 18917–18925 RSC.
- S. Lagergren, About the theory of so-called adsorption of soluble substances, Zur theorie der sogenannten adsorption geloster stoffe, K. Sven. Vetenskapsakad. Handl., 1898, 24, 1–39 Search PubMed.
- Y. S. Ho and G. McKay, Water Res., 1999, 33, 578–584 CrossRef CAS.
- V. Vadivelan and K. V. Kumar, J. Colloid Interface Sci., 2005, 286, 90–100 CrossRef CAS PubMed.
- I. Langmuir, Part I. Solids, J. Am. Chem. Soc., 1916, 38(11), 2221–2295 CrossRef CAS.
- H. Freundlich, J. Phys. Chem., 1906, 57, 385–470 CAS.
- T. W. Weber and R. K. Chakraborty, AIChE J., 1974, 20, 228–238 CrossRef CAS.
- G. Mckay, H. S. Blair and J. R. Gardener, J. Appl. Polym. Sci., 1982, 27, 3043–3057 CrossRef CAS.
- K. Porkodi and K. V. Kumar, J. Hazard. Mater., 2007, 143, 311–327 CrossRef CAS PubMed.
- R. Ahmad, J. Hazard. Mater., 2009, 17, 767–773 CrossRef PubMed.
- F. Chen, P. Fang, Y. Gao, Z. Liu, Y. Liu and Y. Dai, Chem. Eng. J., 2012, 204, 107–113 CrossRef.
- Y. Miyah, A. Lahrichi, M. Idrissi, S. Boujraf, Ha. Taouda and F. Zerrouq, J. Assoc. Arab Univ. Basic Appl. Sci., 2017, 23, 20–28 Search PubMed.
- S. Chakraborty, S. Chowdhury and P. D. Saha, Carbohydr. Polym., 2011, 86(4), 1533–1541, DOI:10.1016/j.carbpol.2011.06.058.
- M. B. Silveira, F. A. Pavan, N. F. Gelos, E. C. Lima and S. L. Dias, Clean: Soil, Air, Water, 2014, 42(7), 939–946 CAS.
- P. D. Saha, S. Chakraborty and S. Chowdhury, Colloids Surf., B, 2012, 92, 262–270 CrossRef PubMed.
- C. Liua, A. M. Omer and X.-k. Ouyang, Int. J. Biol. Macromol., 2018, 106, 823–833 CrossRef PubMed.
- S. K. Maiti, D. Bera, P. Chattopadhyay and L. Ray, Appl. Biochem. Biotechnol., 2009, 159, 488–504 CrossRef CAS PubMed.
- M. Talat, S. Mohan, V. Dixita, D. K. Singh, S. H. Hasan and O. N. Srivastava, Groundw. Sustain. Dev., 2018, 7, 48–55 CrossRef.
- S. Mohan, D. K. Singh and V. Kumar, J. Fluorine Chem., 2017, 194, 40–50 CrossRef CAS.
- E. N. E. Qada, S. J. Allen and G. M. Walker, Chem. Eng. J., 2006, 124, 103–110 CrossRef.
- B. H. Hameed and A. A. Ahmad, J. Hazard. Mater., 2009, b164, 870–875 CrossRef PubMed.
- H. Ren, Z. Gao, D. Wu, J. Jiang, Y. Sun and C. Luo, Carbohydr. Polym., 2016, 137, 402–409 CrossRef CAS PubMed.
- A. I. Abd-Elhamid, M. R. El-Aassar, G. F. El Fawal and H. M. A. Soliman, Environmental Nanotechnology, Monitoring and Management, 2019, 11, 100207 CrossRef.
- D. A. Gopakumar, D. Pasquini, M. A. Henrique, L. C. de Morais, Y. Grohens and S. Thomas, Meldrum's Acid Modified Cellulose Nanofiber-Based Polyvinylidene Fluoride Microfiltration Membrane for Dye Water Treatment and Nanoparticle Removal, ACS Sustainable Chem. Eng., 2017, 5, 2026–2033 CrossRef CAS.
- J. Štefelová, V. Slovák, G. Siqueira, R. T. Olsson, P. Tingaut, T. Zimmermann and H. Sehaqui, Drying and Pyrolysis of Cellulose Nanofibers from Wood, Bacteria, and Algae for Char Application in Oil Absorption and Dye Adsorption, ACS Sustainable Chem. Eng., 2017, 5, 2679–2692 CrossRef.
| This journal is © The Royal Society of Chemistry 2019 |