Open Access Article
Open Access ArticleDehydration of fructose, sucrose and inulin to 5-hydroxymethylfurfural over yeast-derived carbonaceous microspheres at low temperatures†
Xiaofeng Li,
Yi Wang,
Xiaomin Xie,
Changhong Huang and
Sen Yang *
*
Beijing Key Laboratory of Farmland Soil Pollution Prevention and Remediation, College of Resources and Environmental Sciences, China Agricultural University, Beijing 100193, P. R. China. E-mail: syang@cau.edu.cn; Fax: +86-10-62733470; Tel: +86-10-62733470
First published on 19th March 2019
Abstract
This work prepared carbonaceous microspheres by hydrothermal carbonization of yeast cells followed by sulfonation with concentrated sulphuric acid (98%) at room temperature. The obtained carbonaceous product (CM-SO3H) had a high acid density (1.80 mmol g−1). We evaluated CM-SO3H as a solid catalyst for the dehydration of fructose-based carbohydrates to 5-hydroxymethylfurfural (5-HMF) in the ionic liquid 1-butyl-3-methylimidazolium chloride ([BMIM][Cl]). The effects of the catalyst and substrate loadings as well as the reaction temperature and time on the yield of 5-HMF were investigated. Under the optimum conditions, a 5-HMF yield of up to 83.5% was obtained from fructose with a reaction temperature of 80 °C for 30 min. Furthermore, 44.8% and 59.2% 5-HMF yields were obtained from sucrose (80 °C for 30 min) and inulin (80 °C for 60 min), respectively. CM-SO3H and [BMIM][Cl] showed high stability and could be recycled between five and eight times without significant loss of catalytic activity. More importantly, the catalytic system could be applied to high substrate concentrations. CM-SO3H combined with [BMIM][Cl] is a promising system for transforming fructose-based carbohydrates into 5-HMF.
Introduction
Biomass-derived chemicals have attracted interest for their potential to reduce global dependence upon fossil fuels.1 Among these chemicals, 5-hydroxylmethylfurfan (5-HMF) is one of the most important platform chemicals, and has been widely applied in the production of polymers, fine chemicals, pharmaceuticals, and biofuels.2,3 5-HMF can be generated from the dehydration of biomass derivatives such as fructose,4,5 glucose,6 sucrose,7 and cellulose.8 The most efficient method is dehydration of fructose catalysed by acid,9 as the conversion of other feedstocks requires a carbohydrate-to-fructose step.10Sulphuric acid11 and hydrochloric acid12 are efficient homogeneous catalysts for 5-HMF production through carbohydrate dehydration, but they have several drawbacks. These include difficulties with the separation and recycling of the catalyst, corrosion of equipment, and release of toxic waste.13 Heterogeneous catalysts can be used as an alternative to homogeneous catalysts to address these issues, and are more suitable for industrial applications.14,15 Therefore, a variety of heterogeneous catalysts, such as solid superacids16 and acid cation exchange resins,17,18 have been considered for the production of 5-HMF. Functionalization of solid catalysts with –SO3H groups is an efficient and readily available means of improving the reaction rate and selectivity during the dehydration of carbohydrates to 5-HMF.19
SO3H-functionalized carbonaceous materials are very promising solid acid catalysts because they exhibit high stability and surface modifiability.20,21 Recently, several carbonaceous materials, including mesoporous carbon, mesoporous carbon–silica composites, carbon nanotubes, biochar, and carbons prepared by the hydrothermal carbonization of biomass, have been modified with –SO3H groups and have shown good catalytic performance in the conversion of carbohydrates to 5-HMF.19,22,23 However, few studies have used hollow carbonaceous materials as catalysts for the conversion of carbohydrates to 5-HMF. Those carbonaceous materials that have been reported are commonly treated with concentrated sulphuric acid (98%) at high temperatures to introduce –SO3H groups. As an example, biochar requires sulfonation at 150 °C for 12 h,2 and carbonized lignin24 and cellulose-derived carbonaceous solids25 require sulfonation at 200 °C for 5–12 h. The high temperatures required for the sulfonation process are a challenge because they result in harsh operational conditions and can cause equipment corrosion.22 However, any material that can be carbonized can be used to prepare SO3H-functionalized carbonaceous materials.26 One such material is yeast produced as an organic waste by-product of the fermentation industry. The cell walls of yeast primarily consist of a polysaccharide layer constructed from coiled β-1,3-glucan chains, and hollow carbonaceous microspheres (CMs) with meso- and microporous shells have been prepared by the mild hydrothermal treatment of these organisms.27,28 Generally, hollow microspheres exhibit useful characteristics, such as improved mass transport and diffusion, high pile density and good fluidity,29,30 and so can be used as catalysts or catalyst supports. Most importantly, CMs are composed of both carbonized organic matter (that is, aromatic carbon) and non-carbonized organic matter (alkyl carbon), and the surfaces of these materials are covered with oxygenated functional groups, including carbonyl, carboxy, hydroxy, ether, and ester moieties.27,28 Therefore, it is easy to modify the surfaces of CMs by mild sulfonation. Even so, few studies have used CMs fabricated from yeast cells as precursors for solid acid catalysts.
The solvent is crucial in the production of 5-HMF from carbohydrates.24 To date, water and highly polar organic solvents have been used as reaction solvents.14,31 However, the reaction temperatures are generally high (120–180 °C) and high energy inputs are required.7,17,25 As an example, the maximum 5-HMF yield from the dehydration of fructose using sulfonated biochar as the catalyst and water as the solvent is obtained at 160–180 °C.4 With DMSO as the solvent, the transformation of fructose into 5-HMF is generally performed at 120 °C.9 Ionic liquids (ILs) are organic salts that are liquids at room temperature and can be easily recycled.32,33 According to the literature, ILs such as [HMIM][Cl], [OMIM][Cl], and [BEMIM][Cl] can play a positive role in the dehydration of carbohydrates because of their good solvating powers.4,21,22,34 The dehydration of fructose in ILs can give a high 5-HMF yield at temperatures as low as 80 °C.35 Among the ILs, 1-butyl-3-methylimidazolium chloride ([BMIM][Cl]) is a suitable medium for the production of 5-HMF from fructose since it can act as both a proton donor and acceptor.24,36
In this work, we prepared CMs via the hydrothermal treatment of yeast cells, as reported previously.27 SO3H-functionalized microspheres were subsequently obtained after immersion of these CMs in concentrated sulphuric acid (98%) at room temperature. The catalytic activities of the resulting catalysts were estimated during the production of 5-HMF from fructose, sucrose, and inulin.
Experimental
Materials and reagents
Budding yeast (Saccharomyces cerevisiae) was purchased from the Angel Yeast Co., Ltd. (Yichang, China). Fructose (99%) and sucrose (99%) were purchased from the Sigma-Aldrich Co., Ltd. (Missouri, USA). Inulin (90%) was purchased from the Yuan Ye Biotechnology Co., Ltd. (Shanghai, China) and 5-HMF (99%) was purchased from the Aladdin Chemicals Co., Ltd. (California, USA). Acetone (99%), ethyl alcohol (99%), ethyl acetate (99%), and concentrated sulphuric acid (98%) were obtained from the Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Methanol (HPLC grade) was purchased from the Fisher Chemicals Co., Ltd. (New Jersey, USA). [BMIM][Cl] (99%) was purchased from the Linzhou Keneng Materials Technology Co., Ltd. (Linzhou, China). Ultrapure water was used in all experiments. All chemicals were used as obtained without further treatment.Preparation of the catalyst
CMs were synthesized by a modification of the hydrothermal method described in our previous study.27 Typically, S. cerevisiae cells (3.0–4.0 g) were pre-washed with acetone and then dispersed in ultrapure water (40 mL). The mixture was subsequently placed in a 50 mL Teflon-sealed autoclave and maintained at 180 °C for 8 h. The puce-colored solid product was isolated by centrifugation, washed alternately with ultrapure water and ethyl alcohol three times, and oven-dried at 80 °C for 8 h. This material is referred to herein as the CMs.The CMs (1.0 g) were then dispersed in concentrated sulphuric acid (20 mL) at room temperature with stirring at 800 rpm. After a certain time, the resulting black precipitate was removed by filtration and washed with water until the wash water was neutral. The sulfonated material obtained in this manner was dried at 75 °C in a vacuum oven overnight. The CMs sulfonated for 8 h are denoted herein as CM-SO3H.
Characterization
Field-emission scanning electron microscopy (FE-SEM, SU 8020, Hitachi, Japan) was used to obtain images of the CMs and CM-SO3H. Fourier-transform infrared (FTIR) spectra were acquired over the range of 400–4000 cm−1 (IS 10, Nicolet, USA) and the C, H, N, O, and S contents of the samples were determined using an elemental analyser (Flash 2000, Therom, USA). The surface areas and pore sizes of the CMs and CM-SO3H were obtained from N2 adsorption/desorption isotherms acquired at −196.15 °C with a physisorption analyser (ASAP 2020, Micromeritics, USA). The total number of acid sites in the CM-SO3H was estimated by acid–base titration.5,9 Briefly, CM-SO3H (0.10 g) was suspended in 50 mL of aqueous 0.5 M NaCl and stirred for 24 h. During this time, H+ was liberated into the aqueous solution by exchange with Na+. The filtrate was subsequently titrated using a standard NaOH solution (0.01 M).Catalytic dehydration of carbohydrates to 5-HMF
The dehydration reactions of carbohydrates were performed in 10 mL glass tubes. Typically, a feedstock (0.50 g of fructose, 0.25 g of sucrose, or 0.25 g of inulin) was dissolved in 2.50 g of [BMIM][Cl] at 60 °C, after which 50 mg of CM-SO3H was added rapidly with stirring at 800 rpm. The reactor was then heated to 80 °C in an oil bath. After the desired reaction time had elapsed, the products were extracted into ethyl acetate with stirring at room temperature.In an experiment to test the reusability of the catalyst, the 5-HMF produced after 30 min of reaction was extracted by 25 mL ethyl acetate. After four such extractions, the remaining [BMIM][Cl] and CM-SO3H were heated at 80 °C for 12 h in a vacuum drying oven to remove water and residual ethyl acetate. The residue was used directly for the next carbohydrate to 5-HMF conversion with fresh feedstock.
Analytical methods
The 1H nuclear magnetic resonance (NMR) spectrum of 5-HMF was recorded in d4-methanol at 25 °C using a 400 MHz NMR spectrometer (JNM-ECZ, Jeol, Japan). The amount of 5-HMF obtained from each reaction was analysed directly by HPLC with a Zorbax SB-C18 column (4.6 mm × 12.5 mm) and methanol/water (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
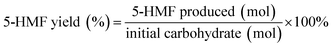
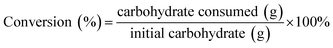
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, v/v) as the mobile phase.37 The flow rate was 0.7 mL min−1 and the column temperature was kept at 30 °C. The carbohydrate content was determined by HPLC with an Aminex HPX-87H column (300 mm × 7.8 mm) in conjunction with a refractive index detector. The mobile phase was 5 mM H2SO4 at a flow rate of 1.0 mL min−1 and the column temperature was maintained at 55 °C.38,39 The 5-HMF yield and carbohydrate conversion were calculated using the following equations.
80, v/v) as the mobile phase.37 The flow rate was 0.7 mL min−1 and the column temperature was kept at 30 °C. The carbohydrate content was determined by HPLC with an Aminex HPX-87H column (300 mm × 7.8 mm) in conjunction with a refractive index detector. The mobile phase was 5 mM H2SO4 at a flow rate of 1.0 mL min−1 and the column temperature was maintained at 55 °C.38,39 The 5-HMF yield and carbohydrate conversion were calculated using the following equations.
 | (1) |
 | (2) |
Each experiment was repeated at least three times with similar results, and all the reported reaction data are averages.
Results and discussion
Catalyst screening
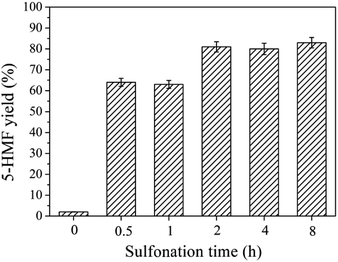
We prepared all solid acid catalysts under the same conditions, except that the sulfonation time was varied from 0 to 8 h. The hydrothermal carbonization was carried out at 180 °C for 8 h and the sulfonation was conducted at room temperature in concentrated sulphuric acid. The catalytic activities of the samples were investigated based on the dehydration of fructose, which was found to be the most reactive substrate (Fig. 1).We carried out a preliminary experiment in the absence of a catalyst in [BMIM][Cl] at 80 °C for 30 min, but no 5-HMF was obtained. It should be noted that, at 120 °C, [BMIM][Cl] can act as both solvent and catalyst during fructose dehydration.24 This indicates that the catalytic activity of [BMIM][Cl] is dependent on the reaction temperature.34 However, at 80 °C, the catalytic activity of the [BMIM][Cl] was negligible. Adding 50 mg of CMs gave a 5-HMF yield of approximately 2.1%, and so it was clear that the CMs exhibited little catalytic activity under the present conditions. However, a large increase in the catalytic activity occurred after the CMs were sulfonated. The yield of 5-HMF increased to 64.0% with the addition of CMs that were sulfonated for 0.5 h, suggesting that –SO3H groups served as active sites on the catalyst.9 A higher 5-HMF yield of 81.0% was obtained when the sulfonation time was increased to 2 h, and a slight further increase in the yield was observed following an additional increase in sulfonation time to 8 h. 1H NMR spectroscopy (Fig. S1†) confirmed the production of 5-HMF. These results show that the sulfonation of CMs at room temperature is an effective approach to preparing highly active catalysts for the synthesis of 5-HMF from fructose. Furthermore, the sulfonation time has a remarkable effect on the catalytic activity of the CMs. We selected the CMs sulfonated for 8 h (CM-SO3H) as the most active and stable catalyst for all the following experiments.
The catalytic activity of the CM-SO3H during the dehydration of glucose, the least reactive substrate, was also assessed.40 A 5-HMF yield of 10.2% was obtained at 120 °C after 2 h. The low 5-HMF yield from glucose may be explained by the lack of Lewis acid sites on CM-SO3H, because 5-HMF synthesis from glucose involves isomerization catalysed by a Lewis acid and dehydration catalysed by a Brønsted acid.41
Characterization of the CM-SO3H catalyst
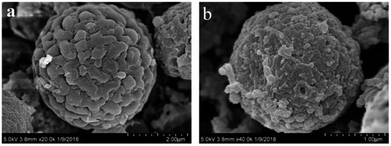
SEM images of the CMs and CM-SO3H are presented in Fig. 2. The obtained CMs were in the form of microspheres with diameters in the 2.0–3.0 μm range, which was consistent with our previous results.27 After sulfonation, the microsphere structure was well preserved but the diameter decreased to 1.0–2.0 μm. The surface area of the CM-SO3H was 12.5 m2 g−1, which was slightly higher than that of the CMs (10.5 m2 g−1) (Table 1). The CMs pore size was 15.5 nm, whereas that of the CM-SO3H was 16.6 nm. From these results, it was clear that the CMs were etched by the concentrated sulphuric acid.| Sample | AD (mmol g−1) | SA (m2 g−1) | W (nm) | Elemental compositions (wt%) | Atomic ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | O/C ratio | (O + N)/C ratio | ||||
| a O/C: atomic ratio of oxygen to carbon. (O + N)/C: atomic ratio of the sum of nitrogen and oxygen to carbon. | ||||||||||
| CMs | 0.20 | 10.5 | 15.5 | 60.06 | 5.33 | 26.68 | 4.69 | 0.20 | 0.33 | 0.40 |
| CM-SO3H | 1.80 | 12.5 | 16.6 | 56.27 | 5.76 | 28.33 | 4.62 | 4.02 | 0.38 | 0.45 |
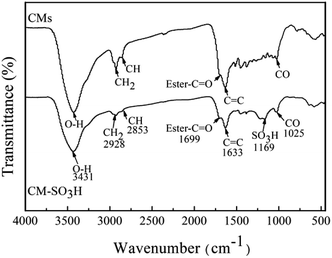
Fig. 3 shows the FTIR spectra of the CMs and CM-SO3H. A new band appearing at 1169 cm−1 in the FTIR spectrum of the CM-SO3H was ascribed to the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of –SO3H groups,42 and this confirmed that –SO3H groups were successfully incorporated into the CMs during the sulfonation process.38,43,44 The band at 3431 cm−1 indicated the presence of –OH groups22,44 on the surfaces of both the CMs and CM-SO3H. Bands at 2928 and 2853 cm−1 were attributed to –CH2 and –CH stretching vibrations,19,27 and the intensities of these bands decreased dramatically after sulfonation of the CMs, indicating that these surface functional groups were removed. This could have been caused by the sulphuric acid, which is a strong oxidizer.
O stretching vibration of –SO3H groups,42 and this confirmed that –SO3H groups were successfully incorporated into the CMs during the sulfonation process.38,43,44 The band at 3431 cm−1 indicated the presence of –OH groups22,44 on the surfaces of both the CMs and CM-SO3H. Bands at 2928 and 2853 cm−1 were attributed to –CH2 and –CH stretching vibrations,19,27 and the intensities of these bands decreased dramatically after sulfonation of the CMs, indicating that these surface functional groups were removed. This could have been caused by the sulphuric acid, which is a strong oxidizer.
The elemental compositions of the CMs and CM-SO3H are shown in Table 1. These results are in good agreement with the FTIR spectra. Following sulfonation, the sulphur percentage greatly increased, from 0.20% in the CMs to 4.02% in the CM-SO3H, which also confirmed the existence of –SO3H groups in the CM-SO3H.45 In addition, the C and O contents of the CMs were decreased after sulfonation. The polarity index, [(O + N)/C], of the CM-SO3H increased, confirming an increase in the number of polar functional groups on the surface.27
Because the acid strength of a solid catalyst is a key parameter for the dehydration of carbohydrates,19,46 we characterized the acidities of the samples by titration.5 The acid density was also greatly increased, from 0.20 mmol g−1 for the CMs to 1.80 mmol g−1 for CM-SO3H (Table 1). Therefore, it is clear that the enhanced catalytic activity of the CM-SO3H may be attributed to its high acid density.
The results from our preliminary experiment inspired us to investigate the synthesis of 5-HMF from fructose and fructose-based carbohydrates (fructose, sucrose, and inulin) over the CM-SO3H catalyst in [BMIM][Cl].
Effect of the reaction temperature and time on 5-HMF yields
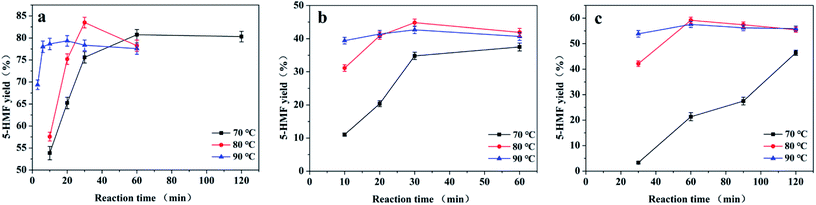
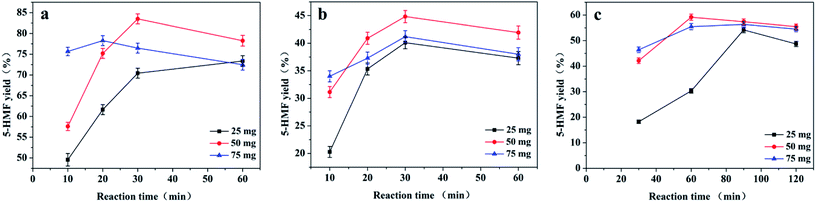
We explored the effects of the reaction temperature and time on the fructose, sucrose, and inulin dehydrations catalysed by CM-SO3H in [BMIM][Cl] (Fig. 4). All experiments were carried out with 50 mg of CM-SO3H and 2.50 g of [BMIM][Cl]. The masses of fructose, sucrose, and inulin were 0.50 g, 0.25 g and 0.25 g, respectively. The reaction temperature played an important role in determining the 5-HMF yield. When the conversion of fructose was carried out at 70 °C, the 5-HMF yield increased to a maximum of 80.7% at 60 min and then reached a plateau. When the reaction temperature was increased to 80 or 90 °C, the reaction rate increased in the initial stages of the reaction. The maximum 5-HMF yields were obtained at 30 min (83.5%) and 20 min (75.6%) with reaction temperatures of 80 °C and 90 °C, respectively. The 5-HMF yields then decreased slightly with further increases in the reaction time at 80 °C and 90 °C.The same tendency was observed for sucrose and inulin dehydration. At 70 °C, the 5-HMF yields were low and increased continuously with the reaction time. In contrast, at higher temperatures (80 and 90 °C), the 5-HMF yields initially increased with the reaction time and then slightly decreased with further increases in the reaction time. The maximum 5-HMF yields from sucrose and inulin were 44.8% (80 °C, 30 min) and 59.2% (80 °C, 60 min), respectively.
The decrease in the 5-HMF yield at higher temperatures suggests that the 5-HMF was consumed by humins.13,44 The formation of humins is inevitable in the acid-catalysed dehydration of carbohydrates because water is produced, and the generation of humins can be simply determined by the observation of a brown colour in the reaction mixture.22,38 To support this, we indirectly observed the colour of the reaction mixture by assessing the ethyl acetate extract (Fig. S2†). Direct observation of the reaction mixture was not possible because the catalyst was black and obscured the colour of the solution. The ethyl acetate extract of the reaction mixture for the reaction at 70 °C for 5 min was colourless. However, when the reaction temperature was increased to 80 or 90 °C, the solution turned very pale yellow or light yellow, respectively. As the reaction time increased, the extract became darker (Fig. S2b†).
These results show that higher reaction temperatures both improve the conversion of fructose to 5-HMF and accelerate the formation of humins and therefore the consumption of 5-HMF.22,44 Thus, an appropriate temperature (80 °C) and time (30 min for fructose and sucrose, and 60 min for inulin) are required to achieve the maximum 5-HMF yield.
Effect of catalyst and substrate loadings on the 5-HMF yield
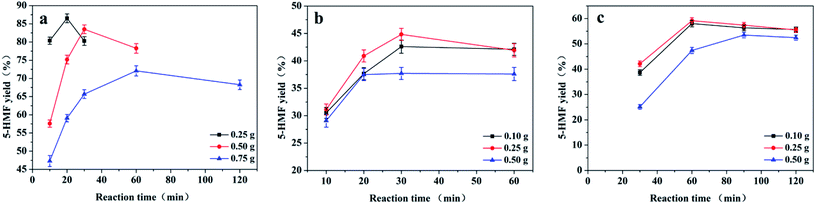
The effect of catalyst loading (25–75 mg) together with that of the reaction time was evaluated at 80 °C in 2.50 g of [BMIM][Cl]. The masses of fructose, sucrose, and inulin were 0.50 g, 0.25 g, and 0.25 g, respectively. A higher 5-HMF yield was obtained from each of the substrates in a shorter reaction time when the mass of catalyst was increased from 25 to 50 mg (Fig. 5). As an example, the yields of 5-HMF from fructose and sucrose steadily increased from 49.6% to 57.6% and from 20.2% to 31.1% after 10 min, respectively. In the case of inulin, the 5-HMF yield steadily increased from 18.2% to 42.2% after 30 min. The increase in the 5-HMF yield with increases in the catalyst loading may be attributed to an increase in the number of active sites and their availability.19,47 However, the maximum yields of 5-HMF from the three substrates were obtained with a catalyst loading of 50 mg, while the 5-HMF yield decreased when the catalyst loading was increased to 75 mg. These results suggest that degradation of the 5-HMF is also catalysed by active acid sites.19,48High substrate loadings are essential to improving the economy of 5-HMF production.17 We investigated the effect of the initial substrate concentration on the production of 5-HMF in reactions catalysed by CM-SO3H in [BMIM][Cl] (Fig. 6). The 5-HMF yield decreased gradually as the fructose mass increased in the initial stages of the reaction. The highest 5-HMF yields were 86.5%, 83.5%, and 72.1% with 0.25, 0.50, and 0.75 g of fructose, respectively. With sucrose and inulin, the 5-HMF yields increased slightly as the mass of substrate was increased from 0.10 to 0.25 g. However, a further increase in the substrate mass to 0.50 g led to a decreased 5-HMF yield. The highest 5-HMF yields were 42.6%, 44.8%, and 37.7% with 0.10, 0.25, and 0.50 g of sucrose, respectively. Using 0.10, 0.25, and 0.50 g of inulin, the highest 5-HMF yields were 58.0%, 59.2%, and 53.5%, respectively.
We compared the data for our catalyst with representative literature data regarding 5-HMF production from various heterogeneous catalysts in ILs (Table 2). CM-SO3H compares favourably with other solid catalysts in terms of the substrate concentration and reaction temperature when [BMIM][Cl] is used as the reaction solvent.
| Car. | Catalyst | Ionic liquid | Ccar. (wt%) | T (°C) | t (min) | Yield (%) | Con. (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Ccar. = concentration of carbohydrate, T = reaction temperature (°C), t = reaction time (min), n.m. = not mentioned, Car. = carbohydrate, Con. = conversion, Ref. = reference. | ||||||||
| Fructose | CM-SO3H | [BMIM][Cl] | 20 | 80 | 30 | 83.5 | 98.1 | — |
| Fructose | CSS | [BMIM][Cl] | 1 | 80 | 10 | 83 | n.m. | 44 |
| Fructose | SBA-15-SO3H | [BMIM][Cl] | 10 | 120 | 60 | ∼81 | ∼100 | 24 |
| Fructose | LCC | [BMIM][Cl]/DMSO | 10 | 110 | 10 | 84 | 98 | 38 |
| Fructose | HTC | [BMIM][Cl] | 1 | 100 | 90 | 88.1 | n.m. | 22 |
| Fructose | H3BO3–SiO2 | [BMIM][HSO4] | 10 | 120 | 90 | 88 | n.m. | 50 |
| Fructose | KL zeolite | [BMIM][Br] | 10 | 120 | 90 | 92.8 | >95.7 | 51 |
| Sucrose | CM-SO3H | [BMIM][Cl] | 10 | 80 | 30 | 44.8 | 99.9 | — |
| Sucrose | H3BO3–SiO2 | [BMIM][HSO4] | 10 | 120 | 180 | 80 | n.m. | 50 |
| Inulin | CM-SO3H | [BMIM][Cl] | 10 | 80 | 60 | 59.2 | 99.6 | — |
| Inulin | H3BO3–SiO2 | [BMIM][HSO4] | 10 | 120 | 300 | 88 | n.m. | 50 |
| Inulin | D265-SO3H | [AMIM][Cl] | 5 | 100 | 60 | 65 | n.m. | 52 |
| Inulin | Amberlyst-15 | [BMIM][HSO4]/[BMIM][Cl] | 2.5 | 80 | 65 | 82 | n.m. | 53 |
Catalyst recycling
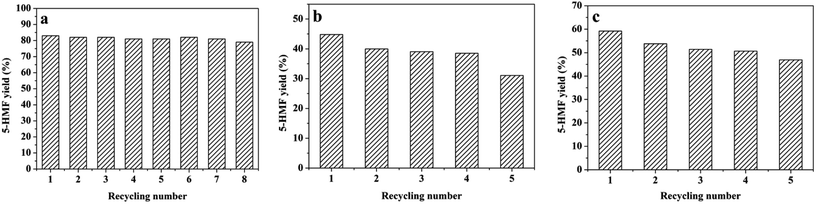
Stability is a key factor for practical applications of solid catalysts. To investigate the reusability of CM-SO3H in the carbohydrate–[BMIM][Cl] reaction system, we conducted recycling experiments at 80 °C according to an established method.44,49 After each reaction, the 5-HMF was recovered from the reaction mixture by extraction into ethyl acetate (4 × 25 mL). Subsequently, the [BMIM][Cl] that contained the CM-SO3H was dried in a vacuum oven at 80 °C for 12 h and reused directly in the next reaction under the same conditions.The yield of 5-HMF from fructose was almost unchanged after eight cycles of the reaction with [BMIM][Cl] and the catalyst (Fig. 7). The recycled catalyst and [BMIM][Cl] were evidently stable during the conversion of fructose to 5-HMF, indicating that the –SO3H groups were tightly bonded to the CM-SO3H. The yields of 5-HMF from sucrose and inulin decreased slightly, from 44.8% to 31.0% and from 59.2% to 46.9%, respectively, after five successive cycles of catalyst reuse. This slight decrease in yield is possibly attributable to the generation of glucose during the hydration of sucrose and inulin (based on HPLC results that are not included herein). This glucose was not removed from the reaction system and thus could have combined with the 5-HMF to promote polymerization to form humins which, in turn, would lower the HMF yield. Moreover, some portion of the humins likely deposited on the catalyst and reduced the catalytic activity of the CM-SO3H.13,38 As shown in Fig. S3,† after five cycles of the sucrose dehydration reaction, humins or other organic residues were deposited on the surface of the CM-SO3H, even after washing alternately with hot water and ethanol three times.
Conclusions
We prepared SO3H-functionalized CMs (CM-SO3H) by the hydrothermal carbonization of yeast cells, followed by sulfonation at room temperature. We applied the CM-SO3H to the synthesis of 5-HMF from fructose-based carbohydrates in [BMIM][Cl]. The CM-SO3H exhibited excellent catalytic activity during the conversions of fructose, sucrose, and inulin to 5-HMF at low reaction temperatures and high substrate concentrations. When 0.50 g of fructose was used, a high 5-HMF yield of 83.5% was obtained in 2.50 g of [BMIM][Cl] after reaction at 80 °C for 30 min. With 0.25 g of sucrose (30 min at 80 °C) or 0.25 g of inulin (60 min at 80 °C), the 5-HMF yields were 44.8% and 59.2%, respectively. More importantly, the CM-SO3H and [BMIM][Cl] system showed high stability and could be reused between five and eight times with minimal loss of catalytic activity.CM-SO3H is a promising catalyst for the acid-catalysed conversion of biomass into value-added chemicals. In particular, in combination with [BMIM][Cl], CM-SO3H transforms fructose-based carbohydrates into 5-HMF.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for financial support provided by the National Key Research and Development Program (Grant No. 2017YFD0801500).Notes and references
- L. Filiciotto, A. M. Balu, J. C. V. d. Waal and R. Luque, Catal. Today, 2018, 302, 2–15 CrossRef CAS.
- L. Cao, I. K. M. Yu, S. S. Chen, D. C. W. Tsang, L. Wang, X. Xiong, S. Zhang, Y. S. Ok, E. E. Kwon, H. Song and C. S. Poon, Bioresour. Technol., 2018, 252, 76–82 CrossRef CAS PubMed.
- M. N. Catrinck, E. S. Ribeiro, R. S. Monteiro, R. M. Ribas, M. H. P. Barbosa and R. F. Teófilo, Fuel, 2017, 210, 67–74 CrossRef CAS.
- X. Xiong, I. K. M. Yu, S. S. Chen, D. C. W. Tsang, L. Cao, H. Song, E. E. Kwon, Y. S. Ok, S. Zhang and C. S. Poon, Catal. Today, 2018, 314, 52–61 CrossRef CAS.
- Y. Dou, S. Zhou, C. Oldani, W. Fang and Q. Cao, Fuel, 2018, 214, 45–54 CrossRef CAS.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS PubMed.
- D. Steinbach, A. Kruse, J. Sauer and P. Vetter, Energies, 2018, 11, 645–660 CrossRef.
- Y. Zhang, M. Liu, J. Zhao, K. Wang, M. Meng and Y. Yan, ChemistrySelect, 2018, 3, 5950–5959 CrossRef CAS.
- J. Wang, Z. Zhang, S. Jin and X. Shen, Fuel, 2017, 192, 102–107 CrossRef CAS.
- F. Parveen and S. Upadhyayula, Fuel Process. Technol., 2017, 162, 30–36 CrossRef CAS.
- Y. Román-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1937 CrossRef PubMed.
- D. Garcés, E. Díaz and S. Ordóñez, Ind. Eng. Chem. Res., 2017, 56, 5221–5230 CrossRef.
- A. Deng, Q. Lin, Y. Yan, H. Li, J. Ren, C. Liu and R. Sun, Bioresour. Technol., 2016, 216, 754–760 CrossRef CAS PubMed.
- M. Ventura, A. Dibenedetto and M. Aresta, Inorg. Chim. Acta, 2018, 470, 11–21 CrossRef CAS.
- B. Agarwal, K. Kailasam, R. S. Sangwan and S. Elumalai, Renewable Sustainable Energy Rev., 2018, 82, 2408–2425 CrossRef CAS.
- H. Mo, X. Chen, X. Liao and T. Zhou, J. Cent. South Univ., 2017, 24, 1745–1753 CrossRef CAS.
- C. Antonetti, A. M. R. Galletti, S. Fulignati and D. Licursi, Catal. Commun., 2017, 97, 146–150 CrossRef CAS.
- W. Li, T. Zhang, H. Xin, M. Su, L. Ma, H. Jameel, H. Chang and G. Pei, RSC Adv., 2017, 7, 27682–27688 RSC.
- R. Liu, J. Chen, X. Huang, L. Chen, L. Ma and X. Li, Green Chem., 2013, 15, 2895–2903 RSC.
- R. Zhong and B. F. Sels, Appl. Catal., B, 2018, 236, 518–545 CrossRef CAS.
- R. Zhong, F. Yu, W. Schutyser, Y. Liao, F. d. Clippel, L. Peng and B. F. Sels, Appl. Catal., B, 2017, 206, 74–88 CrossRef CAS.
- X. Qi, N. Liu and Y. Lian, RSC Adv., 2015, 5, 17526–17531 RSC.
- F. Huang, W. Li, Q. Liu, T. Zhang, S. An, D. Li and X. Zhu, Fuel Process. Technol., 2018, 181, 294–303 CrossRef CAS.
- X. Guo, Q. Cao, Y. Jiang, J. Guan, X. Wang and X. Mu, Carbohydr. Res., 2012, 351, 35–41 CrossRef CAS PubMed.
- X. Du, J. Zhang, Y. Wang and Y. Qu, Catalysts, 2017, 7, 245–256 CrossRef.
- K. Nakajima and M. Hara, ACS Catal., 2012, 2, 1296–1304 CrossRef CAS.
- D. Ni, L. Wang, Y. Sun, Z. Guan, S. Yang and K. Zhou, Angew. Chem., Int. Ed. Engl., 2010, 49, 4223–4227 CrossRef CAS PubMed.
- Z. Guan, L. Liu, L. He and S. Yang, J. Hazard. Mater., 2011, 196, 270–277 CrossRef CAS PubMed.
- Z. Wang, D. Cheng, C. Chen and K. Zhou, Carbon, 2018, 136, 54–62 CrossRef CAS.
- G. Chen, G. Song, W. Zhao, D. Gao, Y. Wei and C. Li, Chem. Eng. J., 2018, 352, 64–70 CrossRef CAS.
- F. Huang, Y. Su, Y. Tao, W. Sun and W. Wang, Fuel, 2018, 226, 417–422 CrossRef CAS.
- A. M. d. C. Lopes and R. Bogel-Łukasik, ChemSusChem, 2015, 8, 947–965 CrossRef PubMed.
- A. M. d. C. Lopes, K. G. João, D. F. Rubik, E. Bogel-Łukasik, L. C. Duarte, J. Andreaus and R. Bogel-Łukasik, Bioresour. Technol., 2013, 142, 198–208 CrossRef PubMed.
- Q. Cao, X. Guo, S. Yao, J. Guan, X. Wang, X. Mu and D. Zhang, Carbohydr. Res., 2011, 346, 956–959 CrossRef CAS PubMed.
- H. Liu, Y. Wang, W. Ma, H. Wang, D. Wang, W. Jiang, M. Zhang, C. Zhou and H. Li, Chem. Pap., 2017, 71, 1541–1549 CrossRef CAS.
- V. Kempter and B. Kirchner, J. Mol. Struct., 2010, 972, 22–34 CrossRef CAS.
- C. Moreau, A. Finiels and L. Vanoye, J. Mol. Catal. A: Chem., 2006, 253, 165–169 CrossRef CAS.
- F. Guo, Z. Fang and T. J. Zhou, Bioresour. Technol., 2012, 112, 313–318 CrossRef CAS PubMed.
- A. H. Jadhav, H. Kim and I. T. Hwang, Catal. Commun., 2012, 21, 96–103 CrossRef CAS.
- J. N. Chheda, Y. Román-Leshkov and J. A. Dumesic, Green Chem., 2007, 9, 342–350 RSC.
- H. Xin, T. Zhang, W. Li, M. Su, S. Li, Q. Shao and L. Ma, RSC Adv., 2017, 7, 41546–41551 RSC.
- P. A. Russo, M. M. Antunes, P. Neves, P. V. Wiper, E. Fazio, F. Neri, F. Barreca, L. Mafra, M. Pillinger, N. Pinna and A. A. Valente, Green Chem., 2014, 16, 4292–4305 RSC.
- Y. Zhang, Y. Shen, Y. Chen, Y. Yan, J. Pan, Q. Xiong, W. Shi and L. Yu, Chem. Eng. J., 2016, 294, 222–235 CrossRef CAS.
- X. Qi, H. Guo, L. Li and J. R. L. Smith, ChemSusChem, 2012, 5, 2215–2220 CrossRef CAS PubMed.
- D. Yamaguchi, K. Watanabe and S. Fukumi, Sci. Rep., 2016, 6, 20327–20334 CrossRef PubMed.
- M. Watanabe, Y. Aizawa, T. Iida, R. Nishimura and H. Inomata, Appl. Catal., A, 2005, 295, 150–156 CrossRef CAS.
- Z. Wang, H. Li, C. Fang, W. Zhao, T. Yang and S. Yang, Energy Technol., 2017, 5, 2046–2054 CrossRef CAS.
- S. K. R. Patil and C. R. F. Lund, Energy Fuels, 2011, 25, 4745–4755 CrossRef CAS.
- X. Qi, M. Watanabe, T. M. Aida and J. R. L. Smith, Green Chem., 2009, 11, 1327–1331 RSC.
- M. Walia, U. Sharma, V. K. Agnihotri and B. Singh, RSC Adv., 2014, 4, 14414–14418 RSC.
- Z. Ma, H. Hu, Z. Sun, W. Fang, J. Zhang, L. Yang, Y. Zhang and L. Wang, ChemSusChem, 2017, 10, 1669–1674 CrossRef CAS PubMed.
- S. Kang, J. Ye, Y. Zhang and J. Chang, RSC Adv., 2013, 3, 7360–7366 RSC.
- X. Qi, M. Watanabe, T. M. Aida and J. R. L. Smith, Green Chem., 2010, 12, 1855–1860 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra10465d |
| This journal is © The Royal Society of Chemistry 2019 |