Open Access Article
Open Access ArticleRubidium ion Capture with a phosphotungstic acid-functionalized finger-citron-residue-based carbon†
Qing Liua,
Guihua Zhaoa,
Yifang Daib,
Na Mac and
Wei Dai *a
*a
aCollege of Chemistry and Life Science, Zhejiang Normal University, Jinhua 321004, Zhejiang Province, PR China. E-mail: daiwei@zjnu.edu.cn; Fax: +86-579-82282325; Tel: +86-579-82282269
bClass 14 of First Grade, Jinhua No.1 High School, Jinhua 321004, Zhejiang Province, PR China
cCollege of Geography and Environmental Sciences, Zhejiang Normal University, Jinhua 321004, Zhejiang Province, PR China
First published on 19th March 2019
Abstract
To solve the contradiction of diffusion and selectivity, we reported a novel finger-citron-residue-based mesoporous carbon (FMC) as a support to prepare a novel adsorbent PTA@FMC (PTA represents phosphotungstic acid) for rubidium ion capture. This new adsorbent was characterized by X-ray diffraction, thermogravimetric analysis, scanning electron microscopy, Fourier transform infrared spectroscopy, and N2 adsorption, and the results showed that the PTA was immobilized in the FMC structure. Based on the results of batch tests, we demonstrated that PTA@FMC is the most distinctive adsorbent with a superior uptake capacity compared with some of those previously reported in the literatures. The adsorption tests in the presence of interfering ions (Na+, K+ and Cs+) showed that the more the added amount of the different types of interfering ions, the more severe is the degree by which the adsorption of Rb+ is weakened. In addition, the Rb+ sorption selectivity was moderately influenced by the co-ion effect in the presence of any ions (K+, Na+ and Cs+) due to PTA doping. In a word, due to its relatively facile preparation process and good uptake capacity, PTA@FMC might be a promising adsorption material for Rb+.
1. Introduction
Rubidium, a typical rare metal, has various applications in the field of photocells, thermoelectric generators, alkali batteries, biological engineering, and medical industries.1–3 In the recent few decades, due to the increasing demand for rubidium from industries, the price of the rubidium metal has been rocketing in comparison to those of other alkali metals.3 Although, the total content of rubidium ions in brine lakes is relatively high, the concentration of rubidium ions in those lakes is in trace amounts.4 Consequently, the separation, condensation and purification of rubidium ions are regarded as hot issues pertinent to scientific research fields.In terms of the methods employed in the capture of rubidium ions, extraction, use of ion exchangers and adsorption are the most frequently used.5–8 Among these, adsorption is considered as a potential method because it has the advantages of strong operability, as it can be carried out in ambient temperature and atmosphere by a simple process, and reproducibility.7 At present, because of their adaptability to the size of rubidium ions and their relatively high specific surface area, the microporous adsorbent materials have wide applications in the field of rubidium ion adsorption. However, the ability of ordinary microporous materials to adsorb rubidium ions is restricted because of the limitations of the simple adsorption process. The adsorption of rubidium ions onto the adsorbents is controlled by the combination of physical adsorption and chemical adsorption. Physical adsorption has a poor adsorption selectivity for ions with rather limited adsorption capacity, while the chemical adsorption process is selective. Hence, amplifying the adsorption advantages of the chemical adsorption process to enhance the adsorption selectivity and thus increase the adsorption capacity of rubidium ions can be a meaningful research task. To be more specific, an effective way to realize this enhancement is to modify the adsorbent materials by introducing the functional groups that have specific affinity towards rubidium ions onto the original adsorbents. Studies in this attempt have been proved to be successful.3–21
Heteropoly acids are certain substances that have adsorption affinities with heavy metal ions.10 The introduction of a heteropoly acid on the adsorbent surface is thought to effectively enhance the chemical adsorption interactions of rubidium ions. One of the most representative MOF materials is HKUST-1 (also named as Cu–BTC), a particular type of MOF containing Cu(II)-paddle wheel-type nodes and 1,3,5-benzenetricarboxylate struts. In our previous study,3 a modified microporous adsorbent, PMA@HKUST-1, which has distinguishing adsorption properties for rubidium ions, was successfully developed by loading molybdophosphoric acid onto the microporous adsorbent HKUST-1. The effect of PMA@HKUST-1 on the adsorption of rubidium ions was gratifying. The use of PMA@HKUST-1 increased the adsorption amount of rubidium ions onto the adsorbents by approximately 28.5%.3 Even then the PMA@HKUST-1 was imperfect. The microporous structure of HKUST-1 was seriously damaged after the introduction of phosphomolybdic acid. In addition, the paradox of the relatively large molecular diameter of PMA and the finite pore size of HKUST-1 rendered the introduction of heteropoly acids a defective practice. The pores of the microporous adsorbents, after the introduction of the heteropoly acid, were blocked, the pore volume of the adsorbents reduced, and the diffusion efficiency of rubidium ions decreased, negatively disturbing the adsorption process. In the face of this undesirability, we came up with the idea of replacing the microporous material with a mesoporous material to enlarge the pore volume, which would be favorable for loading the heteropoly acid and the diffusion of adsorbates.
Supposedly, the pore size characteristics of the mesoporous materials facilitate the loading of heteropoly acid molecules in their pore structure without blocking the porous pathway, which is conducive to enhance the chemical adsorption capacity of rubidium ions. Theoretically, mesoporous materials with high specific area, uniform porous structures and good thermostability are promising support.9 Though few studies have been conducted on the application of mesoporous materials as in the adsorption of rubidium ions, the outcomes drawn from those studies are valuable. In H. Aghayan's study,22 substituted molybdophosphoric acid was loaded onto SBA-15 with various structural morphologies; the as-prepared samples showed a high adsorption ability towards thorium ions and the morphology of SBA-15 was decisively significant in guaranteeing an auspicious adsorption process. This study substantiated the validity of adopting a mesoporous material as the support, onto which heteropoly acids can be introduced. Phosphotungstic acid (PTA), a member of the group of heteropoly acids, has similarity in chemical properties with those of phosphomolybdic acid (PMA).23 In effect, in our previous work trials,12 a finger-citron-residue-based mesoporous carbon (FMC) was synthesized by filling the pores of a siliceous SBA-15 hard template with finger citron residues as the carbon precursors. Herein, we present a study on the rubidium ion capture with a new type of composite material PTA@FMC, including batch equilibrium experiments and adsorption mechanisms.
2. Materials and methods
2.1. Chemicals and reagents
Hydrochloric acid (HCl, ≥99%), phosphotungstic acid hydrate (H3O40PW12·xH2O, ≥99.5%), and ethanol (C2H6O, 99.7%) were purchased from Sinopharm Chemical Reagent Co. Ltd. All the chemicals used in this study were of analytical grade and used without further purification.2.2. Preparation of PTA@FMC
The FMC was facilely synthesized by a similar procedure to that in our reported work.24 The three samples in which different contents of PTA were loaded onto the FMC were prepared as follows: in each container, 1 g of FMC was weighed and equal volumes of PTA of 0.05 M, 0.075 M and 0.1 M were added. The mixtures were kept under agitation and impregnation for 12 h. The subsequent solutions were dried at 100 °C and the products in the form of white powder were obtained. The three samples were designated as 50PTA@FMC, 75PTA@FMC, and 100PTA@FMC.2.3. Characterization
Information on the characterization tests can be found in the ESI (Section 1†), including the results of nitrogen adsorption–desorption, powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), and Fourier transform infrared spectroscopy (FTIR) studies.2.4. Batch equilibrium
2.5. Influence of interfering ions
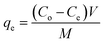 | (1) |
3. Results and conclusion
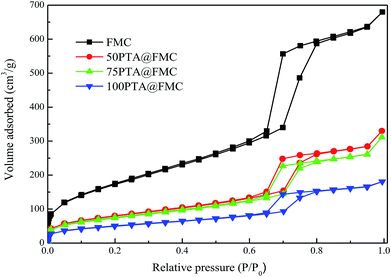
3.1. Characterization
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and the telescopic vibration of the W–O–W bond, respectively.
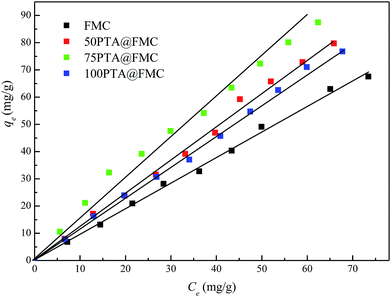
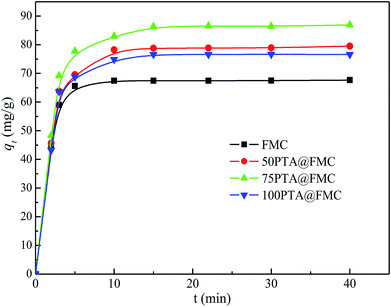
O bond and the telescopic vibration of the W–O–W bond, respectively.3.2. Batch adsorption
3.3. The interference of interfering ions
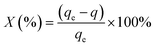 | (2) |
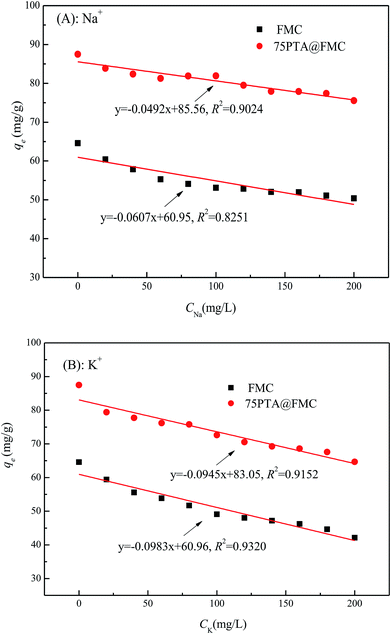 | ||
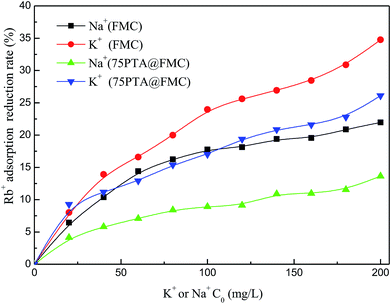
| Fig. 4 Relationship between the adsorption amount of Rb+ onto the FMC or PTA@FMC and the added amount of Na+ and K+. (A): Na+; (B): K+. | ||
The relationship between the reduction rate of the adsorption amount of Rb+ onto the adsorbents (FMC and PTA@FMC materials) and the added concentration of the interfering ions (Na+ and K+) is plotted in Fig. 5. From Fig. 5, we can mainly draw three conclusions. First of all, the reduction rate of the adsorption amount of Rb+ decreases along with the increase in the added amount of interfering ions, which is consistent with the results mentioned above. In addition, the interference effect of K+ on the adsorption of Rb+ onto both the FMC and PTA@FMC is larger than that of Na+, as evidenced by the higher reduction rate of the adsorption amount of Rb+. Finally, regardless that the interference is by Na+ or K+, the reduction in the percentage of adsorption of Rb+ onto the FMC is larger than that onto the corresponding PTA@FMC, indicating that the loading of PTA can abate the influence of the interfering ions on the adsorption of Rb+.
The adsorption of Rb+ ions is achieved depending on various factors like the physical and/or chemical properties of the adsorbents and the mass transfer process.35–42 For example, the Lewis acid–base interaction might be the main factor affecting the K+, Na+ and Rb+ adsorption process in this study. According to Pearson's hard and soft acid–base concept, bases can be classified into two categories. One type of bases is polarizable, and the other type is non-polarizable. These two groups are denoted as “soft” and “hard” bases, respectively. Similarly, acids can be classified based on their preferential interaction with hard or soft bases. That is, acids that form strong interactions with hard and soft bases are called hard and soft acids, respectively. According to this concept, for the same family elements Na, K, and Rb, with the increase in the atomic number, the electron layer increases, and the radius of the atom increases. The attraction of the nucleus to the outermost electrons decreases, and the electron losing ability of the atom increases. Rb+ exhibiting the characteristic of a Lewis acid is relatively soft than Na+ and K+. Thus, the relatively soft Rb+ might be strongly attracted to soft Lewis bases, such as the phosphomolybdic ion. Thus, the uptake of Rb+ is higher than those of Na+ and K+.
4. Conclusion
In this study, we have eliminated the limitation of the diffusion of microporous materials, using the ordered mesoporous material FMC to prepare a novel heteropoly acid-functionalized sorbent (PTA@FMC). The experimental characterization manifests that this composite material shows no significant difference in the structural characteristics from those of the FMC, implying that the introduction of PTA does not disturb the structure of FMC. In addition, according to the batch equilibrium experiments of rubidium ion adsorption by the PTA@FMC materials, we demonstrated that these materials have a satisfactory Rb+ adsorption capacity, among which 75PTA@FMC is the most distinctive. The influence of interfering ions on the adsorption of Rb+ has also been probed into, and several pieces of worthy information have been obtained. Above all, it has been confirmed that Cs+, K+, and Na+ have a competitive adsorption effect on the adsorption of Rb+, and Cs+ is the most competitive one. Furthermore, the amount of interfering ions also has a significant effect on the adsorption of Rb+. We tested the effect on the adsorption of rubidium ion by separately adding one, two, and three types of interfering ions (Na+, K+, Cs+). The results showed that more the added amount of the different types of interfering ions, the more severe is the degree by which the adsorption of Rb+ is weakened. In a word, due to its relatively facile preparation process, low cost and good adsorption capacity, PTA@FMC is a promising adsorption material for Rb+.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Zhejiang Provincial Natural Science Foundation of China under Grant No. LY19B060014.References
- N. Moazezi and M. A. Moosavian, J. Environ. Chem. Eng., 2016, 4, 2440–2449 CrossRef CAS.
- P. Xing, C. Wang, B. Z. Ma, W. J. Zhang and Y. Q. Chen, Chem. Eng., 2018, 6, 4922–4930 CAS.
- W. Dai, Y. Y. Fang, L. Yu, G. H. Zhao and X. Y. Yan, J. Taiwan Inst. Chem. Eng., 2018, 84, 222–228 CrossRef CAS.
- M. R. Awual, M. Khraisheh, N. H. Alharthi, M. Luqman, A. Islam, M. R. Karim, M. M. Rahman and M. A. Khaleque, Chem. Eng. J., 2018, 343, 118–127 CrossRef CAS.
- K. Abbas, H. Znad and M. R. Awuala, Chem. Eng. J., 2018, 334, 432–443 CrossRef CAS.
- A. Shahata, H. M. A. Hassana, H. M. E. Azzazy, E. A. El-Sharkawy, H. M. Abdou and M. R. Awual, Chem. Eng. J., 2018, 332, 377–386 CrossRef.
- M. R. Awual, N. H. Alharthi, M. M. Hasan, M. R. Karim, A. Islam, H. Znad, M. A. Hossain, M. E. Halim, M. M. Rahman and M. A. Khaleque, Chem. Eng. J., 2017, 324, 130–139 CrossRef CAS.
- M. R. Awual, N. H. Alharthi, Y. Okamoto, M. R. Karim, M. E. Halim, M. M. Hasan, M. M. Rahman, M. M. Islam, M. A. Khaleque and M. C. Sheikh, Chem. Eng. J., 2017, 320, 427–435 CrossRef CAS.
- M. R. Awual, Chem. Eng. J., 2017, 307, 85–94 CrossRef CAS.
- M. R. Awual, Chem. Eng. J., 2017, 307, 456–465 CrossRef CAS.
- M. R. Awual, Chem. Eng. J., 2016, 303, 539–546 CrossRef CAS.
- M. R. Awual, Chem. Eng. J., 2016, 300, 264–272 CrossRef CAS.
- M. R. Awual, Y. Miyazaki, T. Taguchi, H. Shiwaku and T. Yaita, Chem. Eng. J., 2016, 291, 128–137 CrossRef CAS.
- M. R. Awual, Chem. Eng. J., 2016, 289, 65–73 CrossRef CAS.
- M. R. Awual, M. M. Hasan, M. A. Khaleque and M. C. Sheikh, Chem. Eng. J., 2016, 288, 368–376 CrossRef CAS.
- M. R. Awual, T. Yaita, H. Shiwaku and S. Suzuki, Chem. Eng. J., 2015, 276, 1–10 CrossRef CAS.
- M. R. Awual, T. Yaita, S. Suzuki and H. Shiwaku, J. Hazard. Mater., 2015, 291, 111–119 CrossRef CAS PubMed.
- M. R. Awual, Chem. Eng. J., 2015, 266, 368–375 CrossRef CAS.
- M. R. Awual, M. M. Hasan and M. A. Khaleque, Sens. Actuators, B, 2015, 209, 194–202 CrossRef CAS.
- M. R. Awual and M. M. Hasan, Sens. Actuators, B, 2015, 206, 692–700 CrossRef CAS.
- M. R. Awual, M. M. Hasan and H. Znad, Chem. Eng. J., 2015, 259, 611–619 CrossRef CAS.
- H. Aghayan, A. R. Khanchi, T. Yousefi and H. Ghasemi, J. Nucl. Mater., 2017, 496, 207–214 CrossRef CAS.
- Y. Fang, G. Zhao, W. Dai, L. Ma and N. Ma, Microporous Mesoporous Mater., 2017, 251, 51–57 CrossRef CAS.
- Y. Fang, W. Dai, L. Chen and N. Ma, Mater. Lett., 2016, 174, 246–248 CrossRef CAS.
- G. Naidu, T. Nur, P. Loganathan, J. Kandasamy and S. Vigneswaran, Sep. Purif. Technol., 2016, 163, 238–246 CrossRef CAS.
- M. R. Awual, M. M. Hasan, T. Ihara and T. Yaita, Microporous Mesoporous Mater., 2014, 197, 331–338 CrossRef.
- M. R. Awual and M. M. Hasan, Microporous Mesoporous Mater., 2014, 196, 261–269 CrossRef CAS.
- M. R. Awual, T. Yaita, T. Taguchi, H. Shiwaku, S. Suzuki and Y. Okamoto, J. Hazard. Mater., 2014, 278, 227–235 CrossRef CAS PubMed.
- M. R. Awual, S. Suzuki, T. Taguchi, H. Shiwaku, Y. Okamoto and T. Yaita, Chem. Eng. J., 2014, 242, 127–135 CrossRef CAS.
- M. R. Awual, I. M. M. Rahman, T. Yaita, M. A. Khaleque and M. Ferdows, Chem. Eng. J., 2014, 236, 100–109 CrossRef CAS.
- M. R. Awual, T. Yaita and H. Shiwaku, Chem. Eng. J., 2013, 228, 327–335 CrossRef CAS.
- G. Naidu, S. Jeong, Y. K. Choi, M. H. Song, U. Oyunchuluun and S. Vigneswaran, J. Cleaner Prod., 2018, 174, 1079–1088 CrossRef CAS.
- X. J. Huang, L. Y. An, X. Y. Zhao and X. Q. Chen, Adv. Mater. Res., 2013, 652, 2524–2528 Search PubMed.
- D. L. Guerra, A. C. Batista, R. R. Viana and A. i. Claudio, Desalination, 2011, 275, 107–117 CrossRef CAS.
- N. Gayathri, L. Paripurnanda, J. Sanghyung, H. J. M. Abu, P. T. Vu Hien, K. Jaya and V. Saravanamuthu, Chem. Eng. J., 2016, 306, 31–42 CrossRef.
- M. R. Awual, M. Ismael, T. Yaita, S. A. El-Safty, H. Shiwaku, Y. Okamoto and S. Suzuki, Chem. Eng. J., 2013, 222, 67–76 CrossRef CAS.
- M. R. Awual, T. Yaita, S. A. El-Safty, H. Shiwaku, Y. Okamoto, S. Suzuki and Y. Okamoto, Chem. Eng. J., 2013, 221, 322–330 CrossRef CAS.
- A. Shahat, M. R. Awual and M. Naushad, Chem. Eng. J., 2015, 271, 155–163 CrossRef CAS.
- M. R. Awual, G. E. Eldesoky, T. Yaita, M. Naushad, H. Shiwaku, Z. A. AlOthman and S. Suzuki, Chem. Eng. J., 2015, 279, 639–647 CrossRef CAS.
- M. R. Awual, J. Ind. Eng. Chem., 2014, 20, 3493–3501 CrossRef CAS.
- Y. Onodera, T. Iwasaki, H. Hayashi and K. Torii, Nippon Kogyo Kaishi, 1988, 104, 277–282 CAS.
- R. Gong, J. J. Ye, W. Dai, X. Y. Yan, J. Hu, X. Hu, S. Li and H. Huang, Ind. Eng. Chem. Res., 2013, 52, 14297–14303 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra10453k |
| This journal is © The Royal Society of Chemistry 2019 |