Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
K3PO4-promoted domino reactions: diastereoselective synthesis of trans-2,3-dihydrobenzofurans from salicyl N-tert-butanesulfinyl imines and sulfur ylides†‡
Minxuan Zhanga,
Tianyu Lua,
Yun Zhaoa,
Guixian Xie*c and
Zhiwei Miao *ab
*ab
aState Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Weijin Road 94, Tianjin 300071, People's Republic of China. E-mail: miaozhiwei@nankai.edu.cn
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300071, People's Republic of China
cCollege of Resources and Environment, Hunan Agricultural University, Changsha 410128, People's Republic of China
First published on 16th April 2019
Abstract
An efficient domino annulation between sulfur ylides and salicyl N-tert-butylsulfinyl imines was developed. The reaction proceeds with a diastereodivergent process, the configuration of the sulfinyl group determining the stereochemical course of the reaction. The method allows the synthesis of a highly substituted trans-2,3-dihydrobenzofuran skeleton with high yield and good chemo- and diastereoselectivity.
Introduction
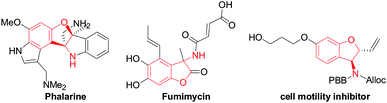
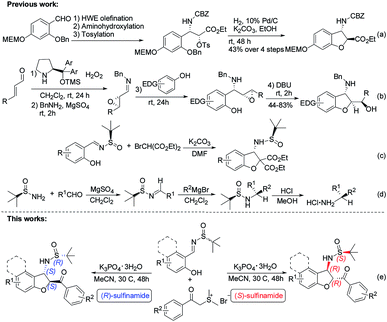
Functionalized chiral benzofuran bearing three carbon stereocenters are the core of many bioactive natural products and pharmaceuticals.1 Such cores also offer valuable chiral building blocks for the enantioselective synthesis of biologically active compounds.2 Especially, the benzofurans with the amino group at the 3-position are vital in drug discovery and considered important for the bioactivity of these molecules.3 For example, phalarine, isolated from Phalaris coerulescens, is a type of important natural product, and has exhibited high structural diversity and a broad bioactivity profile.4 Fumimycin has demonstrated anti-bacterial properties while the synthetic cell motility inhibitor shows good biological effects in cell-based assays (Fig. 1).5Diastereoselective synthesis of such significant chiral benzofurans remains a considerable challenge. 3-Substituted 2,3-dihydrobenzofurans have been synthesized by a variety of methods such as radical cyclizations,6 electrocyclizations,7 anionic cyclizations,8 biomimetic couplings and cycloaddition,9 Lewis acid promoted reactions,10 transition-metal-catalyzed processes,11 and so on.12 In 2008, Sabourin, Arya and co-workers have developed a practical, enantioselective route to access 3-amino-2,3-dihydrobenzofurans (Scheme 1a).13 In 2011, Jørgenson and co-workers developed a highly efficient asymmetric organocatalytic cascade reaction to access 3-amino-2,3-dihydrobenzofurans through four sequential synthetic steps in one pot (Scheme 1b).14 In 2015, Zhao and co-workers reported an efficient domino reaction to chiral synthesize different substituted 2,3-dihydrobenzofuran-derived β-amino esters from ortho-hydroxyl aromatic N-tert-butylsulfinyl imines and diethyl bromomalonate promoted by K2CO3 (Scheme 1c).15 Despite many advances, the development of novel asymmetric catalytic methods to access this class of useful molecules to be highly appealing in modern asymmetric synthesis.
Chiral N-tert-butanesulfinyl imines, one class of the most efficient auxiliaries pioneered by Ellman, have been extensively used for the preparation of various chiral amines including variously substituted benzofuran-derived β-amino esters.16 Enantiomerically pure aldimines and ketoimines generated from an aldehyde or ketone with an alkyl or aryl sulfinamide are versatile building blocks in the construction of chiral amines, and their application has attracted a large amount of interest.17 In 1997, Ellman reported the preparation of tert-butanesulfinimines by direct condensation of a sulfinamide with aldehydes in the presence of MgSO4. Furthermore, tert-butanesulfinimines reacted with Grignard reagents to provide tert-butane sulfinamides in high yields with high diastereoselectivity. Removal of the sulfinyl group is achieved with stoichiometric HCl in methanol to afford the desired α-branched amines (Scheme 1d).18
In spite of the obvious interesting properties of Ellman's chiral auxiliary such as the availability of both enantiomers and the mild conditions required for its cleavage, we speculated that the chirality of the auxiliary of the imine play an important role in the reaction stereoselectivity. Based on our previous study on the chemistry of chiral auxiliary,19 we report the domino annulation of salicyl N-tert-butylsulfinyl imines with sulfur ylides, which allows efficient asymmetric synthesis of trans-2,3-disubstituted 2,3-dihydrobenzofurans with high chemo- and stereoselectivity (Scheme 1e). The anticipated diastereodivergent process was investigated by combining both enantiomeric sulfinylimine auxiliaries employed.
Results and discussion
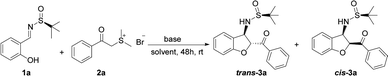
We initiated our investigation by subjecting sulfur ylide precursor 2a (0.50 mmol, 2.5 equiv.)20 to salicyl N-tert-butylsulfinyl imine 1a (0.20 mmol)21 in the presence of K2CO3 (0.50 mmol, 2.5 equiv.) in CH3CN at room temperature. To our delight, the domino reaction proceeded smoothly to provide trans-3-(2-methylpropane-2-sulfinamide)-2,3-dihydrobenzofuran 3a in 78% yield and 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 diastereoselectivity with trans-3a being the major diastereomer (Table 1, entry 1). In accordance with the previous reports on base promoted domino reactions, it was found that bases strongly influenced the yield.22 When KOtBu was used, the reaction became complicated and the product 3a was isolated in low yield (Table 1, entry 2). Similarly, when the reaction was promoted with the weak base KOAc, the reaction rate was very slow and a low yield was obtained after 48 hours (Table 1, entry 3). To our great delight, in the presence of 0.50 mmol K3PO4·3H2O in CH3CN at room temperature, after 48 h, the desired product 3a was isolated in 87% yield (Table 1, entry 4). However, when the organic base DABCO (1,4-diazabicyclo [2.2.2] octane) was employed, and the yield of the desired product was higher than those using K2CO3 and KOAc but still lower than that K3PO4·3H2O (Table 1, entries 5). The base amount was also examined as shown in Table 1. Increasing or decreasing the amount of base resulted in decrease of the efficiency of this reaction (Table 1, entries 6–7).
28 diastereoselectivity with trans-3a being the major diastereomer (Table 1, entry 1). In accordance with the previous reports on base promoted domino reactions, it was found that bases strongly influenced the yield.22 When KOtBu was used, the reaction became complicated and the product 3a was isolated in low yield (Table 1, entry 2). Similarly, when the reaction was promoted with the weak base KOAc, the reaction rate was very slow and a low yield was obtained after 48 hours (Table 1, entry 3). To our great delight, in the presence of 0.50 mmol K3PO4·3H2O in CH3CN at room temperature, after 48 h, the desired product 3a was isolated in 87% yield (Table 1, entry 4). However, when the organic base DABCO (1,4-diazabicyclo [2.2.2] octane) was employed, and the yield of the desired product was higher than those using K2CO3 and KOAc but still lower than that K3PO4·3H2O (Table 1, entries 5). The base amount was also examined as shown in Table 1. Increasing or decreasing the amount of base resulted in decrease of the efficiency of this reaction (Table 1, entries 6–7).
| Entry | Base | Solvent | trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cisb cisb |
Combined yield (%)c |
|---|---|---|---|---|
| a Unless otherwise specified, all reactions were carried out using N-tert-butylsulfinyl imine 1a (0.20 mmol) and sulfur ylide precursor 2a (0.50 mmol, 2.5 equiv.) in 2 mL solvent with 0.50 mmol of base at room temperature.b Determined by 1H NMR (crude reaction mixture).c Combined yield of isolated products of trans-3a and cis-3a after column chromatography.d K3PO4·3H2O loading is 0.10 mmol.e K3PO4·3H2O loading is 0.40 mmol.f The reaction temperature is 30 °C. | ||||
| 1 | K2CO3 | CH3CN | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
78 |
| 2 | KOtBu | CH3CN | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
16 |
| 3 | KOAc | CH3CN | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
34 |
| 4 | K3PO4·3H2O | CH3CN | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
87 |
| 5 | DABCO | CH3CN | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
44 |
| 6d | K3PO4·3H2O | CH3CN | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
46 |
| 7e | K3PO4·3H2O | CH3CN | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
57 |
| 8 | K3PO4·3H2O | Toluene | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
6 |
| 9 | K3PO4·3H2O | CH2Cl2 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
33 |
| 10 | K3PO4·3H2O | ClCH2CH2Cl | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41 |
40 |
| 11 | K3PO4·3H2O | THF | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
54 |
| 12 | K3PO4·3H2O | Acetone | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 39 |
11 |
| 13 | K3PO4·3H2O | AcOEt | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
44 |
| 14 | K3PO4·3H2O | CHCl3 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
4 |
| 15f | K3PO4·3H2O | CH3CN | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
93 |
Subsequently, we investigated the effects of solvent on the reactivity, when K3PO4·3H2O was used as the base. A rather low yield and selectivity was obtained when toluene was used as the solvent (Table 1, entry 8). Among the solvents examined, the use of CH3CN gave the best result (Table 1, entries 9–14). Higher reactivity was observed at elevated a temperature of 30 °C, and the product was obtained in 93% yield and 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 diastereoselectivity (Table 1, entry 15). On the basis of the above experimental results, the optimal reaction conditions for this transformation were determined to be N-tert-butylsulfinyl imine 1a (0.20 mmol), sulfur ylide precursor 2a (0.50 mmol), and K3PO4·3H2O (2.5 equiv.) in CH3CN as solvent at 30 °C.
28 diastereoselectivity (Table 1, entry 15). On the basis of the above experimental results, the optimal reaction conditions for this transformation were determined to be N-tert-butylsulfinyl imine 1a (0.20 mmol), sulfur ylide precursor 2a (0.50 mmol), and K3PO4·3H2O (2.5 equiv.) in CH3CN as solvent at 30 °C.
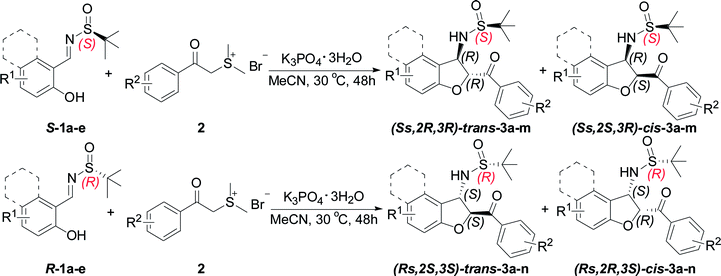
Having established the optimal reaction conditions for the synthesis of chiral sulfonamide 2,3-dihydrobenzofuran-2-yl-phenyl methanone, we next focused on the substrate scope of this transformation and the results are shown in Table 2. Representative N-tert-butylsulfinyl imines 1 with various substitutions were easily prepared by the condensation of either enantiomer of tert-butanesulfinamide with the corresponding aldehydes according to the reported procedure.21 In all instances, imines were prepared in high yield and as single enantiomers. Generally, the domino reactions between a range of readily available ortho-hydroxyl aromatic N-tert-butylsulfinyl imines S-1 and sulfur ylide precursor 2a provided trans-3-(2-methylpropane-2-sulfinamide)-2,3-dihydrobenzofuran 3 in moderate yields with moderate diastereoselectivities (Table 2, entries 1–5). The electronic property of the aromatic substituent has little effect on the yield and stereoselectivity. Even with a sterically hindered substrate, the reaction proceeded smoothly to give the desired product in moderate yield. We were delighted to find that the N-tert-butyl sulfinyl imine of β-naphthylaldehyde S-1e, underwent smooth sequential annulation with 2a, to give the corresponding product trans-(Ss,2R,3R)-3e in moderate yield with moderate diastereoselective (Table 2, entry 5).
| Entry | Major product | R1 | R2 | Yield (%)c | trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cisd cisd |
De (trans) (%)e |
|---|---|---|---|---|---|---|
| a Reaction conditions: N-tert-butylsulfinyl imines 1 (0.20 mmol), sulfur ylide 2 (0.50 mmol), in 2 mL of MeCN at 30 °C in the presence of 250 mol % of K3PO4·3H2O.b The suffix R or S in the numbering refers to the absolute configuration of the sulfinylimine auxiliary.c Isolated yield after silica gel chromatography of 3. trans and cis adducts have been separated by column chromatography and that only the major trans-adducts are described.d Cis/trans ratio determined from the 1H NMR of the crude reaction mixture.e De determined from 1H NMR of the crude reaction mixture.f N-tert-Butyl sulfinyl imine of β-naphthylaldehyde was used. | ||||||
| 1 | trans-(Ss,2R,3R)-3a | H (S-1a) | H (2a) | 67 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
>99 |
| 2 | trans-(Ss,2R,3R)-3b | 5-Cl (S-1b) | H (2a) | 40 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36 |
98 |
| 3 | trans-(Ss,2R,3R)-3c | 5-Br (S-1c) | H (2a) | 65 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
90 |
| 4 | trans-(Ss,2R,3R)-3d | 3,5-Cl (S-1d) | H (2a) | 47 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
98 |
| 5f | trans-(Ss,2R,3R)-3e | H (S-1e) | H (2a) | 41 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
98 |
| 6 | trans-(Ss,2R,3R)-3f | 5-Cl (S-1b) | 4-Cl (2b) | 54 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
98 |
| 7 | trans-(Ss,2R,3R)-3g | 5-Br (S-1c) | 4-Cl (2b) | 41 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
98 |
| 8 | trans-(Ss,2R,3R)-3h | 3,5-Cl (S-1d) | 4-Cl (2b) | 28 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
90 |
| 9f | trans-(Ss,2R,3R)-3i | H (S-1e) | 4-Cl (2b) | 57 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
98 |
| 10f | trans-(Ss,2R,3R)-3j | H (S-1e) | 4-Me (2c) | 53 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
98 |
| 11 | trans-(Ss,2R,3R)-3k | 5-Br (S-1c) | 4-Me (2c) | 53 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
98 |
| 12 | trans-(Ss,2R,3R)-3l | 5-Cl (S-1b) | 4-Me (2c) | 69 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
98 |
| 13 | trans-(Ss,2R,3R)-3m | 5-Br (S-1c) | 4-NO2 (2d) | — | — | — |
| 14 | trans-(Rs,2S,3S)-3a | H (R-1a) | H (2a) | 65 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
90 |
| 15 | trans-(Rs,2S,3S)-3b | 5-Cl (R-1b) | H (2a) | 69 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
98 |
| 16 | trans-(Rs,2S,3S)-3c | 5-Br (R-1c) | H (2a) | 60 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
98 |
| 17f | trans-(Rs,2S,3S)-3d | 3,5-Cl (R-1d) | H (2a) | 55 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32 |
98 |
| 18 | trans-(Rs,2S,3S)-3e | H (R-1e) | H (2a) | 55 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
98 |
| 19 | trans-(Rs,2S,3S)-3f | 5-Cl (R-1b) | 4-Cl (2b) | 69 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
98 |
| 20 | trans-(Rs,2S,3S)-3g | 5-Br (R-1c) | 4-Cl (2b) | 55 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
98 |
| 21 | trans-(Rs,2S,3S)-3h | 3,5-Cl (R-1d) | 4-Cl (2b) | 51 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 39 |
92 |
| 22f | trans-(Rs,2S,3S)-3i | H (R-1e) | 4-Cl (2b) | 49 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
90 |
| 23f | trans-(Rs,2S,3S)-3j | H (R-1e) | 4-Me (2c) | 72 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
98 |
| 24 | trans-(Rs,2S,3S)-3l | 5-Cl (R-1b) | 4-Me (2c) | 62 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
98 |
| 25 | trans-(Rs,2S,3S)-3n | 5-Br (R-1c) | 4-Me (2c) | 63 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36 |
98 |
Further investigations using other sulfur ylide precursors and salicyl N-tert-butyl sulfinyl imines were performed under the optimized conditions. A series of sulfur ylide precursor proved to be suitable for this reaction. Aryl units containing electron-donating or electron-withdrawing substituents in sulfur ylides were readily tolerated, thus giving preferentially the corresponding trans-3-(2-methylpropane-2-sulfinamide)-2,3-dihydrobenzofuran 3 in moderate yields with moderate diastereoselectivities (Table 2, entries 6–12). Notably, when the substituent was at the 4-position with an electron-withdrawing group of the benzene ring of sulfur ylide precursor 2d, the reaction cannot work and no desired product 3m observed (Table 2, entry 13). In contrast, when the enantiomeric (R)-chiral auxiliary (1a–e) was used, the diastereoselectivity was the same as the (S)-configured chiral auxiliary (Table 2, entries 14–25).
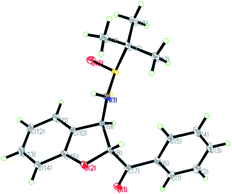
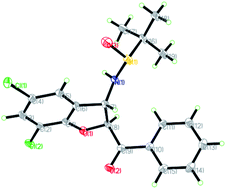
The relative configuration of the major diastereomers obtained from the reaction of sulfur ylide precursor 2a and salicyl N-tert-butylsulfinyl imines S-1a and R-1d could be determined by X-ray crystallographic analysis (Fig. 2 and 3).23 Both products trans-(Ss,2R,3R)-3a and trans-(Rs,2S,3S)-3d are derived from the same N-tert-butyl sulfinylimine enantiomer but with differently configured chiral auxiliaries, and the different configuration of the formed amine stereocenter clearly proved that the diastereoselection was determined by the configuration of the auxiliary. On that basis, the stereochemistry of the other major and minor isomers was assigned.
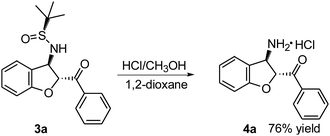
To demonstrate further the synthetic utility of these findings, (2R,3R)-(3-amino-2,3-dihydro-benzofuran-2-yl)-phenyl-methanone 4a was readily synthesized from trans-(Ss,2R,3R)-3a (Scheme 2). The deprotection and hydrolysis of 3a in HCl (12 M) at room temperature gave the desired optically active 4a with anti-relative configuration in 76% yield as the hydrochloride salt.
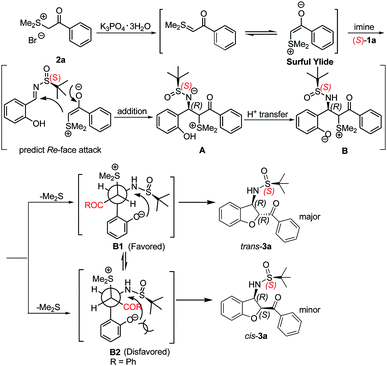
A mechanism for this domino reaction is proposed on the basis of previous literature reports and is shown in Scheme 3.24 The reaction might be initiated by the formation of the surfur ylide via the deprotonation of 2a. Subsequent Re-face attack (for an (S)-configured sulfinylimine) nucleophilic addition of the surfur ylide to the electron-deficient imine 1a yielded the intermediate A. Intermediate A will transform into intermediate B under proton transfer. As shown in Newman projections B1 and B2, intermediate B1 is the favoured one, followed by SN2 substitution to give the trans-configured 2,3-disubstituted dihydrobenzofuran 3a.
Conclusions
In conclusion, we have developed a simple, convenient, and stereoselective domino reaction between salicyl N-tert-butylsulfinyl imines and sulfonium salts that provides a new method for the construction of trans-2,3-disubstituted 2,3-dihydrobenzofurans in good yields and good chemo- and diastereoselectivities. The domino reaction of the former proceeds with diastereodivergent process, with the configuration of the chiral auxiliary determining the stereo induction. Exploration of the scope and limitations of this reaction and the use of such dihydrobenzofurans to provide concise routes to more complex structures are ongoing and will be reported in due course.Experimental
General methods
All reactions were performed under N2 atmosphere in oven-dried glassware with magnetic stirring. Solvents were dried and distilled prior to use according to the standard methods. Unless otherwise indicated, all materials were obtained from commercial sources, and used as purchased without dehydration. Column chromatography was performed on silica gel 200–300 mesh. Nitrogen gas (99.999%) was purchased from Boc Gas Inc. 1H and 13C NMR spectra were measured at 400 and 101 MHz, respectively. The solvents used for NMR spectroscopy were CDCl3 and CD3OD, using tetramethylsilane as the internal reference. Chemical shifts were recorded in parts per million (ppm). Coupling constants were given in Hz. The crystal structure was determined on a Bruker SMART 1000 CCD diffractometer. Mass spectra were obtained using an electrospray ionization (ESI-TOF) mass spectrometer. Melting points were determined on a T-4 melting point apparatus (uncorrected).Preparation of salicyl N-tert-butylsulfinyl imines 115
Under a nitrogen atmosphere, to a mixture of salicylaldehyde (7.2 mmol, 1.2 equiv.) and sulfinamide (6 mmol, 1.0 equiv.) in CH2Cl2 was added Ti(Oi-Pr)4 (3–5 equiv.). After the mixture was stirred at room temperature for 48 h, 15 mL saturated solution of sodium bicarbonate was added. Stirring for a further 15 min was followed by filtration over a pad of MgSO4 and Celite. The filter cake was washed with EtOAc and the filtrate concentrated under reduced pressure. The residue was purified by flash column chromatograph (eluted with 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether/EtOAc) to afford pure salicyl N-tert-butylsulfinyl imine.
1 petroleum ether/EtOAc) to afford pure salicyl N-tert-butylsulfinyl imine.
General procedure for synthesis of trans-2,3-disubstituted 2,3-dihydrobenzofurans 3
Under a nitrogen atmosphere, to a mixture of sulfur ylide precursor 2 (0.5 mmol, 2.5 equiv.), and K3PO4·3H2O (133 mg, 0.5 mmol, 100 mmol %) was added CH3CN (1 mL) via a syringe and allowed to stir for 5 min at room temperature. Salicyl N-tert-butylsulfinyl imine 1 (0.2 mmol, 1.0 equiv.) in CH3CN (1 mL) was added and the reaction was allowed to stir for 48 h at 30 °C. The reaction was monitored by TLC spectroscopy. After the reaction was completed, the reaction mixture was directly purified by flash column chromatograph (eluted with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-heptane/EtOAc) to afford the product 3.
1 n-heptane/EtOAc) to afford the product 3.
General procedure for synthesis of 3-amino-2,3-dihydrobenzofuran-2-yl(aryl)methanone hydrochlorides 5
2The synthesis of 3-amino-2,3-dihydrobenzofuran-2-yl(phenyl)methanone hydrochloride 5a is representative. To a solution of trans-3-(2-methylpropane-2-sulfinamide)-2,3-dihydrobenzofuran 3a (68.7 mg, 0.20 mmol) in dioxane (10 mL) was added dropwise freshly prepared saturated dioxane/HCl (15 mL, ∼20 equiv. HCl). The mixture was allowed to stir for 1 h. Then the reaction mixture was concentrated in vacuo. Precipitation in diethyl ether afforded 42.1 mg (0.15 mmol) of pure 3-amino-2,3-dihydrobenzofuran-2-yl-(phenyl)methanone hydrochloride 5a.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Key Research and Development Program of China (2016YFD0201200), the Rural Affairs Commission of the CPC Tianjin Municipal Committee (2016D01A016) and the Key Technologies R & D Program of Tianjin (17YFZCGX00470) for financial support.Notes and references
- (a) M. Iwahashi, A. Shimabukuro, T. Onoda, Y. Matsunaga, Y. Okada, R. Matsumoto, F. Nambu, H. Nakai and M. Toda, Bioorg. Med. Chem., 2011, 19, 4574 CrossRef CAS PubMed; (b) L. Zhou, Y. F. Xi, W. Wang, B. Lin, X. B. Wang, X. X. Huang and S. J. Song, Fitoterapia, 2018, 127, 56 CrossRef CAS PubMed; (c) H. Sunden, A. Schafer, M. Scheepstra, S. Leysen, M. Malo, J. N. Ma, E. S. Burstein, C. Ottmann, L. Brunsveld and R. Olsson, J. Med. Chem., 2016, 59, 1232 CrossRef CAS PubMed.
- (a) B. Shota, A. Sachie, F. Masaki, A. Kosuke, F. Hitoshi, S. Michinori, I. Tatsushi, I. Kazunobu, T. Katsuhiko and I. Ryo, Eur. J. Org. Chem., 2017, 6914 Search PubMed; (b) M. Wang, X. Zhang, Z. Ling, Z. F. Zhang and W. B. Zhang, Chem. Commun., 2017, 53, 1381 RSC; (c) X. Y. Liu, C. S. Tian, X. Z. Jiao, X. Y. Li, H. G. Yang, Y. Y. Yao and P. Xie, Org. Biomol. Chem., 2016, 14, 7715 RSC.
- (a) J. P. Nandy, B. Rakic, B. V. N. B. Sarma, N. Babu, M. Lefrance, G. D. Enright, D. M. Leek, K. Daniel, L. A. Sabourin and P. Arya, Org. Lett., 2008, 10, 1143 CrossRef CAS PubMed; (b) T. R. Helgren, L. Y. L. Xu, D. Sotelo, Y. R. Mehta, M. A. Korkmaz, I. Pavlinov and L. N. Aldrich, Chem.–Eur. J., 2018, 24, 4509 CrossRef CAS PubMed.
- N. Anderton, P. A. Cockrum, S. M. Colegate, J. A. Edgar, K. Flower, D. Gardner and R. I. Willing, Phytochemistry, 1999, 51, 153 CrossRef CAS.
- (a) A. K. Shaikh and G. Varvounis, RSC Adv., 2015, 5, 14892 RSC; (b) Y. Cheng, X. Q. Hu, S. Gao, L. Q. Lu, J. R. Chen and W. J. Xiao, Tetrahedron, 2013, 69, 3810 CrossRef CAS.
- (a) J. T. Kuethe, A. Wong, M. Journet and I. W. Davies, J. Org. Chem., 2005, 70, 3727 CrossRef CAS PubMed; (b) M. C. Jiménez, M. A. Miranda and R. Tormas, J. Org. Chem., 1998, 63, 1323 CrossRef; (c) G. F. Meijs and A. L. J. Beckwith, J. Am. Chem. Soc., 1986, 108, 5890 CrossRef CAS PubMed; (d) E. M. Rochette, W. Lewis, A. G. Dossetter and R. A. Stockman, Chem. Commun., 2013, 49, 9395 RSC.
- G. A. Salman, A. Ali, M. Hussain, R. A. Khera and P. Langer, Synthesis, 2011, 2208 CAS.
- G. Solladié, D. Boeffel and J. Maignan, Tetrahedron, 1995, 51, 9559 CrossRef.
- (a) J. Mangas-Sánchez, E. Busto, V. Gotor-Fernández and V. Gotor, Org. Lett., 2010, 12, 3498 CrossRef PubMed; (b) J. W. Benbow and R. Katoch-Rouse, J. Org. Chem., 2001, 66, 49652 CrossRef; (c) T. A. Engler, K. D. Combrink and J. E. Ray, J. Am. Chem. Soc., 1988, 110, 7931 CrossRef CAS.
- (a) T. A. Engler, D. Wei, M. A. Letavic, K. D. Combrink and J. P. Reddy, J. Org. Chem., 1994, 59, 6588 CrossRef CAS; (b) B. D. Gates, P. Dalidowicz, A. Tebben, S. Wang and J. S. Swenton, J. Org. Chem., 1992, 57, 2135 CrossRef CAS; (c) S. Wang, B. D. Gates and J. S. Swenton, J. Org. Chem., 1991, 56, 1979 CrossRef CAS; (d) T. Tomakinian, R. Guillot, C. Kouklovsky and G. Vincent, Angew. Chem., Int. Ed., 2014, 53, 11881 CrossRef CAS PubMed; (e) E. Yoshioka, H. Tanaka, S. Kohtani and H. Miyabe, Org. Lett., 2013, 15, 3938 CrossRef CAS PubMed.
- (a) X. Wang, Y. Lu, H. X. Dai and J. Q. Yu, J. Am. Chem. Soc., 2010, 132, 12203 CrossRef CAS PubMed; (b) M. Szlosek-Pinaud, P. Diaz, J. Martinez and F. Lamaty, Tetrahedron, 2007, 63, 3340 CrossRef CAS.
- C. Soldi, K. N. Lamb, R. A. Squitieri, M. Gonzalez-Lopez, M. J. Di Maso and J. T. Shaw, J. Am. Chem. Soc., 2014, 136, 15142 CrossRef CAS PubMed.
- J. P. Nandy, B. Rakic, B. V. N. B. Sarma, N. Babu, M. Lefrance, G. D. Enright, D. M. Leek, K. Daniel, L. A. Sabourin and P. Arya, Org. Lett., 2008, 10, 1143 CrossRef CAS PubMed.
- Ł. Albrecht, L. K. Ransborg, V. Lauridsen, M. Overgaard, T. Zweifel and K. A. Jørgensen, Angew. Chem., Int. Ed., 2011, 50, 12496 CrossRef PubMed.
- Y. H. Zhao, X. F. Ren and H. W. Liu, Synth. Commun., 2015, 45, 1566 CrossRef CAS.
- For reviews on the chemistry of N-tert-butanesulfinyl imines, see: (a) F. A. Davis, P. Zhou and B. C. Chen, Chem. Soc. Rev., 1998, 37, 13 RSC; (b) J. A. Ellman, T. D. Owens and T. P. Tang, Acc. Chem. Res., 2002, 35, 984 CrossRef CAS PubMed; (c) J. A. Ellman, Pure Appl. Chem., 2003, 75, 39 CAS; (d) C. H. Senanayake, D. Krishnamurthy, Z. H. Lu, Z. Han and E. Gallon, Aldrichimica Acta, 2005, 38, 93 CAS; (e) D. Mortan and R. A. Stockman, Tetrahedron, 2006, 62, 8869 CrossRef; (f) G. Q. Lin, M. H. Xu, Y. W. Zhong and X. W. Sun, Acc. Chem. Res., 2008, 41, 831 CrossRef CAS; (g) F. Ferreira, C. Botuha, F. Chemla and A. Pérez-Luna, Chem. Soc. Rev., 2009, 38, 1162 RSC.
- M. T. Robak, M. A. Herbage and J. A. Ellman, Chem. Rev., 2010, 110, 3600 CrossRef CAS PubMed.
- G. C. Liu, D. A. Cogan and J. A. Ellman, J. Am. Chem. Soc., 1997, 119, 9913 CrossRef CAS.
- (a) B. Cui, G. Hou, Y. Cai and Z. W. Miao, Carbohydr. Res., 2013, 374, 1 CrossRef CAS PubMed; (b) H. T. Yang, B. Cui, G. P. Wu, Z. W. Miao and R. Y. Chen, Tetrahedron, 2012, 68, 4830 CrossRef CAS; (c) B. Cui, S. S. Kong, G. P. Wu, Z. W. Miao and R. Y. Chen, Synthesis, 2012, 44, 111 CrossRef; (d) J. P. Yu, Z. W. Miao and R. Y. Chen, Org. Biomol. Chem., 2011, 9, 1756 RSC; (e) Y. D. Wang, Y. Y. Wang, J. P. Yu, Z. W. Miao and R. Y. Chen, Chem.–Eur. J., 2009, 15, 9290 CrossRef CAS; (f) Y. D. Wang, F. Wang, Y. Y. Wang, Z. W. Miao and R. Y. Chen, Adv. Synth. Catal., 2008, 350, 2339 CrossRef CAS.
- (a) Z. B. Yuan, X. X. Fang, X. Y. Li, J. Wu, H. Q. Yao and A. J. Lin, J. Org. Chem., 2015, 80, 11123 CrossRef CAS; (b) L. N. Liu, Z. B. Yuan, R. Pan, Y. Y. Zeng, A. J. Lin, H. Q. Yao and Y. Huang, Org. Chem. Front., 2018, 5, 623 RSC.
- (a) G. Liu, D. A. Cogan, T. D. Owens, T. P. Tang and J. A. Ellman, J. Org. Chem., 1999, 64, 1278 CrossRef CAS; (b) F. A. Davis, Y. Zhang, Y. Andemichael, T. Fang, D. L. Fanelli and H. Zhang, J. Org. Chem., 1999, 64, 1403 CrossRef CAS.
- (a) P. Z. Xie, L. Y. Wang, L. H. Yang, E. Q. Li, J. Z. Ma, Y. Huang and R. Y. Chen, J. Org. Chem., 2011, 76, 7699 CrossRef CAS; (b) Q. B. Li, F. T. Zhou, Z. G. Liu, X. F. Li, W. D. Zhu and J. W. Xie, J. Org. Chem., 2011, 76, 7222 CrossRef CAS PubMed.
- Crystallographic data for the structural analysis of compounds 3a and 4c has been deposited at the Cambridge Crystallographic Data Centre as No. CCDC 1583643 and 1587117.‡.
- (a) X. T. Meng, Y. Huang and R. Y. Chen, Org. Lett., 2009, 11, 137 CrossRef CAS; (b) I. Lantos, J. Flisak, L. Liu, R. Matsuoka, W. Mendelson, D. Stevenson, K. Tubman, K. Tucker, W. Y. Zhang and J. Adams, J. Org. Chem., 1997, 62, 5385 CrossRef CAS; (c) D. Morton, D. Pearson, R. A. Field and R. A. Stockman, Chem. Commun., 2006, 17, 1833 RSC.
Footnotes |
| † Dedicated to 100th anniversary of Nankai University. Dedicated to the 100th birthday anniversary of Professor Ruyu Chen. |
| ‡ Electronic supplementary information (ESI) available. CCDC 1583643 and 1587117. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra00309f |
| This journal is © The Royal Society of Chemistry 2019 |