Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Controlled synthesis of uniform multilayer hexagonal boron nitride films on Fe2B alloy
Zhiyuan Shiabc,
Guangyuan Luac,
Peng Yangd,
Tianru Wu *ac,
Weijun Yina,
Chao Zhangabc,
Ren Jiangae and
Xiaoming Xieabcf
*ac,
Weijun Yina,
Chao Zhangabc,
Ren Jiangae and
Xiaoming Xieabcf
aState Key Laboratory of Functional Materials for Informatics, Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Sciences, Shanghai 200050, China. E-mail: trwu@mail.sim.ac.cn
bSchool of Electronic, Electrical and Communication Engineering, University of Chinese Academy of Sciences, 19 A Yuquan Road, Beijing 100049, China
cCAS Center for Excellence in Superconducting Electronics (CENSE), Shanghai 200050, China
dState Key Laboratory of ASIC and System, School of Information Science and Technology, Fudan University, 220 Handan Road, Shanghai 200433, China
eState Key Laboratory of Precision Spectroscopy, School of Physics and Material Science, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062, China
fSchool of Physical Science and Technology, ShanghaiTech University, Shanghai 200031, China
First published on 1st April 2019
Abstract
Two-dimensional (2D) hexagonal boron nitride (h-BN) is highly appreciated for its excellent insulating performance and absence of dangling bonds, which could be employed to maintain the intrinsic properties of 2D materials. However, controllable synthesis of large scale multilayer h-BN is still very challenging. Here, we demonstrate chemical vapor deposition (CVD) growth of multilayer h-BN by using iron boride (Fe2B) alloy and nitrogen (N2) as precursors. Different from the self-limited growth mechanism of monolayer h-BN on catalytic metal surfaces, with sufficient B source in the bulk, Fe2B alloy promotes the controllable isothermal segregation of multilayer h-BN by reacting with active N atoms on the surface of the substrate. Microscopic and spectroscopic characterizations prove the high uniformity and crystalline quality of h-BN with a highly orientated layered lattice structure. The achievement of large scale multilayer h-BN in this work would facilitate its applications in 2D electronics and optoelectronics in the future.
Introduction
Two-dimensional (2D) materials have attracted enormous attention for future electronic and optoelectronic device integrations.1–3 Hexagonal boron nitride (h-BN), a 2D insulator with a wide band gap (∼6 eV), is in general highly desired for practical device applications, such as gate dielectric layers, tunnelling barriers or encapsulations.4–7 It is widely regarded as an ideal dielectric substrate for 2D materials because of its atomic flatness and absence of dangling bonds, which could maintain the intrinsic properties of the overlying 2D materials.3,5,6,8 Moreover, single phonon emission from isolated point defect sites in h-BN has been investigated recently, which demonstrates potential in quantum photonics and quantum computation.9–11So far, many efforts have been devoted to synthesize high-quality h-BN films. In recent years, layered h-BN flakes, integrated with other layered materials for 2D heterostructure devices, were mechanically exfoliated from high quality h-BN bulk crystal, which was obtained under extremely high temperature and pressure for several days.12,13 However, the limited size and inhomogeneous thickness hinder further development of 2D electronics and photonics. As the most promising technique, chemical vapor deposition (CVD) was employed to achieve large-area monolayer and few layer h-BN on metal or dielectric substrates by using ammonia borane (NH3·BH3) or borazine (B3N3H6) as the precursor.14–23 However, the inhomogeneous thickness, poor crystallinity, toxicity of precursors and complicated procedures limits the application in practical 2D devices. High quality, thickness uniformity, low cost and facile synthesis remain extremely challenging for compound 2D layered materials such as h-BN.
Here, we report a new method to synthesize multilayer h-BN on Fe2B alloy with N2 as a reactant. It is much easier to control the flow rate by using gaseous N2 as a precursor than solid precursors (NH3·BH3 or B3N3H6). Comparing with previous approaches, the new synthesis method yields large scale and high quality multilayer h-BN at ambient pressure. The surface morphology, crystalline quality and defect distributions were investigated by various characterizations. Fe2B, an alloy not only supplies boron source directly, but also reacts with N2 to form multilayer h-BN. The entire growth process breaks the traditional self-limited growth mechanism on catalytic metal substrates and successfully obtains multilayer h-BN on Fe2B alloy. Controllable synthesis of h-BN film establishes a significant approach for further rational catalyst design for other compound 2D materials.
Materials and methods
Multilayer h-BN was synthesized on Fe2B alloy using ambient pressure CVD. Firstly, a Fe2B substrate was heated to 1300 °C and maintained for 60 minutes under Ar and H2 atmosphere at flow rates of 300 and 50 sccm, respectively. Then, N2 and H2 atmosphere at flow rates of 300 sccm and 50 sccm were introduced at 1300 °C for 60 minutes to facilitate h-BN thin film growth. Finally, the furnace was cooled down to room temperature naturally under Ar and H2 flows.The as-grown multilayer h-BN films were transferred to arbitrary substrates through chemical transfer process. The morphology of multilayer h-BN on Fe2B alloy was characterized by scanning electron microscope (SEM, Zeiss Supra 55, operated at 3 kV). Atomic force microscope (AFM, Bruker Icon, Tapping mode) was employed to determine the thickness of multilayer h-BN. Raman spectra were obtained in the spectral range of 1100–1700 (cm−1) by using a laser excitation of 532 nm. The stoichiometry of B and N of h-BN was evaluated by X-ray photoemission spectroscopy (XPS, species, Al Kα). The phase structure of h-BN thin film was identified by X-ray diffraction (XRD, Bruker discover D8). Transmission electron microscope (TEM, JEM-2100F, operated at 200 kV) images and selected area electron diffraction (SAED) confirm the high crystalline quality of as-prepared h-BN. Photoluminescence (PL) mapping and PL spectra were collected by using a laser excitation of 532 nm (WITec Alpha 300R, 5 mW).
Results and discussion
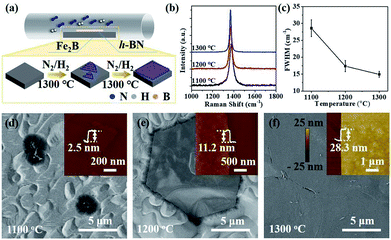
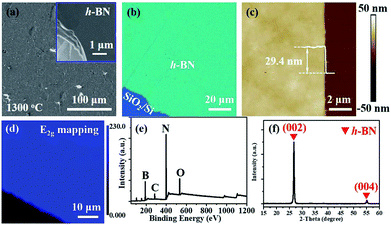
The schematic of ambient-pressure CVD setup for multilayer h-BN growth was depicted in Fig. 1a (details are given in Materials and methods). To demonstrate the growth dynamics, Fe2B alloys were annealed at 1100 °C, 1200 °C and 1300 °C for 60 minutes, respectively. SEM images exhibit h-BN nucleus, isolated h-BN flakes and continuous h-BN film synthesized at different growth temperatures (Fig. 1d–f). Corresponding AFM images and height profiles in the inset verify the thickness of as-grown h-BN transferred on SiO2 (300 nm)/Si substrate. By introducing N2 gas as a reactant, uniform multilayer h-BN films over large areas formed on Fe2B substrate. Fig. 1b displays the Raman spectra of multilayer h-BN films prepared at various growth temperatures. It could be observed that the position of characteristic E2g band presents a slightly shift (from 1372 to 1366 cm−1) with the increasing of growth temperature, indicating the increase of thickness.24 According to earlier reports, the full-width at half maximum (FWHM) of the Raman E2g band is associated with the crystallinity of h-BN.24–26 The FWHM of E2g phonon mode decreased with growth temperature increasing (Fig. 1c). The h-BN synthesized at 1300 °C shows the minimum FWHM value of ∼15 cm−1, which is much smaller than that synthesized by existing catalytic epitaxial approaches.25Multilayer h-BN film obtained at 1300 °C was further characterized to analyse its morphology and crystalline quality. Continuous and uniform h-BN film grown on Fe2B alloy was displayed in Fig. 2a. Layered structure was verified primarily by measuring the edge of h-BN film. To further evaluate the thickness uniformity of h-BN films on Fe2B substrate, the as-grown multilayer h-BN was transferred on a SiO2 (300 nm)/Si substrate. The uniform optical contrast confirms the smooth surface and high uniformity of thickness (Fig. 2b). The thickness of the film is around 30 nm according to the height profile on the edge of h-BN (Fig. 2c). Also, Raman mapping of E2g phonon mode depicts the homogeneity thickness of as-prepared h-BN multilayers (Fig. 2d). Moreover, we investigated the composition and crystal structure of h-BN film. The survey XPS spectrum of h-BN film, displayed in Fig. 2e, indicates that the stoichiometry of B and N atoms is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.06. Further, The XRD spectrum displays sharp (0002) diffraction peak at 26.8° and (0004) diffraction peak at 55.2°, which signifies the layers of h-BN are well aligned with the c axis (Fig. 2f).18 The Inorganic Crystal Structure Database (ICSD) (h-BN, 340421) was used for phase identification. The lattice constant was calculated to be 0.33 nm, which is in good agreement with reported values.18
1.06. Further, The XRD spectrum displays sharp (0002) diffraction peak at 26.8° and (0004) diffraction peak at 55.2°, which signifies the layers of h-BN are well aligned with the c axis (Fig. 2f).18 The Inorganic Crystal Structure Database (ICSD) (h-BN, 340421) was used for phase identification. The lattice constant was calculated to be 0.33 nm, which is in good agreement with reported values.18
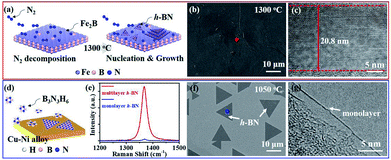
As previous reports, NH3·BH3 or B3N3H6 was always used to synthesize monolayer or few-layer h-BN on metal substrates.14–17,19,21,23 For monolayer h-BN grown on Cu–Ni alloy, B3N3H6 precursor undergoes decompose, polymerization and dehydrogenation on the surface of the substrate (Fig. 3d).19 SEM image displays the morphology of h-BN domains and TEM image signifies the monolayer nature of as-grown h-BN (Fig. 3f and g). The surfaces do not assist the synthesis of multilayer h-BN due to the self-limited growth effect when monolayer h-BN covered the Cu–Ni alloy.19 For multilayer h-BN grown on Fe2B alloy, N2 would firstly decompose into active nitrogen atoms with the catalysis of Fe2B substrate.27 Then, active nitrogen atoms would react with Fe2B to form initial B–N molecular.18,27 High concentration B–N molecules would segregate and induce the nucleation of h-BN isothermally. Finally, h-BN nucleus would grow and coalesce into multilayer h-BN films on the surface of Fe2B alloy. The controllable thickness would be realized by optimizing growth temperature or reaction time (Fig. 3a). SEM image of as-grown multilayer h-BN displays the uniform and smooth surface morphology and TEM image indicates multi-layered h-BN nanostructure (Fig. 3b and c). Further, E2g phonon mode of multilayer h-BN in the Raman spectrum shows much stronger intensity than the E2g band of monolayer h-BN (Fig. 3e). Comparing with the two synthesis approaches mentioned above, the success of thickness controllable growth is mainly due to the change of h-BN growth mechanism from surface-mediated growth to isothermal segregation on Fe2B substrate by using nitrogen as feedstock.
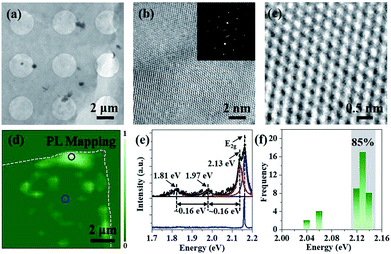
The crystalline quality and atomic structure of the multilayer h-BN is further characterized by TEM. The low-magnified TEM view in Fig. 4a displays continuous and uniform h-BN film suspended on Cu mesh grid. High resolution TEM image exhibits the lattice fringe of h-BN multilayers (Fig. 4b). Corresponding selected area electron diffraction (SAED) presents only one set of hexagonally arranged spots, rather than ring-shaped patterns, assuring the highly oriented stacking order.20 Moreover, Fig. 4c reveals a honeycomb structure with an inter-atomic distance of about 0.25 nm, in good agreement with bulk h-BN.28 Moreover, as earlier theoretically and experimentally analysis, point defects would induce non-resonant absorption and emission within the band gap of materials.9,29–31 Here, PL spectroscopy was used to investigate point defects distributions in h-BN film.9,30,31 PL measurement was carried out at room temperature with a 532 nm sub-bandgap excitation laser. Fig. 4d illustrates the defect distributions in h-BN flake transferred on Si substrate. Optical emissions are accumulated on the edge of as-prepared h-BN multilayers, which may be introduced by the transfer and annealing processes. Typical PL spectra were extracted and fitted by Lorentzian fitting (Fig. 4e). Three peaks centred at 2.13 eV, 1.97 eV and 1.81 eV were observed, which is consisted with zero phonon line (ZPL) and corresponding one- and two-optical-phonon sidebands (PSB), respectively.9,30,31 In addition, Fig. 4f displays a histogram of the emission energy values measured for 40 individual emitters. More than 85% of the emitters were measured to have a ZPL energy localized in a very narrow spectral window, (2.13 ± 0.1) eV. Such a small spectra variability of the ZPLs may be caused by strain and/or local lattice symmetry distortions.31 Moreover, it is observed that no optical emission was found on the large area of h-BN region, indicating the high quality of as-grown h-BN multilayers.
Conclusion
In this work, uniform and continuous multilayer h-BN has been obtained isothermally through the reaction between Fe2B alloy and N2 via CVD method. The thickness could be mediated by controlling diffusion rate of nitrogen in the catalyst at different growth temperatures. Raman shift at 1366 cm−1 and sharp (0002) diffraction peak in the XRD pattern confirm the crystallinity of h-BN. TEM measurements revealed that the h-BN has highly orientated layered lattice structure. Comparing with monolayer h-BN growth on catalytic metal surfaces, this method shows its unique mechanism for achieving multilayer h-BN, which has potential on the synthesis of other layered 2D heterostructures. Therefore, the present achievement of high-quality multilayer h-BN synthesis would give strong impact on ultrathin dielectric-, support-, or barrier-layer in particular for integrated electronics and photonics of 2D materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Xudong Hu for the Raman mapping of multilayer h-BN. We also acknowledge Wei Liu and Ruxiang Shen for the XRD and XPS measurements. This work was financially supported by the National Key R&D program (Grant No. 2017YFF0206106), the National Science Foundation of China (Grant No. 51772317), the Science and Technology Commission of Shanghai Municipality (Grant No. 16ZR1442700, 18511110700), Shanghai Rising-Star Program (A type) (Grant No. 18QA1404800), China Postdoctoral Science Foundation (Grant No. 2017M621563, 2018T110415).Notes and references
- K. S. Novoselov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- K. F. Mak, C. Lee, J. Hone, J. Shan and T. F. Heinz, Phys. Rev. Lett., 2010, 105, 136805 CrossRef PubMed.
- L. Li, F. Yang, G. J. Ye, Z. Zhang, Z. Zhu, W. Lou, X. Zhou, L. Li, K. Watanabe, T. Taniguchi, K. Chang, Y. Wang, X. H. Chen and Y. Zhang, Nat. Nanotechnol., 2016, 11, 593–597 CrossRef CAS.
- K. Watanabe, T. Taniguchi and H. Kanda, Phys. Status Solidi, 2004, 201, 2561–2565 CrossRef CAS.
- A. K. Geim and I. V. Grigorieva, Nature, 2013, 499, 419–425 CrossRef CAS.
- L. Wang, I. Meric, P. Y. Huang, Q. Gao, Y. Gao, H. Tran, T. Taniguchi, K. Watanabe, L. M. Campos, D. A. Muller, J. Guo, P. Kim, J. Hone, K. L. Shepard and C. R. Dean, Science, 2013, 342, 614–617 CrossRef CAS PubMed.
- L. Britnell, R. V. Gorbachev, R. Jalil, B. D. Belle, F. Schedin, M. I. Katsnelson, L. Eaves, S. V. Morozov, A. S. Mayorov, N. M. R. Peres, A. H. Castro Neto, J. Leist, A. K. Geim, L. A. Ponomarenko and K. S. Novoselov, Nano Lett., 2012, 12, 1707–1710 CrossRef CAS PubMed.
- C. R. Dean, A. F. Young, I. Meric, C. Lee, L. Wang, S. Sorgenfrei, K. Watanabe, T. Taniguchi, P. Kim, K. L. Shepard and J. Hone, Nat. Nanotechnol., 2010, 5, 722–726 CrossRef CAS PubMed.
- T. T. Tran, K. Bray, M. J. Ford, M. Toth and I. Aharonovich, Nat. Nanotechnol., 2016, 11, 37–41 CrossRef CAS PubMed.
- S. Kim, J. E. Fröch, J. Christian, M. Straw, J. Bishop, D. Totonjian, K. Watanabe, T. Taniguchi, M. Toth and I. Aharonovich, Nat. Commun., 2018, 9, 2623 CrossRef PubMed.
- A. Sajid, J. R. Reimers and M. J. Ford, Phys. Rev. B, 2018, 97, 064101 CrossRef CAS.
- Y. Kubota, K. Watanabe, O. Tsuda and T. Taniguchi, Science, 2007, 317, 932–934 CrossRef CAS PubMed.
- S. Liu, R. He, Z. Ye, X. Du, J. Lin, H. Jiang, B. Liu and J. H. Edgar, Cryst. Growth Des., 2017, 17, 4932–4935 CrossRef CAS.
- Y. Shi, C. Hamsen, X. Jia, K. K. Kim, A. Reina, M. Hofmann, A. L. Hsu, K. Zhang, H. Li, Z.-Y. Juang, M. S. Dresselhaus, L.-J. Li and J. Kong, Nano Lett., 2010, 10, 4134–4139 CrossRef CAS PubMed.
- K. K. Kim, A. Hsu, X. Jia, S. M. Kim, Y. Shi, M. Hofmann, D. Nezich, J. F. Rodriguez-Nieva, M. Dresselhaus, T. Palacios and J. Kong, Nano Lett., 2012, 12, 161–166 CrossRef PubMed.
- J. H. Park, J. C. Park, S. J. Yun, H. Kim, D. H. Luong, S. M. Kim, S. H. Choi, W. Yang, J. Kong, K. K. Kim and Y. H. Lee, ACS Nano, 2014, 8, 8520–8528 CrossRef CAS.
- S. Caneva, R. S. Weatherup, B. C. Bayer, B. Brennan, S. J. Spencer, K. Mingard, A. Cabrero-Vilatela, C. Baehtz, A. J. Pollard and S. Hofmann, Nano Lett., 2015, 15, 1867–1875 CrossRef CAS.
- S. M. Kim, A. Hsu, M. H. Park, S. H. Chae, S. J. Yun, J. S. Lee, D.-H. Cho, W. Fang, C. Lee, T. Palacios, M. Dresselhaus, K. K. Kim, Y. H. Lee and J. Kong, Nat. Commun., 2015, 6, 8662 CrossRef CAS PubMed.
- G. Lu, T. Wu, Q. Yuan, H. Wang, H. Wang, F. Ding, X. Xie and M. Jiang, Nat. Commun., 2015, 6, 6160 CrossRef CAS PubMed.
- A.-R. Jang, S. Hong, C. Hyun, S. I. Yoon, G. Kim, H. Y. Jeong, T. J. Shin, S. O. Park, K. Wong, S. K. Kwak, N. Park, K. Yu, E. Choi, A. Mishchenko, F. Withers, K. S. Novoselov, H. Lim and H. S. Shin, Nano Lett., 2016, 16, 3360–3366 CrossRef CAS PubMed.
- Y. Uchida, S. Nakandakari, K. Kawahara, S. Yamasaki, M. Mitsuhara and H. Ago, ACS Nano, 2018, 12, 6236–6244 CrossRef CAS PubMed.
- Q. Li, Q. Wu, J. Gao, T. Wei, J. Sun, H. Hong, Z. Dou, Z. Zhang, M. H. Rümmeli, P. Gao, J. Yan, J. Wang, J. Li, Y. Zhang and Z. Liu, Adv. Mater. Interfaces, 2018, 5, 1800662 CrossRef.
- J. S. Lee, S. H. Choi, S. J. Yun, Y. I. Kim, S. Boandoh, J.-H. Park, B. G. Shin, H. Ko, S. H. Lee, Y.-M. Kim, Y. H. Lee, K. K. Kim and S. M. Kim, Science, 2018, 362, 817–821 CrossRef CAS.
- R. V. Gorbachev, I. Riaz, R. R. Nair, R. Jalil, L. Britnell, B. D. Belle, E. W. Hill, K. S. Novoselov, K. Watanabe, T. Taniguchi, A. K. Geim and P. Blake, Small, 2011, 7, 465–468 CrossRef CAS.
- L. Schué, I. Stenger, F. Fossard, A. Loiseau and J. Barjon, 2D Mater., 2016, 4, 015028 CrossRef.
- Q. Cai, D. Scullion, A. Falin, K. Watanabe, T. Taniguchi, Y. Chen, E. J. G. Santos and L. H. Li, Nanoscale, 2017, 9, 3059–3067 RSC.
- H. Tokoro, S. Fujii, T. Oku, T. Segi and S. Nasu, Mater. Trans., 2004, 45, 2941–2944 CrossRef CAS.
- Y. Kobayashi, T. Akasaka and T. Makimoto, J. Cryst. Growth, 2008, 310, 5048–5052 CrossRef CAS.
- K. Huang and A. Rhys, Proc. R. Soc. Lond. A Math. Phys. Sci., 1950, 204, 406–423 CAS.
- N. R. Jungwirth and G. D. Fuchs, Phys. Rev. Lett., 2017, 119, 057401 CrossRef PubMed.
- X. Li, G. D. Shepard, A. Cupo, N. Camporeale, K. Shayan, Y. Luo, V. Meunier and S. Strauf, ACS Nano, 2017, 11, 6652–6660 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |